Viruses in Simuliidae: An Updated Systematic Review of Arboviral Diversity and Vector Potential
Abstract
1. Introduction
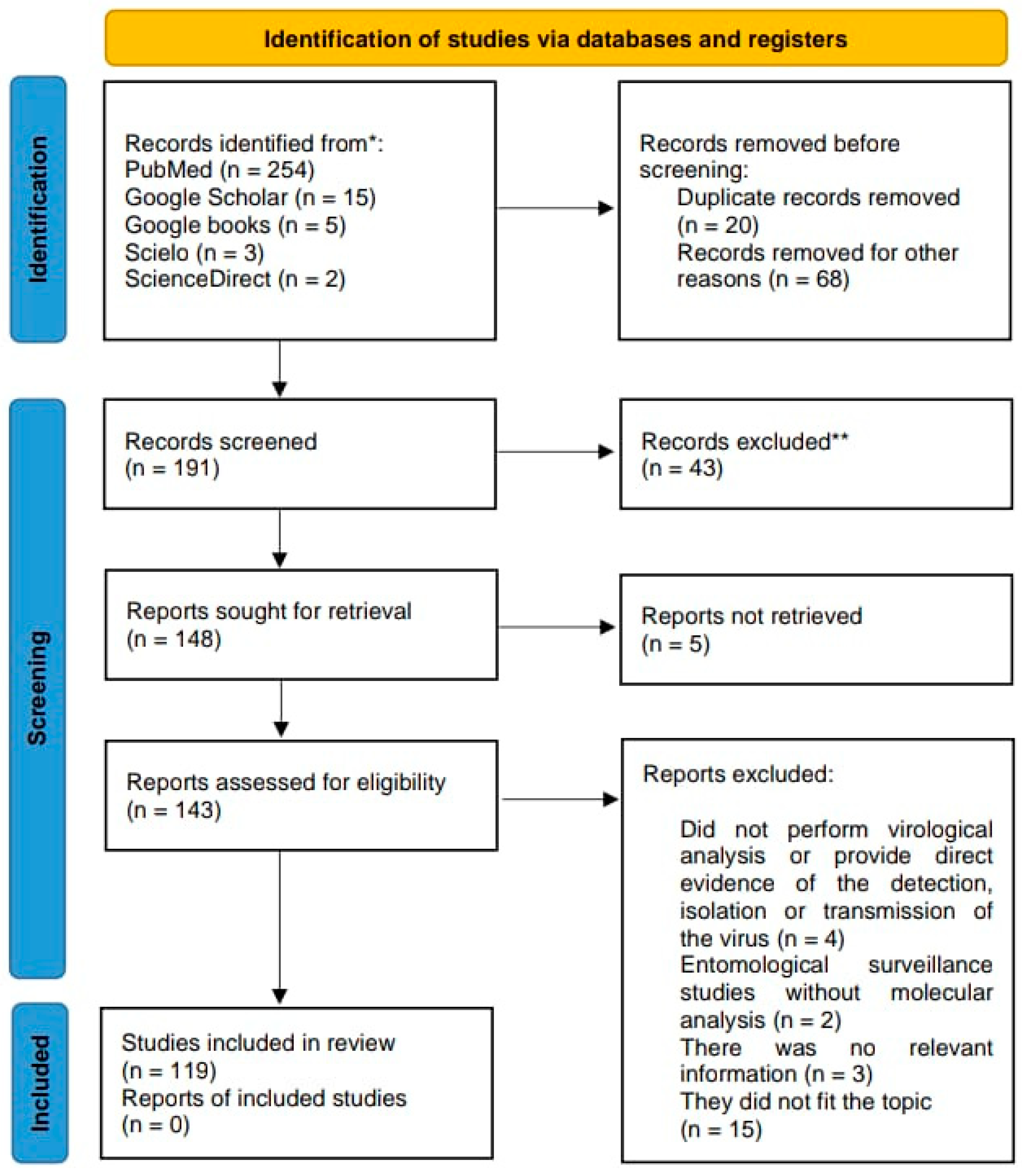
2. Materials and Methods
3. Results Overview
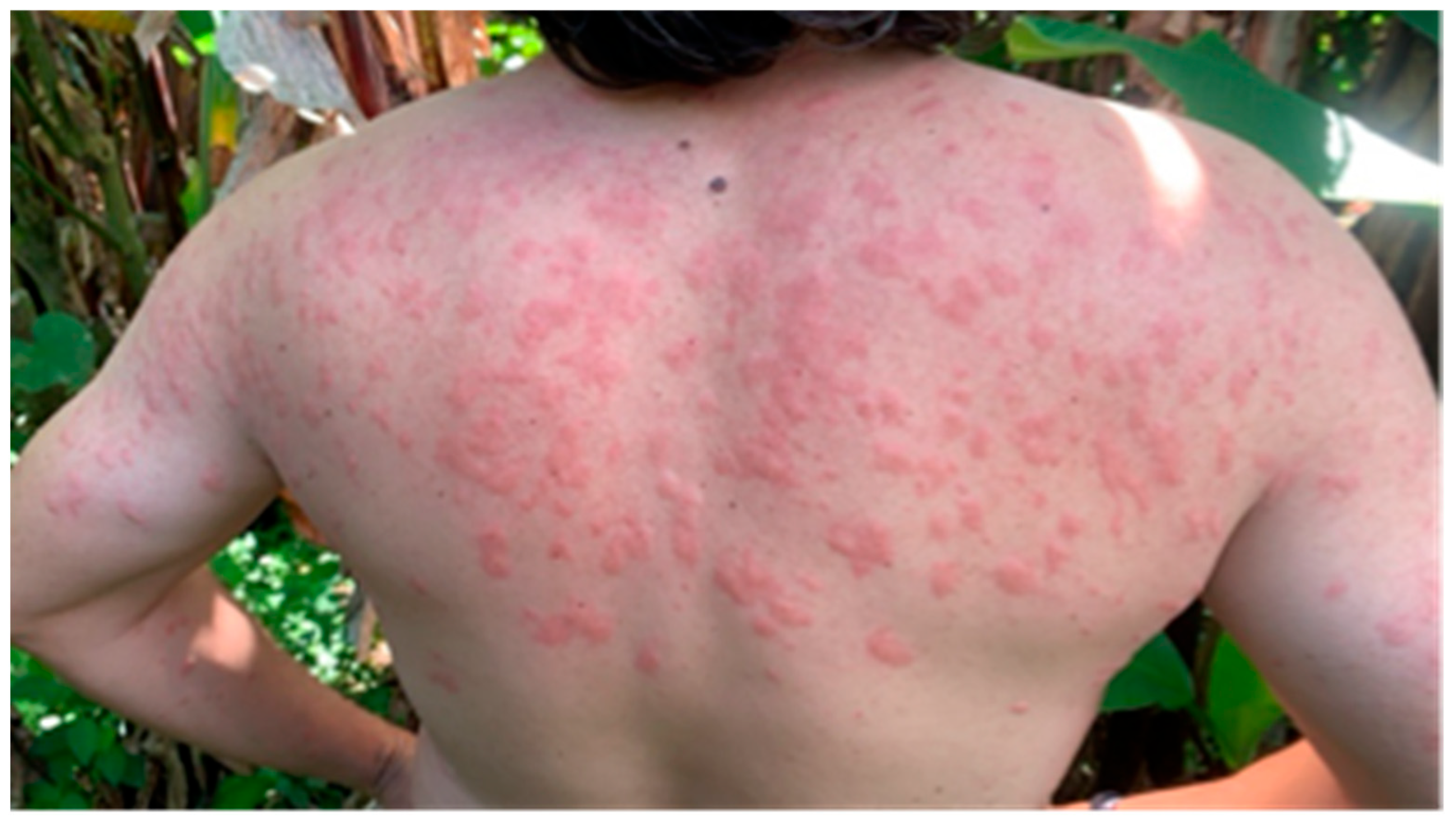
3.1. Simuliidae as Vectors of Public-Health-Related Non-Viral Pathogens
3.2. General Relevance of Arbovirosis in Vector-Borne Diseases
3.3. Detection and Isolation of Viruses in Simuliidae
3.4. Metagenomics of Viromes as a Tool for the Detection of Zoonotic Arboviruses
3.5. Metagenomic Studies of Viruses in Simuliidae
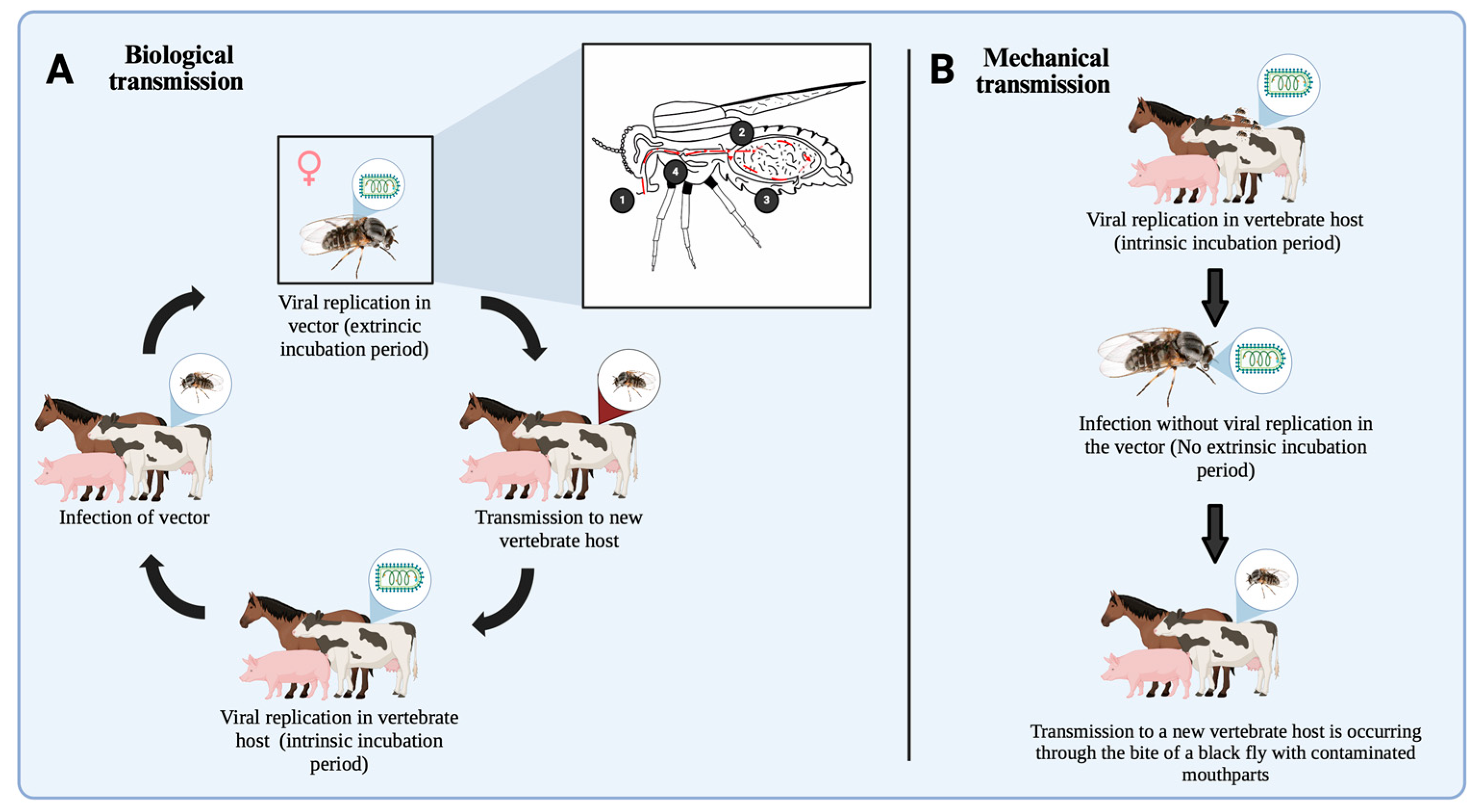
3.6. Exploring Causal and Casual Modes of Arbovirus Transmission Mechanisms in Simuliidae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ta-Tang, T.H.; Crainey, J.L.; Post, R.J.; Luz, S.L.; Rubio, J.M. Mansonellosis: Current perspectives. Res. Rep. Trop. Med. 2018, 9, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Howerth, E.W.; Carter, D.; Gray, E.W.; Noblet, R.; Mead, D.G. Mechanical Transmission of Vesicular Stomatitis New Jersey Virus by Simulium vittatum (Diptera: Simuliidae) to Domestic Swine (Sus scrofa). J. Med. Entomol. 2009, 46, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.H.; Currie, D.C.; Madera, D.M. Las Moscas Negras (Simuliidae) de América Del Norte; Cornell University Press: Ítaca, NY, USA, 2004. [Google Scholar]
- Cunze, S.; Jourdan, J.; Klimpel, S. Ecologically and medically important black flies of the genus Simulium: Identification of biogeographical groups according to similar larval niches. Sci. Total Environ. 2024, 917, 170454. [Google Scholar] [CrossRef]
- Negredo Antón, A.I.; de Ory Manchón, F.; Sánchez-Seco Fariñas, M.P.; Franco Narváez, L.; Gegúndez Cámara, M.I.; Navarro Mari, J.M.; Tenorio Matanzo, A. Diagnóstico microbiológico de arbovirosis y robovirosis emergentes. Enfermedades Infecc. Y Microbiol. Clin. 2015, 33, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Miller, S.A. Clinical metagenomics. Nat. Rev. Genet. 2019, 20, 341–355. [Google Scholar] [CrossRef]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Unraveling the viral dark matter through viral metagenomics. Front. Immunol. 2022, 13, 1005107. [Google Scholar] [CrossRef]
- Ghurye, J.S.; Cepeda-Espinoza, V.; Pop, M. Metagenomic Assembly: Overview, Challenges and Applications. Yale J. Biol. Med. 2016, 89, 353–362. [Google Scholar]
- Taş, N.; de Jong, A.E.; Li, Y.; Trubl, G.; Xue, Y.; Dove, N.C. Metagenomic tools in microbial ecology research. Curr. Opin. Biotechnol. 2021, 67, 184–191. [Google Scholar] [CrossRef]
- Pan, Y.F.; Zhao, H.; Gou, Q.Y.; Shi, P.B.; Tian, J.H.; Feng, Y.; Li, K.; Yang, W.H.; Wu, D.; Tang, G.; et al. Metagenomic analysis of individual mosquitos reveals the ecology of insect viruses. bioRxiv 2023. bioRxiv:2023.08.28.555221. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Adler, P.H.; Crosskey, R.W. World Blackflies (Diptera: Simuliidae): A Comprehensive Revision of the Taxonomic and Geographical Inventory [2019]. Available online: https://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf (accessed on 10 January 2025).
