Association Between Soil Patterns and Mortality with Distinct Types of Cancers and CVD Across the USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Design
2.2. Database
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sigel, A.; Sigel, H.; Sigel, R.K.O. (Eds.) Interrelations Between Essential Metal Ions and Human Diseases; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; Volume 13, ISBN 978-94-007-7499-5. [Google Scholar]
- Mertz, W. The Essential Trace Elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Daghbouche-Rubio, N.; López-López, J.R.; Pérez-García, M.T.; Cidad, P. Vascular Smooth Muscle Ion Channels in Essential Hypertension. Front. Physiol. 2022, 13, 1016175. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and Human Health: An Update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Liu, K.; Wang, Y.; Jiang, Y.; Zhang, C. Association of Dietary Intake of Zinc and Selenium with Breast Cancer Risk: A Case-Control Study in Chinese Women. Nutrients 2023, 15, 3253. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, J.; Nie, G.; Xie, D.; Zhu, X.; Niu, J.; Li, X. Association and Mediation Analyses among Multiple Metal Exposure, Mineralocorticoid Levels, and Serum Ion Balance in Residents of Northwest China. Sci. Rep. 2024, 14, 8023. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, Y.; Hao, H.; Zhu, W.; Zou, M.; Liu, Q.; Wang, M.; Han, B.; Bao, L.; Niu, Y.; et al. Associations of Multiple Serum Metals with the Risk of Metabolic Syndrome among the Older Population in China Based on a Community Study: A Mediation Role of Peripheral Blood Cells. Ecotoxicol. Environ. Saf. 2024, 284, 116981. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lv, J.; Zhang, A. Depletion of S-Adenosylmethionine Induced by Arsenic Exposure Is Involved in Liver Injury of Rat through Perturbing Histone H3K36 Trimethylation Dependent Bile Acid Metabolism. Environ. Pollut. 2023, 334, 122228. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, D.; Long, K.; Xiong, W.; Man, J.; Zhang, Q.; Zhang, Z. N6-Methyladenosine Promotes Aberrant Redox Homeostasis Required for Arsenic Carcinogenesis by Controlling the Adaptation of Key Antioxidant Enzymes. J. Hazard. Mater. 2024, 465, 133329. [Google Scholar] [CrossRef]
- Wadgaonkar, P.; Wang, Z.; Chen, F. Endoplasmic Reticulum Stress Responses and Epigenetic Alterations in Arsenic Carcinogenesis. Environ. Pollut. 2024, 347, 123565. [Google Scholar] [CrossRef]
- Yamamoto, T.; Gi, M.; Yamashita, S.; Suzuki, S.; Fujioka, M.; Vachiraarunwong, A.; Guo, R.; Qiu, G.; Kakehashi, A.; Kato, M.; et al. DNA Methylation Aberrations in Dimethylarsinic Acid-Induced Bladder Carcinogenesis. Cancers 2023, 15, 5274. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium Distribution in the Chinese Environment and Its Relationship with Human Health: A Review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Zha, X.; An, J.; Gao, X.; Tian, Y. Dietary and Drinking Water Intake of Essential Trace Elements in a Typical Kashin-Beck Disease Endemic Area of Tibet, China. Environ. Health 2022, 21, 86. [Google Scholar] [CrossRef]
