Correlation Between Sarcopenia and Oral Health in Patients on Chronic Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Oral Status
2.3. Body Composition Analysis
2.4. Grip Strength
2.5. Questionnaires
2.6. Data Analysis
3. Results
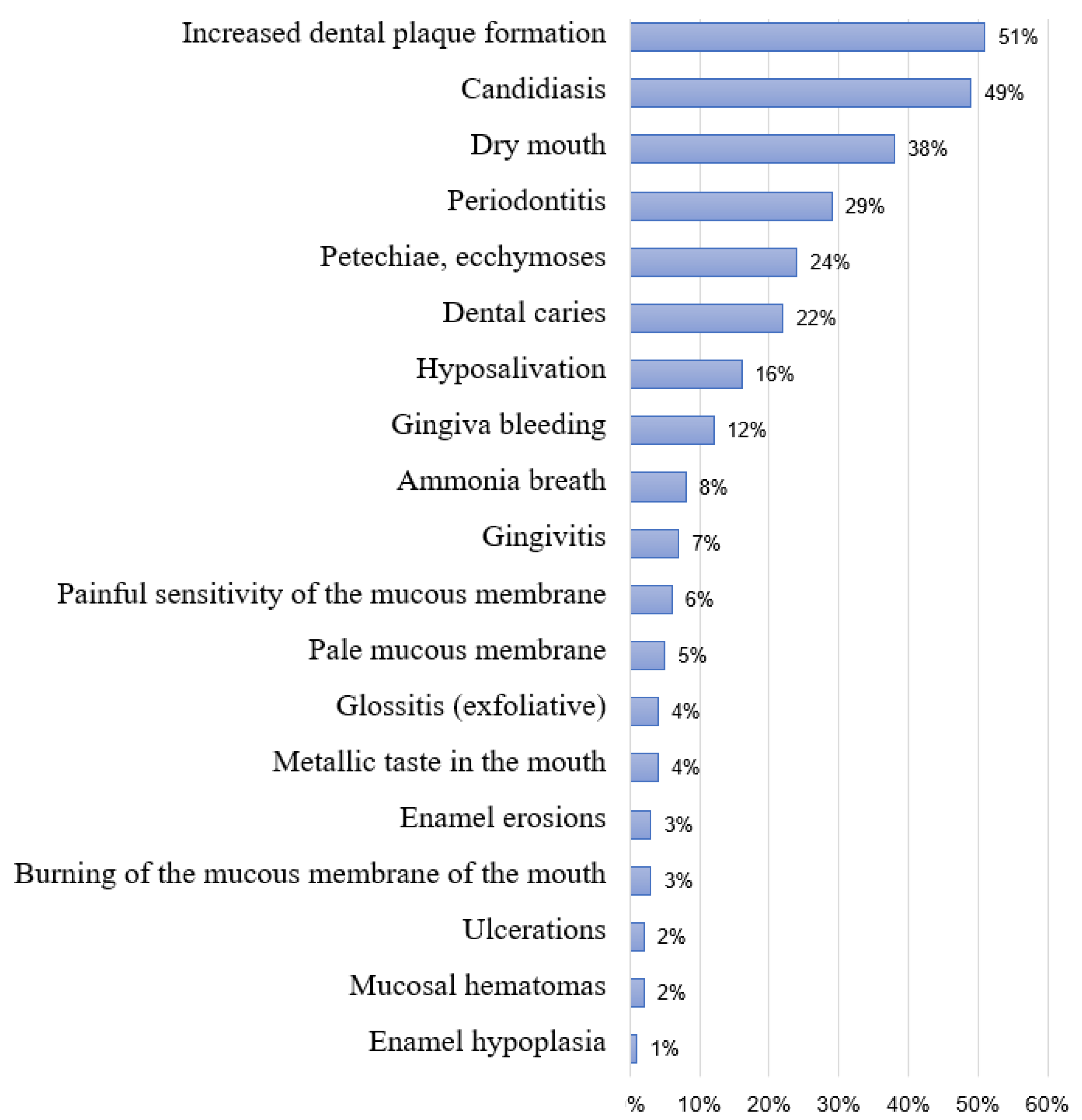
4. Discussion
Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Erratum: Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Rizzoli, R.; Reginster, J.Y.; Arnal, J.F.; Bautmans, I.; Beaudart, C.; Bischoff-Ferrari, H.; Biver, E.; Boonen, S.; Brandi, M.L.; Chines, A.; et al. Quality of life in sarcopenia and frailty. Calcif. Tissue Int. 2013, 93, 101–120. [Google Scholar] [CrossRef]
- Rantanen, T. Muscle strength, disability and mortality. Scand. J. Med. Sci. Sport. 2003, 13, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and Clinical Correlates of Sarcopenia, Identified According to the EWGSOP Definition and Diagnostic Algorithm, in Hospitalized Older People: The GLISTEN Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef]
- McIntyre, C.W.; Selby, N.M.; Sigrist, M.; Pearce, L.E.; Mercer, T.H.; Naish, P.F. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 2210–2216. [Google Scholar] [CrossRef]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Bárány, P.; Heimbürger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.R.; Choi, M.J.; Kim, S.G.; Lee, Y.K.; Noh, J.W.; Kim, H.J.; Song, Y.R. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin. Nutr. 2014, 33, 64–68. [Google Scholar] [CrossRef]
- Lamarca, F.; Carrero, J.J.; Rodrigues, J.C.D.; Bigogno, F.G.; Fetter, R.L.; Avesani, C.M. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: The impact of different diagnostic criteria. J. Nutr. Health Aging 2014, 18, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Bataille, S.; Serveaux, M.; Carreno, E.; Pedinielli, N.; Darmon, P.; Robert, A. The diagnosis of sarcopenia is mainly driven by muscle mass in hemodialysis patients. Clin. Nutr. 2017, 36, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J. Cachexia. Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef]
- Kaizu, Y.; Ohkawa, S.; Odamaki, M.; Ikegaya, N.; Hibi, I.; Miyaji, K.; Kumagai, H. Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am. J. Kidney Dis. 2003, 42, 295–302. [Google Scholar] [CrossRef]
- Visser, W.J.; de Mik-Van Egmond, A.M.E.; Timman, R.; Severs, D.; Hoorn, E.J. Risk factors for muscle loss in hemodialysis patients with high comorbidity. Nutrients 2020, 12, 2494. [Google Scholar] [CrossRef] [PubMed]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef]
- Song, Y.H.; Li, Y.; Du, J.; Mitch, W.E.; Rosenthal, N.; Delafontaine, P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J. Clin. Investig. 2005, 115, 451–458. [Google Scholar] [CrossRef]