- Adler, P.H.; Cheke, R.A.; Post, R.J. Evolution, epidemiology, and population genetics of black flies (Diptera: Simuliidae). Infect. Genet. Evol. 2010, 10, 846–865. [Google Scholar] [CrossRef]
- Boatin, B.A.; Richards, F.O. Control of Onchocerciasis. Adv. Parasitol. 2006, 61, 349–394. [Google Scholar] [PubMed]
- World Health Organization (WHO). Elimination of human onchocerciasis: Progress report, 2023–2024. Wkly. Epidemiol. Rec. 2024, 41, 577–590. [Google Scholar]
- Shelley, A.J.; Coscarón, S. Simuliid blackflies (Diptera: Simuliidae) and ceratopogonid midges (Diptera: Ceratopogonidae) as vectors of Mansonella ozzardi (Nematoda: Onchocercidae) in northern Argentina. Mem. Inst. Oswaldo Cruz 2001, 96, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Manzanell, R.; Stocker, A.S.; Deplazes, P.; Mathis, A. Morphological description and multilocus genotyping of Onchocerca spp. in red deer (Cervus elaphus) in Switzerland. Int. J. Parasitol. Parasites Wildl. 2022, 19, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Sazmand, A.; Bahari, A.; Papi, S.; Otranto, D. Parasitic diseases of equids in Iran (1931–2020): A literature review. Parasit. Vectors 2020, 13, 586. [Google Scholar] [CrossRef]
- Michalski, M.L.; Bain, O.; Fischer, K.; Fischer, P.U.; Kumar, S.; Foster, J.M. Identification and phylogenetic analysis of Dirofilaria ursi (Nematoda: Filarioidea) from Wisconsin black bears (Ursus americanus) and its Wolbachia endosymbiont. J. Parasitol. 2010, 96, 412–419. [Google Scholar] [CrossRef]
- Yamada, M.; Shishito, N.; Nozawa, Y.; Uni, S.; Nishioka, K.; Nakaya, T. A combined human case of Dirofilaria ursi infection in dorsal subcutaneous tissue and Anisakis simplex sensu stricto (s.s.) infection in ventral subcutaneous tissue. Trop. Med. Health 2017, 45, 26. [Google Scholar] [CrossRef]
- Anderson, R.C. The simuliid vectors of Splendidofilaria fallisensis of ducks. Can. J. Zool. 1968, 46, 610–611. [Google Scholar] [CrossRef]
- Jumpato, W.; Tangkawanit, U.; Wongpakam, K.; Pramual, P. Molecular detection of Leucocytozoon (Apicomplexa: Haemosporida) in black flies (Diptera: Simuliidae) from Thailand. Acta Trop. 2019, 190, 228–234. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, M.A.; Garza-Hernández, J.A.; Salinas-Carmona, M.C.; Fernández-Salas, I.; Reyes-Villanueva, F.; Real-Najarro, O.; Unnasch, T.R. The esperanza window trap reduces the human biting rate of Simulium ochraceum sl in formerly onchocerciasis endemic foci in Southern Mexico. PLOS Negl. Trop. Dis. 2017, 11, e0005686. [Google Scholar] [CrossRef]
- Ruiz-Arrondo, I.; Garza-Hernández, J.A.; Reyes-Villanueva, F.; Lucientes-Curdi, J.; Rodríguez-Pérez, M.A. Human-landing rate, gonotrophic cycle length, survivorship, and public health importance of Simulium erythrocephalum in Zaragoza, northeastern Spain. Parasites Vectors 2017, 10, 175. [Google Scholar] [CrossRef]
- Sitarz, M.; Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. Risk of attacks by blackflies (Diptera: Simuliidae) and occurrence of severe skin symptoms in bitten patients along the Eastern border of the European Union. Int. J. Environ. Res. Public Health 2022, 19, 7610. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R. Arboviruses: A Family on the Move. Adv. Exp. Med. Biol. 2018, 1062, 1–10. [Google Scholar] [PubMed]