- Qu, B.; Wu, S.; Zhao, P.; Ma, Z.F.; Goodacre, R.; Yuan, L.; Chen, Y. Geographical Pattern of Minerals and Its Association with Health Disparities in the USA. Environ. Geochem. Health 2023, 45, 4407–4424. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B.; Russell, M.W.; Huse, D.M.; Ellison, R.C.; Silbershatz, H.; Wilson, P.W.F.; Hartz, S.C. Primary and Subsequent Coronary Risk Appraisal: New Results from the Framingham Study. Am. Heart J. 2000, 139, 272–281. [Google Scholar] [CrossRef]
- Cuomo, A.; Pirozzi, F.; Attanasio, U.; Franco, R.; Elia, F.; De Rosa, E.; Russo, M.; Ghigo, A.; Ameri, P.; Tocchetti, C.G.; et al. Cancer Risk in the Heart Failure Population: Epidemiology, Mechanisms, and Clinical Implications. Curr. Oncol. Rep. 2021, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Mookadam, F.; Sharma, A.; Lee, H.R.; Northfelt, D.W. Intersection of Cardiology and Oncology Clinical Practices. Front. Oncol. 2014, 4, 259. [Google Scholar] [CrossRef]
- Narayan, V.; Thompson, E.W.; Demissei, B.; Ho, J.E.; Januzzi, J.; Ky, B. Mechanistic Biomarkers Informative of Both Cancer and Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 2726–2737. [Google Scholar] [CrossRef]
- Smith, D.B.; Smith, S.M.; Horton, J.D. History and Evaluation of National-Scale Geochemical Data Sets for the United States. Geosci. Front. 2013, 4, 167–183. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. United States Cancer Mortality Rates by County 1980–2014. Seattle, United States; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2017. [Google Scholar]
- Chen, Y.; Zhao, P.; Xu, Q.; Qu, B.; Li, D.; Clement, S.; Li, L. Relating Biodiversity with Health Disparities of Human Population: An Ecological Study across the United States. One Health 2023, 16, 100548. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Li, H.; Yang, L.; Li, Y.; Kong, C. Spatial Distribution and Determinants of Health Loss from Kashin-Beck Disease in Bin County, Shaanxi Province, China. BMC Public Health 2021, 21, 387. [Google Scholar] [CrossRef] [PubMed]
- Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Kutz, M.J.; Huynh, C.; Barber, R.M.; Shackelford, K.A.; Mackenbach, J.P.; van Lenthe, F.J.; et al. US County-Level Trends in Mortality Rates for Major Causes of Death, 1980-2014. JAMA 2016, 316, 2385–2401. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Mackenbach, J.P.; van Lenthe, F.J.; Mokdad, A.H.; Murray, C.J.L. Inequalities in Life Expectancy Among US Counties, 1980 to 2014: Temporal Trends and Key Drivers. JAMA Intern. Med. 2017, 177, 1003. [Google Scholar] [CrossRef]
- GBD 2019 Respiratory Tract Cancers Collaborators. Global, Regional, and National Burden of Respiratory Tract Cancers and Associated Risk Factors from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet. Respir. Med. 2021, 9, 1030–1049. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Santoni, M.; Mollica, V.; Fiorentino, M.; Brandi, G.; Massari, F. Microbiota and Prostate Cancer. Semin. Cancer Biol. 2022, 86, 1058–1065. [Google Scholar] [CrossRef]