- Cholewa, M.; Madziarska, K.; Radwan-Oczko, M. The association between periodontal conditions, inflammation, nutritional status and calcium-phosphate metabolism disorders in hemodialysis patients. J. Appl. Oral Sci. 2018, 26, e20170495. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, A.; AlBakr, S.; Alanazi, T.; Alshahrani, F.; Chalisserry, E.; Anil, S. Prevalence of Periodontitis in Patients Undergoing Hemodialysis: A Case Control Study. Mater. Socio Medica 2018, 30, 58. [Google Scholar] [CrossRef]
- Miyata, Y.; Obata, Y.; Mochizuki, Y.; Kitamura, M.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Mukae, H.; Nishino, T.; Yoshimura, A.; et al. Periodontal disease in patients receiving dialysis. Int. J. Mol. Sci. 2019, 20, 3805. [Google Scholar] [CrossRef]
- Chen, L.P.; Chiang, C.K.; Chan, C.P.; Hung, K.Y.; Huang, C.S. Does Periodontitis Reflect Inflammation and Malnutrition Status in Hemodialysis Patients? Am. J. Kidney Dis. 2006, 47, 815–822. [Google Scholar] [CrossRef]
- Cerveró, A.J.; Bagán, J.V.; Soriano, Y.J.; Roda, R.P. Dental management in renal failure: Patients on dialysis. Med. Oral Patol. Oral Cir. Bucal 2008, 13, 419–426. [Google Scholar]
- Egbring, L.C.; Lang, T.; Kreft, B.; Weich, K.W.; Gaengler, P. Xerostomia in Dialysis Patients—Oral Care to Reduce Hyposalivation, Dental Biofilms and Gingivitis in Patients with Terminal Renal Insufficiency: A Randomized Clinical Study. Kidney Dial. 2023, 3, 111–120. [Google Scholar] [CrossRef]
- Ruospo, M.; Palmer, S.C.; Craig, J.C.; Gentile, G.; Johnson, D.W.; Ford, P.J.; Tonelli, M.; Petruzzi, M.; De Benedittis, M.; Strippoli, G.F.M. Prevalence and severity of oral disease in adults with chronic kidney disease: A systematic review of observational studies. Nephrol. Dial. Transplant. 2014, 29, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Hatta, K.; Ikebe, K. Association between oral health and sarcopenia: A literature review. J. Prosthodont. Res. 2021, 65, 131–136. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic in Longitudinal Studies: Development. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef]
- Radić, M.; Benjak, T.; Vukres, V.D.; Rotim, Ž.; Zore, I.F. Prikaz kretanja KEP indeksa u Hrvatskoj i Europi. Acta Stomatol. Croat. 2015, 49, 275–284. [Google Scholar] [CrossRef]
- Kramer, I.R.H.; Pindborg, J.J.; Bezroukov, V.; Sardo Infirri, J. Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. Community Dent. Oral Epidemiol. 1980, 8, 1–24. [Google Scholar]
- Cvijetić, S.; Keser, I.; Boschiero, D.; Ilich, J.Z. Osteosarcopenic Adiposity and Nutritional Status in Older Nursing Home Residents during the COVID-19 Pandemic. Nutrients 2023, 15, 227. [Google Scholar] [CrossRef]