- Kuno, G. Host range specificity of flaviviruses: Correlation with in vitro replication. J. Med. Entomol. 2007, 44, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z. History of Arbovirus Research in the Czech Republic. Viruses 2021, 13, 2334. [Google Scholar] [CrossRef]
- Kuno, G. Transmission of arboviruses without involvement of arthropod vectors. Acta Virol. 2001, 45, 139–150. [Google Scholar]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Gubler, D.J. Resurgent Vector-Borne Diseases as a Global Health Problem. Emerg. Infect. Dis. 1998, 4, 442. [Google Scholar] [CrossRef]
- Gratz, N.G. Emerging and Resurging Vector-Borne Diseases. Annu. Rev. Entomol. 1999, 44, 51. [Google Scholar] [CrossRef]
- Pavlovsky, E.N. On Natural Foci of Infection and Parasitic Diseases. Vestn. Akad. Nauk. SSSR 1939, 10, 98–108. [Google Scholar]
- Keesing, F.; Ostfeld, R.S. Impacts of Biodiversity and Biodiversity Loss on Zoonotic Diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023540118. [Google Scholar] [CrossRef]
- de Oliveira, J.G.; Netto, S.A.; Francisco, E.O.; Vieira, C.P.; Variza, P.F.; Iser, B.P.M.; Lima-Camara, T.N.; Lorenz, C.; Prophiro, J.S. Aedes aegypti in Southern Brazil: Spatiotemporal Distribution Dynamics and Association with Climate and Environmental Factors. Trop. Med. Infect. Dis. 2023, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Kolimenakis, A.; Heinz, S.; Wilson, M.L.; Winkler, V.; Yakob, L.; Michaelakis, A.; Papachristos, D.; Richardson, C.; Horstick, O. The Role of Urbanisation in the Spread of Aedes Mosquitoes and the Diseases They Transmit—A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009631. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T. The role of co-infection and swarm dynamics in arbovirus transmission. Virus Res. 2019, 265, 88–93. [Google Scholar] [CrossRef]
- Snyder, J.; Jose, J.; Kuhn, R. The Togaviridae and the Flaviviridae; Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Xu, Z.; Peng, Y.; Yang, M.; Li, X.; Wang, J.; Zou, R.; Liang, J.; Fang, S.; Liu, Y.; Yang, Y. Simultaneous Detection of Zika, Chikungunya, Dengue, Yellow Fever, West Nile, and Japanese Encephalitis Viruses by a Two-Tube Multiplex Real-Time RT-PCR Assay. J. Med. Virol. 2022, 94, 2528–2536. [Google Scholar] [CrossRef]
- Latanova, A.; Starodubova, E.; Karpov, V. Flaviviridae Nonstructural Proteins: The Role in Molecular Mechanisms of Triggering Inflammation. Viruses 2022, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W. Other Positive-Strand RNA Viruses. In Molecular Virology of Human Pathogenic Viruses; Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2017; pp. 177–184. [Google Scholar]
- Toribio, R.E. Arboviral Equine Encephalitides. Vet. Clin. N. Am. Equine Pract. 2022, 38, 299–321. [Google Scholar] [CrossRef]
- Chen, R.; Mukhopadhyay, S.; Merits, A.; Bolling, B.; Nasar, F.; Coffey, L.L.; Powers, A.; Weaver, S.C. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Togaviridae. J. Gen. Virol. 2018, 99, 761–762. [Google Scholar] [CrossRef] [PubMed]
- AbudAbudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar]