- Kelly, S.P.; Anderson, W.F.; Rosenberg, P.S.; Cook, M.B. Past, Current, and Future Incidence Rates and Burden of Metastatic Prostate Cancer in the United States. Eur. Urol. Focus 2018, 4, 121–127. [Google Scholar] [CrossRef]
- Sharma, V.K. Cerebrovascular Disease. In International Encyclopedia of Public Health; Elsevier: Amsterdam, The Netherlands, 2017; pp. 455–470. ISBN 978-0-12-803708-9. [Google Scholar]
- Díez, J.; Butler, J. Growing Heart Failure Burden of Hypertensive Heart Disease: A Call to Action. Hypertension 2023, 80, 13–21. [Google Scholar] [CrossRef]
- Maleczek, M.; Reszeć-Giełażyn, J.; Szymulewska-Konopko, K. Beneficial Effects of Selenium and Its Supplementation on Carcinogenesis and the Use of Nanoselenium in the Treatment of Malignant Tumors. Int. J. Mol. Sci. 2024, 25, 11285. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, B.; Tang, B.; Wei, Q. Selenium in Prostate Cancer: Prevention, Progression, and Treatment. Pharmaceuticals 2023, 16, 1250. [Google Scholar] [CrossRef]
- He, L.; Zhang, L.; Peng, Y.; He, Z. Selenium in Cancer Management: Exploring the Therapeutic Potential. Front. Oncol. 2025, 14, 1490740. [Google Scholar] [CrossRef]
- Zhuo, H.; Smith, A.H.; Steinmaus, C. Selenium and Lung Cancer: A Quantitative Analysis of Heterogeneity in the Current Epidemiological Literature. Cancer Epidemiol. Biomark. Prev. 2004, 13, 771–778. [Google Scholar] [CrossRef]
- Peters, U.; Takata, Y. Selenium and the Prevention of Prostate and Colorectal Cancer. Mol. Nutr. Food Res. 2008, 52, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Health. Rpt. 2018, 5, 464–485. [Google Scholar] [CrossRef]
- Qi, L.; Wang, Y.; Su, S.; Wang, M.; Jablonska, E.; Jia, Y.; Wang, R.; Hao, S.; Feng, C.; Li, G.; et al. Sodium Selenite Inhibits Cervical Cancer Growth via ROS Mediated AMPK/FOXO3a /GADD45a Axis. Chem. Biol. Interact. 2022, 367, 110171. [Google Scholar] [CrossRef]
- Chen, D.; Cai, B.; Zhu, Y.; Ma, Y.; Yu, X.; Xiong, J.; Shen, J.; Tie, W.; Zhang, Y.; Guo, F. Targeting Histone Demethylases JMJD3 and UTX: Selenium as a Potential Therapeutic Agent for Cervical Cancer. Clin. Epigenetics 2024, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Yu, P.; Gui, L.; Wang, N.; Pan, D.; Wang, S. Relationship between Serum Levels of Selenium and Thyroid Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 75, 14–23. [Google Scholar] [CrossRef]
- Ma, J.; Huang, X.; Xu, J.; Li, Z.; Lai, J.; Shen, Y.; Zhao, J.; Sun, X.; Ma, L. SBP1 Promotes Tumorigenesis of Thyroid Cancer through TXN/NIS Pathway. Mol. Med. 2023, 29, 121. [Google Scholar] [CrossRef]
- Chalcarz, M.; Grabarek, B.O.; de Mezer, M.; Krokowicz, P.; Patera, J.; Czupryna, K.; Żurawski, J. Serum Selenium Concentration as a Potential Diagnostic Marker for Early-Stage Colorectal Cancer: A Comparative Study. Med. Sci. Monit. 2024, 30, e942882-1–e942882-8. [Google Scholar] [CrossRef]