- Slade, G.D. Derivation and validation of a short-form oral health impact profile. Community Dent. Oral Epidemiol. 1997, 25, 284–290. [Google Scholar] [CrossRef]
- Topal, R. Impact of malocclusion on Oral Health-Related Quality of Life (OHRQoL) using the Oral Health Impact Profile (OHIP-14). J. Med. Dent. Investig. 2023, 4, e230354. [Google Scholar] [CrossRef]
- Beaudart, C.; Biver, E.; Reginster, J.Y.; Rizzoli, R.; Rolland, Y.; Bautmans, I.; Petermans, J.; Gillain, S.; Buckinx, F.; Dardenne, N.; et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. J. Cachexia. Sarcopenia Muscle 2017, 8, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Demonceau, C.; Voz, B.; Bruyère, O.; Reginster, J.Y.; Beaudart, C. Content validity of SarQoL, a quality of life questionnaire specific to sarcopenia. Aging Clin. Exp. Res. 2024, 36, 101. [Google Scholar] [CrossRef]
- Guillamón-Escudero, C.; Diago-Galmés, A.; Zuazua Rico, D.; Maestro-González, A.; Tenías-Burillo, J.M.; Soriano, J.M.; Fernández-Garrido, J.J. SarQoL Questionnaire in Community-Dwelling Older Adults under EWGSOP2 Sarcopenia Diagnosis Algorithm: A New Screening Method? Int. J. Environ. Res. Public Health 2022, 19, 8473. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Goh, Z.S.; Griva, K. Anxiety and depression in patients with end-stage renal disease: Impact and management challenges—A narrative review. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 93–102. [Google Scholar] [CrossRef]
- Nagy, E.; Tharwat, S.; Elsayed, A.M.; Shabaka, S.A.E.G.; Nassar, M.K. Anxiety and depression in maintenance hemodialysis patients: Prevalence and their effects on health-related quality of life. Int. Urol. Nephrol. 2023, 55, 2905–2914. [Google Scholar] [CrossRef]
- Furuta, M.; Yamashita, Y. Oral Health and Swallowing Problems. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 216–222. [Google Scholar] [CrossRef]
- Massini, G.; Caldiroli, L.; Molinari, P.; Carminati, F.M.I.; Castellano, G.; Vettoretti, S. Nutritional Strategies to Prevent Muscle Loss and Sarcopenia in Chronic Kidney Disease: What Do We Currently Know? Nutrients 2023, 15, 3107. [Google Scholar] [CrossRef]
- Basha, M.M.; Al-Kadasi, B.A.; Al-Hajri, M.; Al-Sharani, H.M.; Elayah, S.A. Exploring the correlation between periodontal disease and serum biomarkers in haemodialysis patients. BMC Oral Health 2024, 24, 1066. [Google Scholar] [CrossRef]
- Borawski, J.; Wilczyńska-Borawska, M.; Stokowska, W.; Myśliwiec, M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol. Dial. Transplant. 2007, 22, 457–464. [Google Scholar] [CrossRef]