- Zhang, Y.; Liu, X.; Wu, Z.; Feng, S.; Lu, K.; Zhu, W.; Sun, H.; Niu, G. Oropouche Virus: A Neglected Global Arboviral Threat. Virus Res. 2024, 341, 199318. [Google Scholar] [CrossRef]
- Sasaya, T.; Palacios, G.; Briese, T.; Di Serio, F.; Groschup, M.H.; Neriya, Y.; Song, J.; Tomitaka, Y. ICTV Virus Taxonomy Profile: Phenuiviridae 2023. J. Gen. Virol. 2023, 104, e001990. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Alkhovsky, S.V.; Avšič-Županc, T.; Bergeron, É.; Burt, F.; Ergünay, K.; Garrison, A.R.; Marklewitz, M.; Mirazimi, A.; Papa, A.; et al. ICTV Virus Taxonomy Profile: Nairoviridae 2024. J. Gen. Virol. 2024, 105, e002012. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Guthrie, A.J. Re-emergence of bluetongue, African horse sickness, and other orbivirus diseases. Vet. Res. 2010, 41, 35. [Google Scholar] [CrossRef]
- Vasilakis, N.; Tesh, R.B. Insect-Specific Viruses and Their Potential Impact on Arbovirus Transmission. Curr. Opin. Virol. 2015, 15, 69–74. [Google Scholar] [CrossRef]
- Lledó, L.; Giménez-Pardo, C.; Gegúndez, M.I. Epidemiological Study of Thogoto and Dhori Virus Infection in People Bitten by Ticks, and in Sheep, in an Area of Northern Spain. Int. J. Environ. Res. Public Health 2020, 17, 2254. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Freitas-Astúa, J.; Bejerman, N.; Blasdell, K.R.; Breyta, R.; Dietzgen, R.G.; Fooks, A.R.; Kondo, H.; Kurath, G.; Kuzmin, I.V.; et al. ICTV Virus Taxonomy Profile: Rhabdoviridae 2022. J. Gen. Virol. 2022, 103, 001689. [Google Scholar] [CrossRef]
- Mykytowycz, R. The Transmission of Myxomatosis by Simulium melatum Wharton (Diptera: Simuliidae). CSIRO Wildl. Res. 1957, 2, 1–4. [Google Scholar] [CrossRef]
- Kalarani, I.B.; Thasneem, K.; Veerabathiran, R. Oncoviruses: Future Prospects of Molecular Mechanisms and Therapeutic Strategies. In Oncogenic Viruses; Elsevier eBooks; Academic Press: Cambridge, MA, USA, 2023; pp. 81–107. [Google Scholar]
- Young, K.I.; Valdez, F.; Vaquera, C.; Campos, C.; Zhou, L.; Vessels, H.K.; Hanley, K.A. Surveillance Along the Rio Grande During the 2020 Vesicular Stomatitis Outbreak Reveals Spatio-Temporal Dynamics of and Viral RNA Detection in Black Flies. Pathogens 2021, 10, 1264. [Google Scholar] [CrossRef]
- Pelzel-McCluskey, A.M. Vesicular Stomatitis Virus. Vet. Clin. N. Am. Equine Pract. 2023, 39, 147–155. [Google Scholar] [CrossRef]
- Cupp, E.W.; Maré, C.J.; Cupp, M.S.; Ramberg, F.B. Biological Transmission of Vesicular Stomatitis Virus (New Jersey) by Simulium vittatum (Diptera: Simuliidae). J. Med. Entomol. 1992, 29, 137–140. [Google Scholar] [CrossRef]
- Rodríguez, L.L. Emergence and Re-Emergence of Vesicular Stomatitis in the United States. Virus Res. 2002, 85, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, G.F. Insect transmission of equine encephalomyelitis studies. Bull. Utah Agric. Exp. Stn. 1934, 250, 47. [Google Scholar]
- Ferris, D.H.; Hanson, R.P.; Dickie, R.J.; Roberts, R.H. Experimental transmission of vesicular stomatitis virus by Diptera. J. Infect. Dis. 1955, 96, 184. [Google Scholar] [CrossRef] [PubMed]
- Van Velden, D.J.; Meyer, J.D.; Olivier, J.; Gear, J.H.; McIntosh, B. Rift Valley Fever Affecting Humans in South Africa: A Clinicopathological Study. S. Afr. Med. J. 1977, 51, 867–871. [Google Scholar]