- Shimada, B.K.; Alfulaij, N.; Seale, L.A. The Impact of Selenium Deficiency on Cardiovascular Function. Int. J. Mol. Sci. 2021, 22, 10713. [Google Scholar] [CrossRef] [PubMed]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and Its Supplementation in Cardiovascular Disease—What Do We Know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, Y.; Sui, W. Association Between Blood Selenium Levels and Stroke: A Study Based on the NHANES (2011–2018). Biol. Trace. Elem. Res. 2024, 202, 25–33. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Adaramoye, O.; Akinloye, O.; Olatunji, I. Trace Elements and Vitamin E Status in Nigerian Patients with Prostate Cancer. Afr. Health Sci. 2010, 10, 2–8. [Google Scholar]
- Amadi, C.; Aleme, B.M. The Prevalence of Zinc Deficiency among Men with and without Prostate Cancer in Port Harcourt, Nigeria. Nutr. Cancer 2020, 72, 1018–1025. [Google Scholar] [CrossRef]
- Mahabir, S.; Spitz, M.R.; Barrera, S.L.; Beaver, S.H.; Etzel, C.; Forman, M.R. Dietary Zinc, Copper and Selenium, and Risk of Lung Cancer. Int. J. Cancer 2007, 120, 1108–1115. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Li, A.; Zhang, Y. Association between Serum Zinc Levels and Lung Cancer: A Meta-Analysis of Observational Studies. World J. Surg. Oncol. 2019, 17, 78. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Fu, W.; Lu, Y.; Wei, W.; Chen, W.; Wu, X.; Meng, H.; Feng, Y.; Liu, Y.; et al. Circulating Essential Metals and Lung Cancer: Risk Assessment and Potential Molecular Effects. Environ. Int. 2019, 127, 685–693. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Zhang, Y.; Li, H.; Zhang, H.; Huo, Y.; Li, J.; Liu, X.; Wang, X.; Qin, X.; et al. Baseline Plasma Zinc and Risk of First Stroke in Hypertensive Patients: A Nested Case-Control Study. Stroke 2019, 50, 3255–3258. [Google Scholar] [CrossRef]
- Lin, R.; Feng, W.; Yang, Y.; Xu, J.; Yang, H.; Wu, J.; Li, J.; Qin, G.; Yu, Y.; Chen, J. Association of Dietary Calcium with Mortality from All Causes, Cardiovascular Disease and Cancer in People with Hypertension. J. Clin. Hypertens. 2023, 25, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Tang, Y.; Fang, S.; Gong, K.; Liu, D.; Xie, Y.; Liu, G.; Tian, Y.; Zhang, L.; Li, Y.; et al. Total, Dietary, and Supplemental Calcium Intake and Risk of All-Cause, Cardiovascular, and Cancer Mortality among U.S. Adults: A Prospective Cohort Study from the National Health and Nutrition Examination Survey. Arch. Osteoporos. 2024, 19, 114. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil Microbiomes and One Health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liao, X.; Tao, H.; Li, Y. Chain Modeling for the Biogeochemical Nexus of Cadmium in Soil–Rice–Human Health System. Environ. Int. 2022, 167, 107424. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hu, M.; Cheng, N.; Shi, R.; Ma, T.; Wang, W.; Huang, W. Prediction of the Bioaccessibility and Accumulation of Cadmium in the Soil-Rice-Human System Based on Optimized DGT and BCR Coupled Models. Ecotoxicol. Environ. Saf. 2024, 280, 116509. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.M.; Pullabhotla, H.; Bevis, L.; Lobell, D.B. Soil Micronutrients Linked to Human Health in India. Sci. Rep. 2023, 13, 13591. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.-N.; Huang, Y.; Yuan, G.-L.; Sun, Y.-C.; Li, J.; Li, H.; Zhang, B. Uptake of Zinc from the Soil to the Wheat Grain: Nonlinear Process Prediction Based on Artificial Neural Network and Geochemical Data. Sci. Total Environ. 