- Pardo, A.; Signoriello, A.; Signoretto, C.; Messina, E.; Carelli, M.; Tessari, M.; De Manna, N.D.; Rossetti, C.; Albanese, M.; Lombardo, G.; et al. Detection of periodontal pathogens in oral samples and cardiac specimens in patients undergoing aortic valve replacement: A pilot study. J. Clin. Med. 2021, 10, 3874. [Google Scholar] [CrossRef]
- Bassani, B.; Cucchiara, M.; Butera, A.; Kayali, O.; Chiesa, A.; Palano, M.T.; Olmeo, F.; Gallazzi, M.; Dellavia, C.P.B.; Mortara, L.; et al. Neutrophils’ Contribution to Periodontitis and Periodontitis-Associated Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 15370. [Google Scholar] [CrossRef]
- Antonoglou, G.N.; Romandini, M.; Meurman, J.H.; Surakka, M.; Janket, S.J.; Sanz, M. Periodontitis and edentulism as risk indicators for mortality: Results from a prospective cohort study with 20 years of follow-up. J. Periodontal Res. 2023, 58, 12–21. [Google Scholar] [CrossRef]
- Sharma, P.; Dietrich, T.; Ferro, C.J.; Cockwell, P.; Chapple, I.L.C. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J. Clin. Periodontol. 2016, 43, 104–113. [Google Scholar] [CrossRef]
- Bengtsson, V.W.; Persson, G.R.; Berglund, J.S.; Renvert, S. Periodontitis related to cardiovascular events and mortality: A long-time longitudinal study. Clin. Oral Investig. 2021, 25, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, Y.; Chen, L.; Chen, S.; Tang, R.; Li, Q.; Wei, W.; Bao, K.; Huang, Z.; Lai, W.; et al. Independent and joint effects of high-sensitivity c-reactive protein and hypoalbuminemia on long-term all-cause mortality among coronary artery disease: A prospective and multicenter cohort study. BMC Cardiovasc. Disord. 2021, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.; Kumar, N.; Stein, A.; Moles, D.; Porter, S. Oral and Dental Aspects of Chronic Renal Failure. Crit. Rev. Oral Biol. Med. 2005, 84, 199–208. [Google Scholar] [CrossRef]
- Davidovich, E.; Davidovits, M.; Peretz, B.; Shapira, J.; Aframian, D.J. The correlation between dental calculus and disturbed mineral metabolism in paediatric patients with chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 2439–2445. [Google Scholar] [CrossRef]
- Epstein, S.R.; Mandel, I.; Scopp, I.W. Salivary Composition and Calculus Formation in Patients Undergoing Hemodialysis. J. Periodontol. 1980, 51, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.H.; Hsieh, J.F.; Tsai, S.C.; Ho, Y.J.; Chang, H.R. Decreased salivary function in patients with end-stage renal disease requiring hemodialysis. Am. J. Kidney Dis. 2000, 36, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Boonyapratheeprat, N.; Pimolbutr, K.; Rungraungrayabkul, D.; Meenetkum, S.; Boongird, S.; Chuengsaman, P.; Okuma, N.; Thanakun, S.; Kitiyakara, C.; Sangkhamanee, S.S. Impact of Dry Mouth and Factors Associated with Sarcopenia on Oral Health-Related Quality of Life in Peritoneal Dialysis Patients. Eur. J. Dent. 2025. epub ahead of print. [Google Scholar] [CrossRef]