- Palmer, R.W. Biological and Chemical Control of Blackflies (Diptera: Simuliidae) in the Orange River; Report to the Water Research Commission by the Onderstepoort Veterinary Institute; Water Research Commission: Pretoria, South Africa, 1995; pp. 1–10. [Google Scholar]
- Austin, F.J. The arbovirus vector potential of a simuliid. Ann. Trop. Med. Parasitol. 1967, 61, 189–199. [Google Scholar] [CrossRef]
- Sanmartí, N.C.; Mackenzi, E.R.B.; Trapid, O.H.; Barret, O.P.; Mullena, X.C.; Gutierrez, E.; Lesmes, S.C. Encefalitis Equina Venezolana en Colombia 1967. Bol. Ofic. Sanit. Panamer. 1973, 74, 108–137. [Google Scholar]
- Homan, E.J.; Zuluaga, F.N.; Yuill, T.M.; Lorbacher, H. Studies on the Transmission of Venezuelan Equine Encephalitis Virus by Colombian Simuliidae (Diptera). Am. J. Trop. Med. Hyg. 1985, 34, 799–804. [Google Scholar] [CrossRef]
- Gibbs, E.P.J.; Long, M.T. Equine Alphaviruses; Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2013; pp. 191–197. [Google Scholar]
- Mitchell, C.J.; Monath, T.P.; Sabattini, M.S.; Cropp, C.B.; Daffner, J.F.; Calisher, C.H.; Jakob, W.L.; Christensen, H.A. Arbovirus Investigations in Argentina, 1977–1980. II. Arthropod Collections and Virus Isolations from Argentine Mosquitoes. Am. J. Trop. Med. Hyg. 1985, 34, 945–955. [Google Scholar] [CrossRef]
- Sommerman, K.M. Biting fly-arbovirus probe in interior Alaska (Culicidae) (Simuliidae)—(SSH: California complex) (Northway: Bunyamwera group). Mosq. News 1977, 37, 90–103. [Google Scholar]
- Kramer, W.L.; Jones, R.H.; Holbrook, F.R.; Walton, T.E.; Calisher, C.H. Isolation of arboviruses from Culicoides midges (Diptera: Ceratopogonidae) in Colorado during an epizootic of vesicular stomatitis New Jersey. J. Med. Entomol. 1990, 27, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Chanteau, S.; Sechan, Y.; Moulia-Pelat, J.P.; Luquiaud, P.; Spiegel, A.; Boutin, J.P.; Roux, J.F. The Blackfly Simulium buissoni and Infection by Hepatitis B Virus on a Holoendemic Island of the Marquesas Archipelago in French Polynesia. Am. J. Trop. Med. Hyg. 1993, 48, 763–770. [Google Scholar] [CrossRef]
- Mead, D.G.; Mare, C.J.; Cupp, E.W. Vector Competence of Select Black Fly Species for Vesicular Stomatitis Virus (New Jersey Serotype). Am. J. Trop. Med. Hyg. 1997, 57, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mead, D.G.; Maré, C.J.; Ramberg, F.B. Bite Transmission of Vesicular Stomatitis Virus (New Jersey Serotype) to Laboratory Mice by Simulium vittatum (Diptera: Simuliidae). J. Med. Entomol. 1999, 36, 410–413. [Google Scholar] [CrossRef]
- Mead, D.G.; Ramberg, F.B.; Maré, C.J. Laboratory Vector Competence of Black Flies (Diptera: Simuliidae) for the Indiana Serotype of Vesicular Stomatitis Virus. Ann. N. Y. Acad. Sci. 2000, 916, 437–443. [Google Scholar] [CrossRef]
- Mead, D.G.; Gray, E.W.; Noblet, R.; Murphy, M.D.; Howerth, E.W.; Stallknecht, D.E. Biological Transmission of Vesicular Stomatitis Virus (New Jersey Serotype) by Simulium vittatum (Diptera: Simuliidae) to Domestic Swine (Sus scrofa). J. Med. Entomol. 2004, 41, 78–82. [Google Scholar] [CrossRef]