2024, 947, 174582. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, M.; Darvishiyan, M.; Momeni, M.; Eslami, H.; Fallahzadeh, R.A.; Zarei, A. Ecological Risk Assessment of Trace Elements (TEs) Pollution and Human Health Risk Exposure in Agricultural Soils Used for Saffron Cultivation. Sci. Rep. 2023, 13, 4556. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.C.J.; Maggi, P.; Pirard, C.; Charlier, C.; Ruttens, A.; Liénard, A.; Colinet, G.; Remy, S. Human Biomonitoring Survey (Pb, Cd, As, Cu, Zn, Mo) for Urban Gardeners Exposed to Metal Contaminated Soils. Environ. Pollut. 2022, 312, 120028. [Google Scholar] [CrossRef]
- Lu, T.; Peng, H.; Yao, F.; Nadine Ferrer, A.S.; Xiong, S.; Niu, G.; Wu, Z. Trace Elements in Public Drinking Water in Chinese Cities: Insights from Their Health Risks and Mineral Nutrition Assessments. J. Environ. Manag. 2022, 318, 115540. [Google Scholar] [CrossRef]
- Chakraborty, P.; Wood, D.A.; Singh, S.; Hazra, B. Trace Element Contamination in Soils Surrounding the Open-Cast Coal Mines of Eastern Raniganj Basin, India. Environ. Geochem. Health 2023, 45, 7275–7302. [Google Scholar] [CrossRef] [PubMed]
- Baidourela, A.; Cheng, S.; Halik, Ü.; Sun, Q.; Zhayimu, K.; Zhang, C.; Cui, K.; Liu, L.; Sun, G.; Baiketuerhan, Y.; et al. Bio-Availability of Potential Trace Elements in Urban Dust, Soil, and Plants in Arid Northwest China. Int. J. Phytoremed. 2024, 26, 1981–1995. [Google Scholar] [CrossRef] [PubMed]
- Baraza, T.; Cassidy, K.J.; Hasenmueller, E.A. Road Salt Applications Mobilize Trace Elements from Roadside Soil to Shallow Groundwater. Sci. Total Environ. 2024, 942, 173435. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Vashisht, R.; Butte, A.J. From Genes to Geography, from Cells to Community, from Biomolecules to Behaviors: The Importance of Social Determinants of Health. Biomolecules 2022, 12, 1449. [Google Scholar] [CrossRef]
- Lee, J.K.; Lin, L.; Magee, C. Investigating the Relationships between Social Capital, Chronic Health Conditions and Health Status among Australian Adults: Findings from an Australian National Cohort Survey. BMC Public Health 2020, 20, 329. [Google Scholar] [CrossRef]
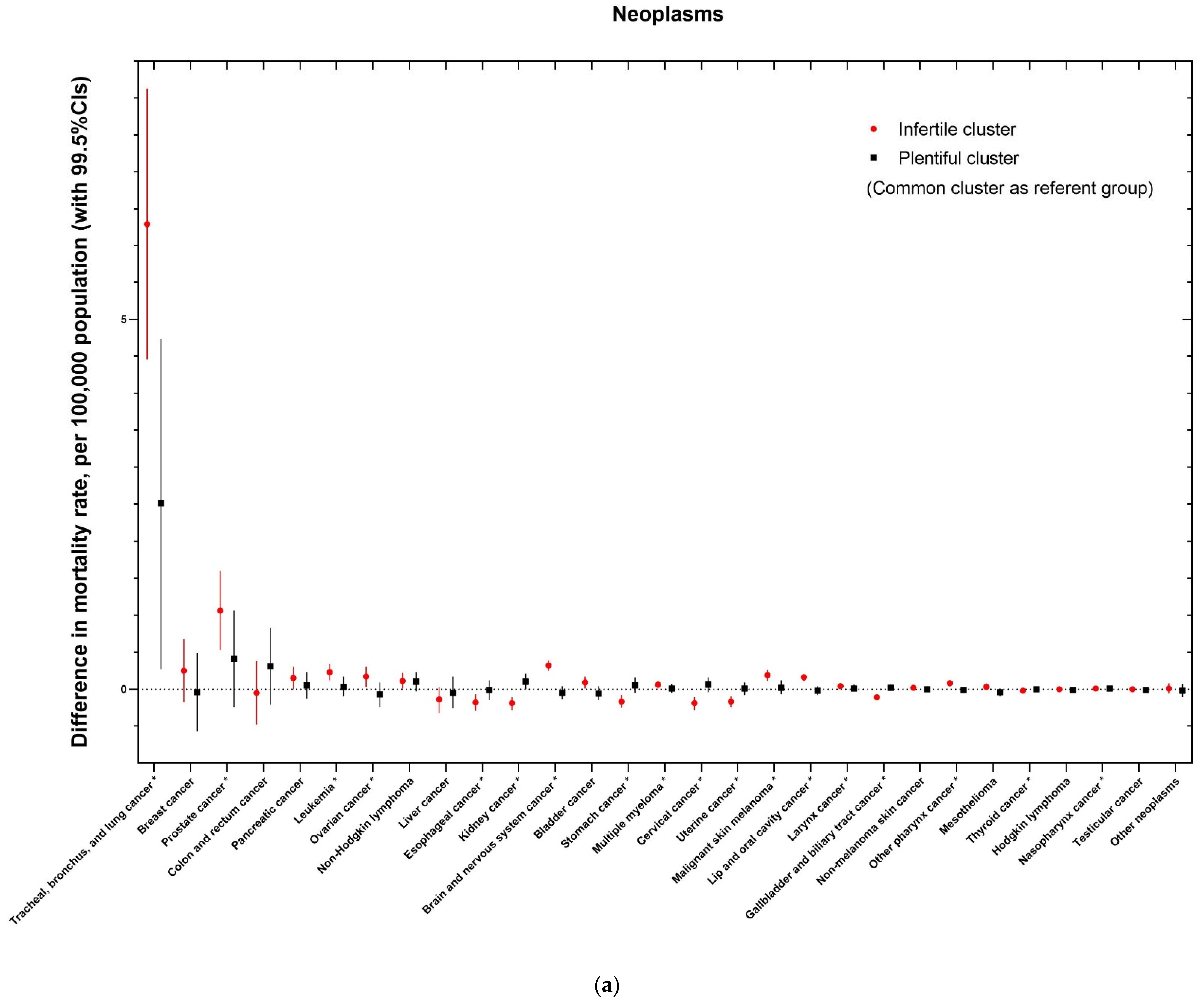
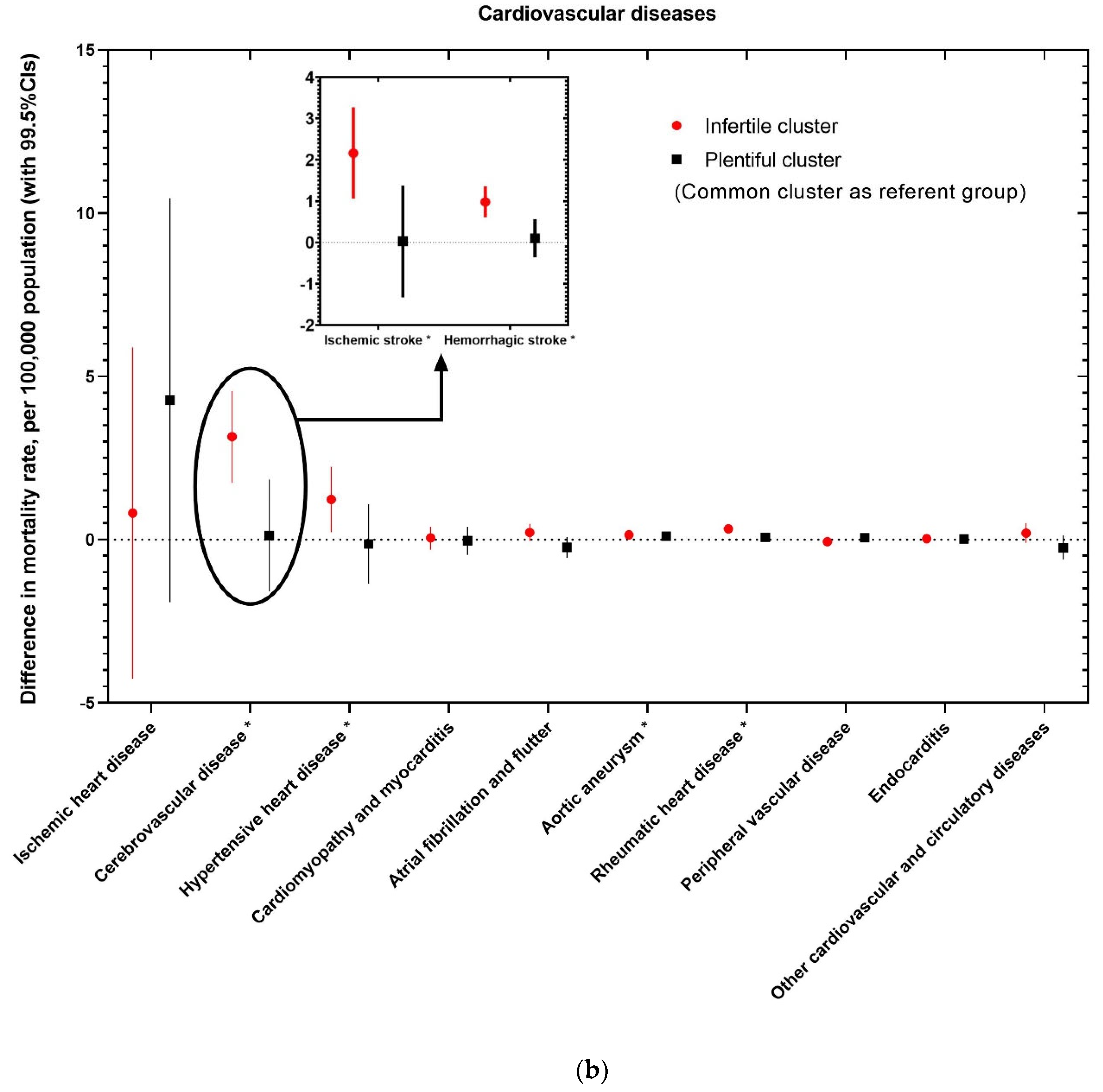
| Characteristics | Number (%), or Mean (Standard Deviation) | Number (%), or Mean (Standard Deviation) | Number (%), or Mean (Standard Deviation) | Number (%), or Mean (Standard Deviation) | p-Value 1 | |
|---|---|---|---|---|---|---|
| Total n = 3080 | The “Common” Cluster n = 2056 | The “Infertile” Cluster n = 739 | The “Plentiful” Cluster n = 285 | |||
| Mineral concentration (mg/kg) | ||||||
| Arsenic (As) | 6.61 (5.45) | 6.91 (3.24) | 3.30 (1.72) | 13.0 (12.99) | <0.001 | |
| Calcium (Ca) | 17,167.71 (20,641.96) | 17,787.26 (12,492.62) | 2669.94 (2435.21) | 50,290.75 (42,709.36) | <0.001 | |
| Selenium (Se) | 0.34 (0.26) | 0.34 (0.17) | 0.20 (0.07) | 0.68 (0.57) | <0.001 | |
| Sodium (Na) | 6318.26 (4680.04) | 7972.08 (4403.47) | 1716.39 (1564.57) | 6320.13 (3985.59) | <0.001 | |