- Oksala, E. Factors predisposing to oral yeast infections. Acta Odontol. Scand. 1990, 48, 71–74. [Google Scholar] [CrossRef]
- Yeter, H.H.; Erten, Y.; Sevmez, H.; Korucu, B.; Kalkanci, A.; Elbeg, S.; Altok, K.; Bali, M.; Yilmaz, H. Oral Candida Colonization as a Risk Factor for Chronic Inflammation and Atherosclerosis in Hemodialysis Patients. Ther. Apher. Dial. 2019, 23, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Vettor, R.; Milan, G.; Franzin, C.; Sanna, M.; De Coppi, P.; Rizzuto, R.; Federspil, G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E987–E998. [Google Scholar] [CrossRef]
- Heimbürger, O.; Qureshi, A.R.; Blaner, W.S.; Berglund, L.; Stenvinkel, P. Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am. J. Kidney Dis. 2000, 36, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Suliman, M.E.; Ryu, K.; Qureshi, A.R.; Li, X.; Avesani, C.M.; Barany, P.; Heimbürger, O.; Stenvinkel, P.; Lindholm, B. Handgrip strength and mortality in a cohort of kidney failure patients: Comparative analysis of different normalization methods. Nutrition 2024, 125, 112470. [Google Scholar] [CrossRef]
- Paśnik-Chwalik, B.; Konopka, T. Impact of periodontitis on the oral health impact profile: A systematic review and meta-analysis. Dent. Med. Probl. 2020, 57, 423–431. [Google Scholar] [CrossRef]
- Schmalz, G.; Kollmar, O.; Vasko, R.; Müller, G.A.; Haak, R.; Ziebolz, D. Oral health-related quality of life in patients on chronic haemodialysis and after kidney transplantation. Oral Dis. 2016, 22, 665–672. [Google Scholar] [CrossRef]

| N | % | ||
|---|---|---|---|
| Sarcopenia | No | 72 | 72.0% |
| Yes | 28 | 28.0% | |
| Gender | Male | 61 | 61.0% |
| Female | 39 | 39.0% | |
| Last visit to the dentist | Regularly every 6 months | 32 | 32.0% |
| During last 12 months | 22 | 22.0% | |
| Several years ago | 46 | 46.0% | |
| Can’t remember | 0 | 0.0% | |
| Oral hygiene | Poor | 39 | 39.0% |
| Satisfactory | 52 | 52.0% | |
| Good | 8 | 8.0% | |
| Excellent | 1 | 1.0% | |
| Dentition | All teeth present | 8 | 8.0% |
| Partial dentition | 80 | 80.0% | |
| Total edentulousness | 12 | 12.0% | |
| Dental restorations | No dental restorations | 37 | 37.0% |
| Total prosthesis | 17 | 17.0% | |
| Partial prosthesis | 7 | 7.0% | |
| Dental bridge on one jaw | 17 | 17.0% | |
| Dental bridge on both jaws | 5 | 5.0% | |
| Combination | 4 | 4.0% | |
| Total + partial prosthesis | 13 | 13.0% | |