- Drolet, B.S.; Reeves, W.K.; Bennett, K.E.; Pauszek, S.J.; Bertram, M.R.; Rodriguez, L.L. Identical Viral Genetic Sequence Found in Black Flies (Simulium bivittatum) and the Equine Index Case of the 2006 U.S. Vesicular Stomatitis Outbreak. Pathogens 2021, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Mead, D.G.; Lovett, K.R.; Murphy, M.D.; Pauszek, S.J.; Smoliga, G.; Gray, E.W.; Noblet, R.; Overmyer, J.; Rodríguez, L.L. Experimental Transmission of New Jersey Vesicular Stomatitis Virus by Simulium vittatum to Cattle: Clinical Outcome Influenced by Feeding Site. J. Med. Entomol. 2009, 46, 866–872. [Google Scholar] [CrossRef]
- Howerth, E.W.; Mead, D.G.; Stallknecht, D.E. Immunolocalization of Vesicular Stomatitis Virus in Black Flies (Simulium vittatum). Ann. N.Y. Acad. Sci. 2002, 969, 340–345. [Google Scholar] [CrossRef]
- Mesquita, L.P.; Diaz, M.H.; Howerth, E.W.; Stallknecht, D.E.; Noblet, R.; Gray, E.W.; Mead, D.G. Pathogenesis of Vesicular Stomatitis New Jersey Virus Infection in Deer Mice (Peromyscus maniculatus) Transmitted by Black Flies (Simulium vittatum). Vet. Pathol. 2017, 54, 74–81. [Google Scholar] [CrossRef]
- Zhou, L.H.; Valdez, F.; Lopez Gonzalez, I.; Freysser Urbina, W.; Ocaña, A.; Tapia, C.; Zambrano, A.; Hernandez Solis, E.; Peters, D.P.C.; Mire, C.E.; et al. Vesicular Stomatitis Virus Transmission Dynamics Within Its Endemic Range in Chiapas, Mexico. Viruses 2024, 16, 1742. [Google Scholar] [CrossRef] [PubMed]
- Scroggs, S.L.P.; Swanson, D.A.; Steele, T.D.; Hudson, A.R.; Reister-Hendricks, L.M.; Gutierrez, J.; Shults, P.; McGregor, B.L.; Taylor, C.E.; Davis, T.M.; et al. Vesicular Stomatitis Virus Detected in Biting Midges and Black Flies during the 2023 Outbreak in Southern California. Viruses 2024, 16, 1428. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Rozo-Lopez, P.; Davis, T.M.; Drolet, B.S. Detection of Vesicular Stomatitis Virus Indiana from Insects Collected during the 2020 Outbreak in Kansas, USA. Pathogens 2021, 10, 1126. [Google Scholar] [CrossRef]
- Milby, M.M.; Reeves, W.C. Natural infection in arthropod vectors. In Epidemiology and Control of Mosquito-Borne Arboviruses in California, 1953–1987; Reeves, W.C., Ed.; California Mosquito and Vector Control Association: Sacramento, CA, USA, 1990; pp. 128–144. [Google Scholar]
- Cavallaro, M.C.; Risley, E.; Lockburner, P. Evaluation of Partially Submerged Sticky Traps on Lake Spillways for Adult Black Fly (Diptera: Simuliidae) Surveillance and Arbovirus Detection. J. Am. Mosq. Control Assoc. 2018, 34, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.H. Biting flies collected from recumbent bluetongue-infected sheep in Idaho. Mosq. News 1981, 41, 183. [Google Scholar]
- Colebunders, R.; Hendy, A.; Nanyunja, M.; Wamala, J.F.; van Oijen, M. Nodding Syndrome—A New Hypothesis and New Direction for Research. Int. J. Infect. Dis. 2014, 27, 74–77. [Google Scholar] [CrossRef]
- Arndts, K.; Kegele, J.; Ritter, M.; Prazeres da Costa, C.; Hoerauf, A.; Winkler, A.S. Active Infection with Onchocerca volvulus and the Linkage to Epilepsy/Nodding Syndrome. PLoS Negl. Trop. Dis. 2024, 18, e0012076. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Arbovirus Catalog. Available online: https://wwwn.cdc.gov/arbocat/ (accessed on 12 May 2025).