| Zinc (Zn) | 59.64 (48.18) | 63.15 (22.57) | 28.24 (14.55) | 115.75 (122.96) | <0.001 | |
| Cause-specific mortality rate, number of deaths/100,000 population, 2014, (frequent ranking) 2 | ||||||
| Neoplasms | ||||||
| Tracheal, bronchus, and lung cancer | (1) | 62.58 (18.08) | 59.12 (16.69) | 73.81 (17.03) | 58.48 (18.55) | <0.001 |
| Breast cancer (females only) | (2) | 26.31 (4.02) | 25.53 (3.71) | 28.89 (4.02) | 25.25 (3.30) | <0.001 |
| Prostate cancer (males only) | (3) | 26.23 (4.98) | 25.47 (4.13) | 28.83 (6.43) | 24.99 (3.72) | <0.001 |
| Colon and rectum cancer | (4) | 24.74 (4.29) | 24.16 (4.06) | 26.80 (4.33) | 23.53 (4.09) | <0.001 |
| Pancreatic cancer | (5) | 12.86 (1.38) | 12.66 (1.28) | 13.58 (1.43) | 12.49 (1.33) | <0.001 |
| Leukemia | (6) | 9.55 (0.9) | 9.53 (0.89) | 9.66 (0.83) | 9.36 (1.10) | <0.001 |
| Acute lymphoid leukemia | - | 0.71 (0.12) | 0.69 (0.12) | 0.76 (0.11) | 0.69 (0.12) | <0.001 |
| Chronic lymphoid leukemia | - | 2.91 (0.37) | 2.93 (0.37) | 2.88 (0.31) | 2.84 (0.44) | <0.001 |
| Acute myeloid leukemia | - | 5.32 (0.51) | 5.30 (0.50) | 5.37 (0.46) | 5.22 (0.63) | <0.001 |
| Chronic myeloid leukemia | - | 0.61 (0.06) | 0.60 (0.05) | 0.64 (0.05) | 0.59 (0.06) | <0.001 |
| Ovarian cancer (females only) | (7) | 8.63 (0.94) | 8.62 (0.92) | 8.70 (0.99) | 8.44 (0.91) | <0.001 |
| Non-Hodgkin lymphoma | (8) | 8.62 (0.94) | 8.65 (0.93) | 8.53 (0.85) | 8.57 (1.09) | 0.006 |
| Liver cancer | (9) | 6.36 (1.67) | 6.07 (1.66) | 7.21 (1.41) | 6.20 (1.61) | <0.001 |
| Esophageal cancer | (10) | 5.49 (0.94) | 5.55 (0.93) | 5.43 (0.97) | 5.28 (0.94) | <0.001 |
| Kidney cancer | (11) | 5.20 (0.76) | 5.17 (0.75) | 5.28 (0.71) | 5.17 (0.87) | 0.004 |
| Brain and nervous system cancer | (12) | 5.15 (0.61) | 5.14 (0.58) | 5.21 (0.69) | 4.99 (0.58) | <0.001 |
| Bladder cancer | (13) | 5.10 (0.68) | 5.12 (0.70) | 5.05 (0.57) | 4.99 (0.76) | 0.001 |
| Stomach cancer | (14) | 4.43 (1.07) | 4.21 (1.01) | 5.09 (0.98) | 4.27 (0.97) | <0.001 |
| Multiple myeloma | (15) | 3.99 (0.48) | 3.92 (0.40) | 4.24 (0.36) | 3.84 (0.40) | <0.001 |
| Cervical cancer (females only) | (16) | 3.83 (1.08) | 3.61 (0.99) | 4.50 (1.05) | 3.60 (0.96) | <0.001 |
| Uterine cancer (females only) | (17) | 3.74 (0.65) | 3.77 (0.59) | 3.63 (0.76) | 3.67 (0.64) | <0.001 |
| Malignant skin melanoma | (18) | 3.39 (0.63) | 3.38 (0.62) | 3.40 (0.65) | 3.40 (0.69) | 0.703 |
| Lip and oral cavity cancer | (19) | 1.96 (0.48) | 1.85 (0.39) | 2.35 (0.51) | 1.78 (0.36) | <0.001 |
| Larynx cancer | (20) | 1.39 (0.4) | 1.30 (0.36) | 1.68 (0.40) | 1.26 (0.35) | <0.001 |
| Gallbladder and biliary tract cancer | (21) | 1.15 (0.23) | 1.17 (0.24) | 1.08 (0.14) | 1.17 (0.24) | <0.001 |
| Non-melanoma skin cancer | (22) | 1.08 (0.22) | 1.05 (0.21) | 1.14 (0.22) | 1.07 (0.23) | <0.001 |
| Other pharynx cancer | (23) | 1.02 (0.27) | 0.95 (0.22) | 1.24 (0.30) | 0.91 (0.20) | <0.001 |
| Mesothelioma | (24) | 0.98 (0.35) | 1.01 (0.34) | 0.88 (0.39) | 0.95 (0.28) | <0.001 |
| Thyroid cancer | (25) | 0.55 (0.05) | 0.55 (0.05) | 0.53 (0.04) | 0.55 (0.05) | <0.001 |
| Hodgkin lymphoma | (26) | 0.4 (0.05) | 0.38 (0.04) | 0.41 (0.05) | 0.37 (0.04) | <0.001 |