| Min | Max | Centile | |||
|---|---|---|---|---|---|
| 25th | Median | 75th | |||
| Age (years) | 21.00 | 93.00 | 60.00 | 68.50 | 78.00 |
| Weigh (kg) | 43.50 | 122.00 | 65.00 | 75.75 | 89.00 |
| Height (cm) | 146.00 | 198.00 | 162.00 | 169.00 | 176.00 |
| BMI (kg/m2) | 16.80 | 72.00 | 23.06 | 26.12 | 30.89 |
| FFM % | 45.00 | 81.00 | 60.00 | 66.00 | 71.00 |
| FFM kg | 28.00 | 77.60 | 44.00 | 49.55 | 55.53 |
| FM % | 19.00 | 55.00 | 29.00 | 34.00 | 40.00 |
| FM kg | 8.70 | 64.90 | 18.95 | 25.05 | 35.38 |
| IMAT % | 1.20 | 3.50 | 2.13 | 2.50 | 2.80 |
| IMAT kg | 0.60 | 4.10 | 1.50 | 1.90 | 2.30 |
| wSMI % | 11.00 | 31.00 | 18.00 | 22.50 | 26.00 |
| hSMI kg/m2 | 2.50 | 10.50 | 4.65 | 6.10 | 7.10 |
| Skeletal muscle % | 18.20 | 45.50 | 29.05 | 34.70 | 38.30 |
| Skeletal muscle kg | 6.00 | 35.30 | 12.90 | 17.55 | 20.90 |
| Hand grip of the dominant hand (kg) | 6.00 | 48.00 | 19.38 | 25.65 | 31.53 |
| Hand grip of the non-dominant hand (kg) | 0.00 | 47.60 | 15.60 | 20.30 | 26.60 |
| Min | Max | Centile | |||
|---|---|---|---|---|---|
| 25th | Median | 75th | |||
| Albumin (g/L) | 10.90 | 46.80 | 37.18 | 39.70 | 42.45 |
| CRP (mg/L) | 0.30 | 86.50 | 1.38 | 3.10 | 9.23 |
| ALST (kg) | 5.80 | 30.60 | 11.78 | 15.80 | 18.70 |
| Dialysis vintage (months) | 6.00 | 460.00 | 24.00 | 43.00 | 84.00 |
| Charlson index | 2.00 | 14.00 | 5.00 | 6.00 | 8.00 |
| OHIP-14 | 0.00 | 33.00 | 4.00 | 12.00 | 17.75 |
| SarQoL D1 | 27.77 | 99.97 | 47.77 | 59.97 | 73.03 |
| SarQoL D2 | 13.89 | 100.00 | 38.89 | 55.56 | 65.98 |
| SarQoLl D3 | 29.17 | 100.00 | 41.67 | 50.00 | 66.67 |
| SarQoL D4 | 13.46 | 100.00 | 44.23 | 65.38 | 83.62 |
| SarQoL D5 | 0.00 | 100.00 | 30.69 | 51.67 | 70.00 |
| SarQoL D6 | 0.00 | 83.12 | 33.25 | 33.25 | 33.25 |
| SarQoL D7 | 62.50 | 100.00 | 75.00 | 87.50 | 100.00 |
| SarQoL in total | 22.65 | 96.30 | 43.87 | 60.37 | 70.61 |
| Sarcopenia | p | |||||
|---|---|---|---|---|---|---|
| Yes N = 72 | No N = 28 | |||||
| N | % | N | % | |||
| Gender | Male | 44 | 61.1% | 17 | 60.7% | 0.971 |
| Female | 28 | 38.9% | 11 | 39.3% | ||
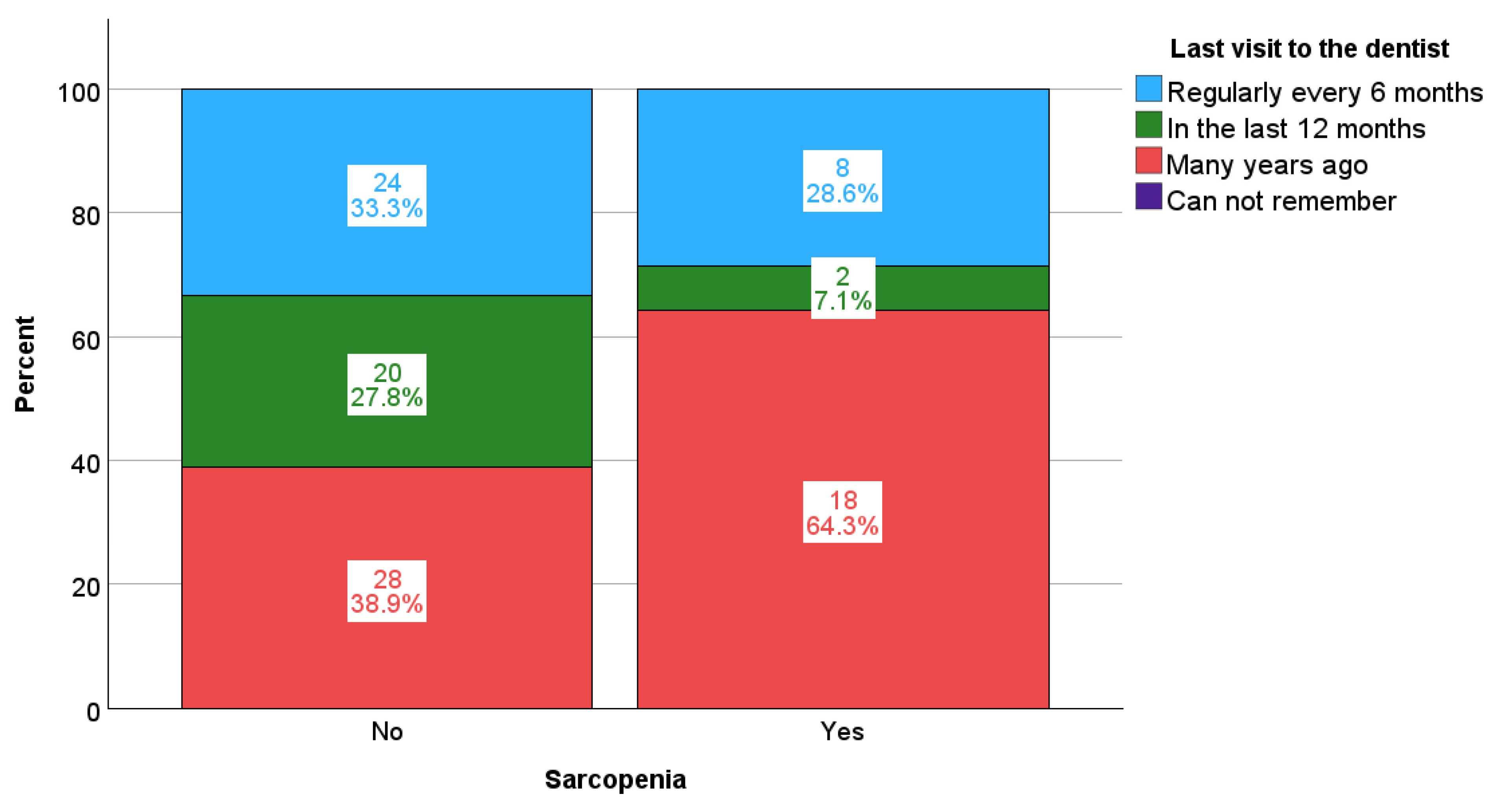
| Last visit to the dentist | Regularly every 6 months | 24 | 33.3% | 8 | 28.6% | 0.030 |
| In the last 12 months | 20 | 27.8% | 2 | 7.1% | ||
| Many years ago | 28 | 38.9% | 18 | 64.3% | ||
| Can’t remember | 0 | 0.0% | 0 | 0.0% | ||
| Oral hygiene | Poor | 27 | 37.5% | 12 | 42.9% | 0.760 |
| Satisfactory | 39 | 54.2% | 13 | 46.4% | ||
| Good | 5 | 6.9% | 3 | 10.7% | ||
| Excellent | 1 | 1.4% | 0 | 0.0% | ||
| Dentition | All teeth present | 6 | 8.3% | 2 | 7.1% | 0.496 |
| Partial dentition | 59 | 81.9% | 21 | 75.0% | ||
| Toothlessness | 7 | 9.7% | 5 | 17.9% | ||