- Cutler, S.J.; Fooks, A.R.; van der Poel, W.H. Public Health Threat of New, Reemerging, and Neglected Zoonoses in the Industrialized World. Emerg. Infect. Dis. 2010, 16, 1–7. [Google Scholar] [CrossRef]
- Woolhouse, M.; Scott, F.; Hudson, Z.; Howey, R.; Chase-Topping, M. Human Viruses: Discovery and Emergence. Philos. Trans. R. Soc. B 2012, 367, 2864–2871. [Google Scholar] [CrossRef]
- Kuno, G.; Mackenzie, J.S.; Junglen, S.; Hubálek, Z.; Plyusnin, A.; Gubler, D.J. Vertebrate Reservoirs of Arboviruses: Myth, Synonym of Amplifier, or Reality? Viruses 2017, 9, 185. [Google Scholar] [CrossRef]
- Kirya, B.G.; Okia, N.O. A Yellow Fever Epizootic in the Zika Forest, Uganda, 1972: Part 2—Serology in Monkeys. Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 300–303. [Google Scholar] [CrossRef]
- Temmam, S.; Davoust, B.; Berenger, J.M.; Raoult, D.; Desnues, C. Viral Metagenomics on Animals as a Tool for the Detection of Zoonoses Prior to Human Infection? Int. J. Mol. Sci. 2014, 15, 10377–10397. [Google Scholar] [CrossRef]
- Ward, V.K.; Marriott, A.C.; Booth, T.F.; El-Ghorr, A.A.; Nuttall, P.A. Detection of an Arbovirus in an Invertebrate and a Vertebrate Host Using the Polymerase Chain Reaction. J. Virol. Methods 1990, 30, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Shi, M.; Holmes, E.C. Using Metagenomics to Characterize an Expanding Virosphere. Cell 2018, 172, 1168–1172. [Google Scholar] [CrossRef]
- Li, C.-X.; Shi, M.; Tian, J.-H.; Lin, X.-D.; Kang, Y.-J.; Chen, L.-J.; Qin, X.-C.; Xu, J.; Holmes, E.C.; Zhang, Y.-Z. Unprecedented Genomic Diversity of RNA Viruses in Arthropods Reveals the Ancestry of Negative-Sense RNA Viruses. eLife 2015, 4, e05378. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Sadeghi, M.; Altan, E.; Deng, X.; Barker, C.M.; Fang, Y.; Coffey, L.L.; Delwart, E. Virome of >12 Thousand Culex Mosquitoes from Throughout California. Virology 2018, 523, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gou, Q.Y.; Yang, W.H.; Wu, W.C.; Wang, J.; Holmes, E.C.; Liang, G.; Shi, M. A Time-Series Meta-Transcriptomic Analysis Reveals the Seasonal, Host, and Gender Structure of Mosquito Viromes. Virus Evol. 2022, 8, veac006. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.d.O.; Ribeiro, G.d.O.; da Couto, R.; Ramos, E.D.S.F.; Morais, V.d.S.; Telles-De-Deus, J.; Helfstein, V.C.; dos Santos, J.M.; Deng, X.; Delwart, E.; et al. Exploring Mosquito Virome Dynamics Within São Paulo Zoo: Insights into Mosquito-Virus-Environment Interactions. Front. Cell. Infect. Microbiol. 2025, 14, 1496126. [Google Scholar] [CrossRef]
- Charles, J.; Tangudu, C.S.; Hurt, S.L.; Tumescheit, C.; Firth, A.E.; Garcia-Rejon, J.E.; Machain-Williams, C.; Blitvich, B.J. Detection of Novel and Recognized RNA Viruses in Mosquitoes from the Yucatan Peninsula of Mexico Using Metagenomics and Characterization of Their In Vitro Host Ranges. J. Gen. Virol. 2018, 99, 1729. [Google Scholar] [CrossRef]
- Cigarroa-Toledo, N.; Baak-Baak, C.M.; Cetina-Trejo, R.C.; Cordova-Fletes, C.; Martinez-Nuñez, M.A.; Talavera-Aguilar, L.G.; Garcia-Rejon, J.E.; Torres-Chable, O.M.; Suzan, G.; Blitvich, B.J.; et al. Complete Genome Sequence of Houston Virus, a Newly Discovered Mosquito-Specific Virus Isolated from Culex quinquefasciatus in Mexico. Microbiol. Resour. Announc. 2018, 7, e00808-18. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.; Tangudu, C.S.; Hurt, S.L.; Tumescheit, C.; Firth, A.E.; Garcia-Rejon, J.E.; Machain-Williams, C.; Blitvich, B.J. Discovery of a Novel Tymoviridae-Like Virus in Mosquitoes from Mexico. Arch. Virol. 2019, 164, 649–652. [Google Scholar] [CrossRef]
- Tangudu, C.S.; Hargett, A.M.; Laredo-Tiscareño, S.V.; Smith, R.C.; Blitvich, B.J. Isolation of a Novel Rhabdovirus and Detection of Multiple Novel Viral Sequences in Culex Species Mosquitoes in the United States. Arch. Virol. 2022, 167, 2577–2590. [Google Scholar] [CrossRef] [PubMed]
- Mirza, J.D.; de Oliveira Guimarães, L.; Wilkinson, S.; Rocha, E.C.; Bertanhe, M.; Helfstein, V.C.; de-Deus, J.T.; Claro, I.M.; Cumley, N.; Quick, J.; et al. Tracking Arboviruses, Their Transmission Vectors and Potential Hosts by Nanopore Sequencing of Mosquitoes. Microb. Genom. 2024, 10, 001184. [Google Scholar] [CrossRef]
- Kraberger, S.; Schmidlin, K.; Fontenele, R.S.; Walters, M.; Varsani, A. Unravelling the Single-Stranded DNA Virome of the New Zealand Blackfly. Viruses 2019, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Murota, K.; Faizah, A.N.; Amoa-Bosompem, M.; Higa, Y.; Hayashi, T.; Tsuda, Y.; Sawabe, K.; Isawa, H. RNA Virome Analysis of Hematophagous Chironomoidea Flies (Diptera: Ceratopogonidae and Simuliidae) Collected in Tokyo, Japan. Med. Entomol. Zool. 2020, 71, 225–243. [Google Scholar] [CrossRef]