| Nasopharynx cancer | (27) | 0.29 (0.10) | 0.26 (0.09) | 0.38 (0.10) | 0.26 (0.09) | <0.001 |
| Testicular cancer (males only) | (28) | 0.28 (0.06) | 0.28 (0.05) | 0.28 (0.06) | 0.26 (0.05) | <0.001 |
| Other neoplasms | - | 6.22 (0.64) | 6.17 (0.59) | 6.43 (0.67) | 5.99 (0.70) | <0.001 |
| Cardiovascular diseases | ||||||
| Ischemic heart disease | (1) | 173.81 (47.17) | 166.80 (45.42) | 196.54 (45.70) | 165.33 (44.63) | <0.001 |
| Cerebrovascular disease | (2) | 53.37 (11.4) | 51.47 (10.27) | 59.80 (12.31) | 50.34 (10.4) | <0.001 |
| Ischemic stroke | - | 36.24 (8.2) | 35.31 (7.64) | 39.44 (8.89) | 34.60 (8.08) | <0.001 |
| Hemorrhagic stroke | - | 17.13 (4.06) | 16.16 (3.36) | 20.35 (4.46) | 15.74 (3.01) | <0.001 |
| Hypertensive heart disease | (3) | 10.18 (7.85) | 8.85 (6.35) | 14.39 (10.55) | 8.74 (5.14) | <0.001 |
| Cardiomyopathy and myocarditis | (4) | 7.92 (3.12) | 7.36 (2.81) | 9.71 (3.38) | 7.21 (2.73) | <0.001 |
| Atrial fibrillation and flutter | (5) | 7.56 (1.85) | 7.72 (1.81) | 7.20 (1.38) | 7.35 (2.74) | <0.001 |
| Aortic aneurysm | (6) | 4.33 (0.62) | 4.35 (0.62) | 4.27 (0.54) | 4.25 (0.70) | 0.002 |
| Rheumatic heart disease | (7) | 3.43 (0.83) | 3.28 (0.65) | 3.92 (1.09) | 3.20 (0.67) | <0.001 |
| Peripheral vascular disease | (8) | 2.68 (0.69) | 2.62 (0.71) | 2.85 (0.59) | 2.63 (0.65) | <0.001 |
| Endocarditis | (9) | 2.59 (0.59) | 2.59 (0.60) | 2.63 (0.51) | 2.51 (0.61) | 0.021 |
| Other cardiovascular and circulatory diseases | - | 12.44 (2.23) | 12.43 (2.28) | 12.72 (2.01) | 11.74 (2.28) | <0.001 |
| Socio-demographics | ||||||
| Population characteristics | ||||||
| Size, n | 101,310 (324,395) | 95,968 (320,031) | 79,131 (211,914) | 197,354 (521,857) | <0.001 | |
| Gender, male % | 50.04 (2.22) | 50.09 (1.96) | 49.79 (2.68) | 50.26 (2.59) | 0.002 | |
| Ethnicity, white alone % | 85.53 (15.78) | 89.01 (13.15) | 74.42 (18.42) | 89.25 (11.99) | <0.001 | |
| Age, % | ||||||
| 0–9 years | 12.22 (2.09) | 12.22 (2.19) | 12.18 (1.77) | 12.31 (2.10) | 0.642 | |
| 10–19 years | 12.89 (1.67) | 12.91 (1.67) | 12.78 (1.57) | 13.01 (1.86) | 0.090 | |
| 20–29 years | 12.31 (3.31) | 12.01 (3.08) | 12.68 (2.96) | 12.13 (2.86) | <0.001 | |
| 30–39 years | 11.53 (1.64) | 11.39 (1.58) | 11.76 (1.61) | 11.88 (1.95) | <0.001 | |
| 40–49 years | 12.2 (1.46) | 12.0 (1.42) | 12.63 (1.33) | 12.49 (1.68) | <0.001 | |
| 50–59 years | 14.61 (1.65) | 14.73 (1.69) | 14.24 (1.43) | 14.63 (1.74) | <0.001 | |
| 60–69 years | 12.28 (2.45) | 12.38 (2.50) | 12.05 (2.20) | 12.08 (2.54) | 0.003 | |
| 70–79 years | 7.44 (1.99) | 7.49 (1.97) | 7.41 (2.05) | 7.05 (1.88) | 0.002 | |
| ≥80 years | 4.53 (1.50) | 4.75 (1.53) | 3.98 (1.18) | 4.30 (1.61) | <0.001 | |
| Socio-economics | ||||||
| Educational level (age ≥ 25), % | ||||||
| Less than a high school diploma | 13.42 (6.32) | 12.42 (6.26) | 16.73 (5.51) | 11.99 (5.61) | <0.001 | |
| A high school diploma only | 34.32 (7.18) | 34.0 (7.27) | 35.72 (6.41) | 32.98 (7.75) | <0.001 | |
| Completing a college or associate’s degree | 30.72 (5.19) | 31.38 (5.41) | 28.92 (4.20) | 30.55 (4.74) | <0.001 | |