| Dental prostheses | Without prostheses | 27 | 37.5% | 10 | 35.7% | 0.443 |
| Total prostheses | 12 | 16.7% | 5 | 17.9% | ||
| Partial prostheses | 5 | 6.9% | 2 | 7.1% | ||
| Bridge on one jaw | 15 | 20.8% | 2 | 7.1% | ||
| Bridge on both jaws | 4 | 5.6% | 1 | 3.6% | ||
| Combined prostheses | 2 | 2.8% | 2 | 7.1% | ||
| Total + partial prostheses | 7 | 9.7% | 6 | 21.4% | ||
| Sarcopenia | Min | Max | Centile | p | |||
|---|---|---|---|---|---|---|---|
| 25th | Median | 75th | |||||
| Age (years) | No | 21.00 | 93.00 | 52.25 | 66.00 | 72.00 | <0.001 |
| Yes | 52.00 | 91.00 | 69.25 | 78.50 | 81.00 | ||
| BMI (kg/m2) | Ne | 17.90 | 72.00 | 22.67 | 25.88 | 31.21 | 0.902 |
| Da | 16.80 | 35.09 | 23.68 | 26.43 | 30.85 | ||
| FFM % | No | 45.00 | 81.00 | 60.00 | 68.00 | 71.75 | 0.069 |
| Yes | 53.00 | 79.00 | 59.25 | 63.00 | 67.75 | ||
| FM % | No | 19.00 | 55.00 | 28.00 | 32.00 | 39.75 | 0.047 |
| Yes | 21.00 | 47.00 | 32.25 | 37.00 | 40.75 | ||
| IMAT % | No | 1.20 | 3.50 | 2.10 | 2.40 | 2.70 | <0.001 |
| Yes | 2.30 | 3.20 | 2.50 | 2.75 | 2.90 | ||
| wSMI % | No | 11.00 | 31.00 | 19.00 | 23.50 | 26.75 | 0.001 |
| Yes | 13.00 | 26.00 | 16.00 | 20.00 | 22.75 | ||
| hSMI kg/m2 | No | 2.50 | 10.50 | 5.10 | 6.30 | 7.10 | 0.008 |
| Yes | 3.00 | 8.10 | 4.23 | 5.30 | 6.25 | ||
| Skeletal muscle % | No | 18.20 | 45.50 | 30.78 | 35.90 | 38.85 | 0.003 |
| Yes | 19.40 | 39.30 | 25.83 | 30.95 | 35.08 | ||
| Hand grip of dominant hand | No | 12.00 | 48.00 | 23.00 | 28.00 | 34.45 | <0.001 |
| Yes | 6.00 | 26.00 | 12.38 | 17.45 | 21.83 | ||
| Hand grip of non-dominant hand | No | 0.00 | 47.60 | 17.70 | 24.30 | 30.00 | <0.001 |
| Yes | 4.00 | 26.00 | 12.00 | 15.60 | 17.60 | ||
| Sarcopenia | Min | Max | Centile | p | |||
|---|---|---|---|---|---|---|---|
| 25th | Median | 75th | |||||
| Albumin (g/L) | No | 10.90 | 46.80 | 38.08 | 40.15 | 43.10 | 0.002 |
| Yes | 29.30 | 43.40 | 35.10 | 37.50 | 40.30 | ||
| CRP | No | 0.30 | 86.50 | 1.18 | 2.70 | 7.40 | 0.035 |
| Yes | 0.60 | 57.00 | 1.93 | 3.45 | 27.85 | ||
| ALST (kg) | No | 5.80 | 30.60 | 12.75 | 17.00 | 19.58 | 0.003 |
| Yes | 7.00 | 19.90 | 10.53 | 13.30 | 16.15 | ||
| Dialysis vintage (months) | No | 6.00 | 208.00 | 20.00 | 39.00 | 71.00 | 0.072 |
| Yes | 9.00 | 460.00 | 32.00 | 61.00 | 94.75 | ||
| Charlson index | Ne | 2.00 | 14.00 | 4.00 | 6.00 | 8.00 | 0.110 |
| Da | 3.00 | 11.00 | 6.00 | 6.00 | 9.00 | ||
| OHIP-14 | No | 0.00 | 29.00 | 3.25 | 12.00 | 16.75 | 0.214 |
| Yes | 2.00 | 33.00 | 7.00 | 13.50 | 21.00 | ||
| SarQol D1 | No | 27.77 | 99.97 | 49.43 | 64.42 | 76.63 | 0.003 |
| Yes | 27.77 | 85.53 | 34.43 | 49.99 | 61.93 | ||
| SarQol D2 | No | 13.89 | 100.00 | 41.67 | 58.33 | 69.44 | 0.002 |