| A bachelor’s degree or higher | 21.54 (9.41) | 22.19 (9.21) | 18.62 (8.63) | 24.46 (10.83) | <0.001 | |
| Median household income (annual), USD | 46,963.46 (12,008.42) | 48,207.46 (11,663.56) | 41,724.81 (10,910.38) | 51,572.92 (12,874.3) | <0.001 | |
| Unemployment rate, % | 6.22 (2.22) | 5.91 (2.26) | 7.33 (1.87) | 5.50 (1.67) | <0.001 | |
| Poverty rate, % | 16.87 (6.43) | 15.78 (5.94) | 20.74 (6.51) | 14.69 (5.42) | <0.001 | |
| Gross domestic product per capita (annual, USD) | 48,919 (136,007) | 52,529 (158,554) | 34,368 (32,101) | 60,605 (123,203) | <0.001 | |
| Healthcare service | ||||||
| Medical insured population (age < 65), % | 85.72 (5.04) | 86.63 (5.04) | 83.24 (3.96) | 85.51 (5.38) | <0.001 | |
| Physicians (per 1000 population), n | 1.21 (1.62) | 1.23 (1.75) | 1.03 (1.14) | 1.48 (1.66) | 0.001 | |
| Residential environment and location | ||||||
| Rural–Urban Continuum Code | <0.001 | |||||
| 1 (Metro areas, 1 million population or more) | 428 (13.90) | 242 (11.77) | 102 (13.80) | 84 (29.47) | ||
| 2 (Metro areas, 250 thousand to 1 million population) | 375 (12.18) | 248 (12.06) | 104 (14.07) | 23 (8.07) | ||
| 3 (Metro areas, population fewer than 250 thousand) | 350 (11.36) | 234 (11.38) | 102 (13.8) | 14 (4.91) | ||
| 4 (Urban population of 20 thousand or more, adjacent to a metro area) | 213 (6.92) | 152 (7.39) | 43 (5.82) | 18 (6.32) | ||
| 5 (Urban population of 20 thousand or more, not adjacent to a metro area) | 88 (2.86) | 61 (2.97) | 17 (2.30) | 10 (3.51) | ||
| 6 (Urban population of 2500 to 19,999, adjacent to a metro area) | 587 (19.06) | 362 (17.61) | 174 (23.55) | 51 (17.89) | ||
| 7 (Urban population of 2500 to 19,999, not adjacent to a metro area) | 418 (13.57) | 306 (14.88) | 78 (10.55) | 34 (11.93) | ||
| 8 (Completely rural or less than 2500 urban population, adjacent to a metro area) | 217 (7.05) | 145 (7.05) | 57 (7.71) | 15 (5.26) | ||
| 9 (Completely rural or less than 2500 urban population, not adjacent to a metro area) | 404 (13.12) | 306 (14.88) | 62 (8.39) | 36 (12.63) | ||
| Latitude | 38.3 (4.83) | 39.79 (4.43) | 34.01 (3.04) | 38.75 (4.87) | <0.001 | |
| Longitude | −91.51 (11.41) | −93.16 (12.37) | −86.09 (5.95) | −93.77 (10.61) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, B.; Xu, Q.; Yuan, L.; Chen, Y. Association Between Soil Patterns and Mortality with Distinct Types of Cancers and CVD Across the USA. Life 2025, 15, 832. https://doi.org/10.3390/life15060832
Qu B, Xu Q, Yuan L, Chen Y. Association Between Soil Patterns and Mortality with Distinct Types of Cancers and CVD Across the USA. Life. 2025; 15(6):832. https://doi.org/10.3390/life15060832
Chicago/Turabian StyleQu, Bingjie, Qiaochu Xu, Linxi Yuan, and Ying Chen. 2025. "Association Between Soil Patterns and Mortality with Distinct Types of Cancers and CVD Across the USA" Life 15, no. 6: 832. https://doi.org/10.3390/life15060832
APA StyleQu, B., Xu, Q., Yuan, L., & Chen, Y. (2025). Association Between Soil Patterns and Mortality with Distinct Types of Cancers and CVD Across the USA. Life, 15(6), 832. https://doi.org/10.3390/life15060832