| Yes | 13.89 | 97.22 | 25.70 | 43.06 | 57.64 | ||
| SarQol D3 | No | 29.17 | 100.00 | 41.67 | 54.17 | 66.67 | 0.042 |
| Yes | 29.17 | 79.17 | 41.67 | 50.00 | 57.29 | ||
| SarQol D4 | No | 32.69 | 100.00 | 57.69 | 73.08 | 86.78 | <0.001 |
| Yes | 13.46 | 94.64 | 31.73 | 44.23 | 64.08 | ||
| SarQol D5 | No | 11.67 | 100.00 | 38.34 | 58.63 | 73.33 | <0.001 |
| Yes | 0.00 | 75.00 | 19.11 | 41.67 | 55.42 | ||
| SarQol D6 | No | 0.00 | 83.12 | 33.25 | 33.25 | 33.25 | 0.070 |
| Yes | 0.00 | 66.50 | 16.62 | 33.25 | 33.25 | ||
| SarQol D7 | No | 62.50 | 100.00 | 87.50 | 87.50 | 100.00 | <0.001 |
| Yes | 62.50 | 100.00 | 62.50 | 75.00 | 87.50 | ||
| SarQol in total | No | 24.35 | 96.30 | 49.83 | 62.78 | 74.24 | <0.001 |
| Yes | 22.65 | 83.24 | 30.10 | 43.63 | 60.56 | ||
| Sarcopenia | Min | Max | Centile | p | |||
|---|---|---|---|---|---|---|---|
| 25th | Median | 75th | |||||
| Erupted incisors | No | 0.00 | 8.00 | 0.00 | 1.00 | 4.75 | 0.063 |
| Yes | 0.00 | 8.00 | 0.25 | 4.00 | 8.00 | ||
| Erupted canines | No | 0.00 | 4.00 | 0.00 | 0.00 | 2.00 | 0.117 |
| Yes | 0.00 | 4.00 | 0.00 | 1.50 | 3.00 | ||
| Erupted premolars | No | 0.00 | 8.00 | 2.00 | 3.00 | 7.00 | 0.035 |
| Yes | 0.00 | 8.00 | 2.25 | 6.50 | 8.00 | ||
| Erupted molars | No | 0.00 | 8.00 | 4.00 | 6.00 | 8.00 | 0.061 |
| Yes | 0.00 | 8.00 | 5.00 | 7.50 | 8.00 | ||
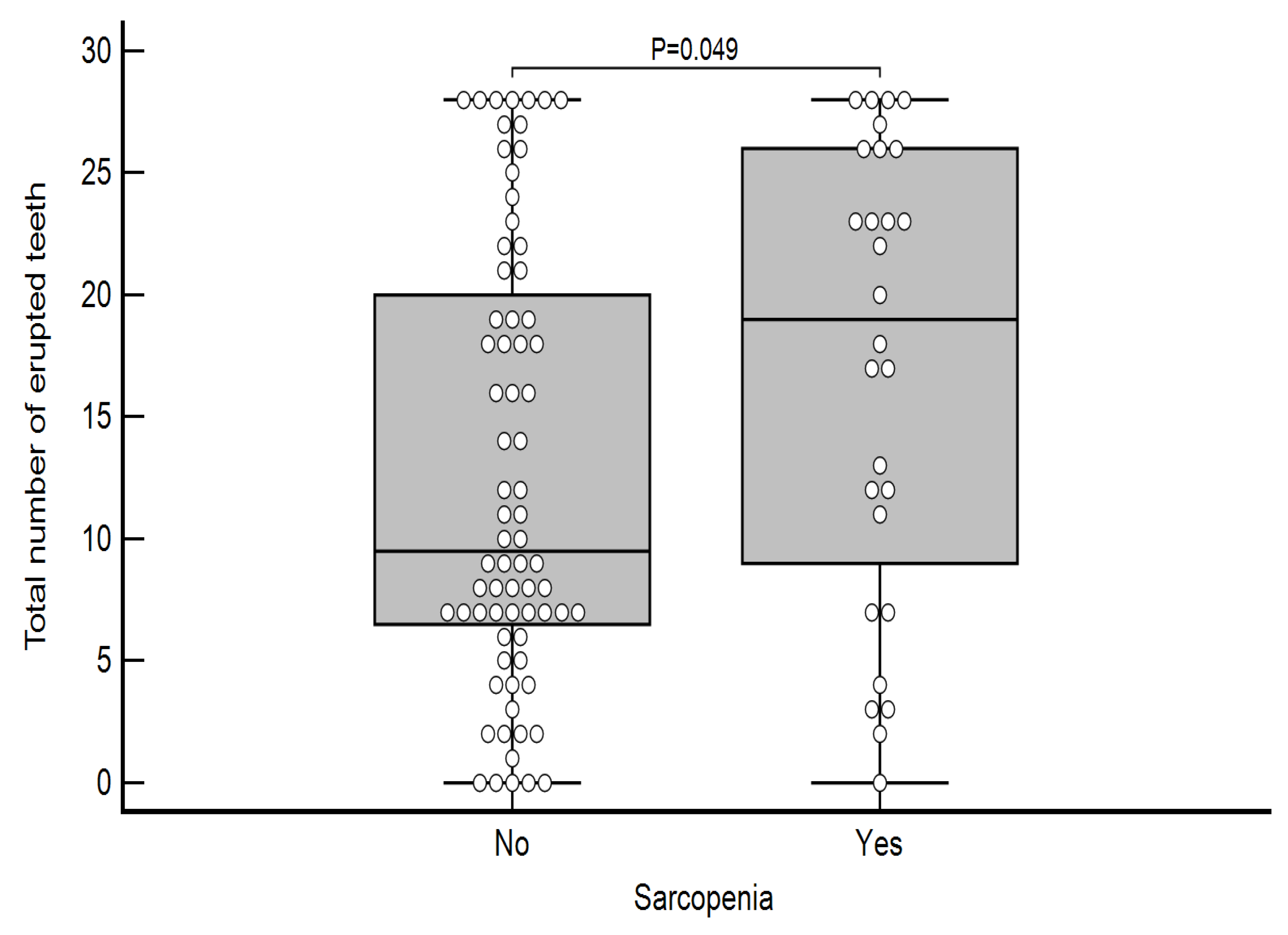
| Total number of erupted teeth | No | 0.00 | 28.00 | 6.25 | 9.50 | 20.50 | 0.049 |
| Yes | 0.00 | 28.00 | 8.00 | 19.00 | 26.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević Totić, P.; Klarić Puđa, I.; Kovačević Čorak, K.; Altabas, V.; Milošević, M.; Cvijetić Avdagić, S.; Altabas, K. Correlation Between Sarcopenia and Oral Health in Patients on Chronic Hemodialysis. Life 2025, 15, 823. https://doi.org/10.3390/life15050823
Kovačević Totić P, Klarić Puđa I, Kovačević Čorak K, Altabas V, Milošević M, Cvijetić Avdagić S, Altabas K. Correlation Between Sarcopenia and Oral Health in Patients on Chronic Hemodialysis. Life. 2025; 15(5):823. https://doi.org/10.3390/life15050823
Chicago/Turabian StyleKovačević Totić, Petra, Iva Klarić Puđa, Karla Kovačević Čorak, Velimir Altabas, Milan Milošević, Selma Cvijetić Avdagić, and Karmela Altabas. 2025. "Correlation Between Sarcopenia and Oral Health in Patients on Chronic Hemodialysis" Life 15, no. 5: 823. https://doi.org/10.3390/life15050823
APA StyleKovačević Totić, P., Klarić Puđa, I., Kovačević Čorak, K., Altabas, V., Milošević, M., Cvijetić Avdagić, S., & Altabas, K. (2025). Correlation Between Sarcopenia and Oral Health in Patients on Chronic Hemodialysis. Life, 15(5), 823. https://doi.org/10.3390/life15050823