- De Coninck, L.; Hadermann, A.; Ingletto, L.; Colebunders, R.; Njamnshi, K.G.; Njamnshi, A.K.; Mokili, J.L.; Fodjo, J.N.S.; Matthijnssens, J. Cameroonian Blackflies (Diptera: Simuliidae) Harbour a Plethora of (RNA) Viruses. Virus Evol. 2025, 11, veaf024. [Google Scholar] [CrossRef]
- Turell, M.J. Horizontal and vertical transmission of viruses by insect and tick vectors. In The Arboviruses; Maramorosch, K., Murphy, F.A., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 127–152. [Google Scholar]
- Hardy, J.L.; Houk, E.J.; Kramer, L.D.; Reeves, W.C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983, 28, 229–262. [Google Scholar] [CrossRef]
- Salazar, M.I.; Richardson, J.H.; Sánchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef]
- Lewis, J.; Gallichotte, E.N.; Randall, J.; Glass, A.; Foy, B.D.; Ebel, G.D.; Kading, R.C. Intrinsic factors driving mosquito vector competence and viral evolution: A review. Front. Cell. Infect. Microbiol. 2023, 13, 1330600. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.-J. Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 2005, 18, 608–637. [Google Scholar] [CrossRef] [PubMed]
- Blanc, S.; Gutiérrez, S. The specifics of vector transmission of arboviruses of vertebrates and plants. Curr. Opin. Virol. 2015, 15, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A. Rift Valley fever. Clin. Lab. Med. 2017, 37, 285. [Google Scholar] [CrossRef]
- Pépin, M.; Bouloy, M.; Bird, B.H.; Kemp, A.; Paweska, J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 2010, 41, 61. [Google Scholar] [CrossRef]
- Hadermann, A.; Amaral, L.J.; Van Cutsem, G.; Fodjo, J.N.S.; Colebunders, R. Onchocerciasis-associated epilepsy: An update and future perspectives. Trends Parasitol. 2023, 39, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Cambra-Pellejà, M.; Gandasegui, J.; Balaña-Fouce, R.; Muñoz, J.; Martínez-Valladares, M. Zoonotic implications of Onchocerca species on human health. Pathogens 2020, 9, 761. [Google Scholar] [CrossRef]
- Cassedy, A.; Parle-McDermott, A.; O’Kennedy, R. Virus detection: A review of the current and emerging molecular and immunological methods. Front. Mol. Biosci. 2021, 8, 637559. [Google Scholar] [CrossRef]
- Rozo-Lopez, P.; Drolet, B.S.; Londoño-Rentería, B. Vesicular stomatitis virus transmission: A comparison of incriminated vectors. Insects 2018, 9, 190. [Google Scholar] [CrossRef]

| * Viral Family (Order) | Virion Size (nm) | Nuclei Acid Type | Number of Segments | Genome Size | Example of Arbovirus | References |
|---|---|---|---|---|---|---|
| Flaviviridae (Amarillovirales) | 40–60 nm | ssRNA + | Not segmented | 9–13 kb | Dengue virus, Zika virus, Yellow fever virus, Japanese encephalitis virus, Saint Louis encephalitis virus, Murray Valley encephalitis virus | [37,38,39,40] |
| Togaviridae (Martellivirales) | 65–70 nm | ssRNA + | Not segmented | 10–12 kb | Chikungunya virus, Ross River virus, O’nyong-nyong virus, Sindbis virus, Barmah Forest virus, Mayaro virus, Western, Eastern, and Venezuelan equine encephalitis viruses | [37,41,42,43] |
| Peribunyaviridae (Elliovirales) | 80–120 nm | ssRNA − | Segmented (3 segments) | 10.7–12.5 kb | La Crosse virus, Oropouche virus, Akabane virus | [30,44,45] |
| Phenuiviridae (Hareavirales) | 80–120 nm | ssRNA − | Segmented (3 segments) | 8.1–25.1 kb | Rift Valley fever virus | [46] |
| Nairoviridae (Hareavirales) | 80–120 nm | ssRNA − | Segmented (3 segments) | 17.2–21.1 kb | Crimean-Congo hemorrhagic fever virus | [47] |
| Sedoreoviridae (Reovirales) | 60–85 nm | dsRNA | Segmented (10–12 segments) | 18–30 kb | Bluetongue virus, African horse sickness virus, Equine encephalitis virus, Epizootic hemorrhagic disease virus | [48,49] |
| Ortomixoviridae (Articulavirales) | 80–120 nm | ssRNA − | Segmented (6–8 segments) | 10–15 kb | Thogoto virus Dhori virus | [50] |
| Rhabdoviridae (Mononegavirales) | 100–180 nm | ssRNA − | Not segmented | 10–16 kb | Vesicular stomatitis virus Bovine Ephemeral Fever | [51] |
| Asfarviridae (Asfuvirales) | 175–215 nm | dsDNA | Not segmented | 17–19 kb | African Swine Fever Virus | [52] |
| Virus Name | Family | Genome Size and Type | Methodology Used in the Report | Reference |
|---|---|---|---|---|
| Myxoma virus | Poxviridae | 160 kb, dsDNA | In vivo studies | [53] |
| Vesicular stomatitis viruses (VSV) (Serotype New Jersey and Indiana) | Rhabdoviridae | ~11 kb, −ssRNA | Cytopathic effect in Vero-M cells Titration with fluorescent antibodies Plaque assay | [57] |
| Plaque assay Microplate neutralization Virus re-isolation in cell culture RT-PCR | [72] | |||
| RT-PCR Sequencing | [55] | |||
| Real-time RT-PCR Sequencing | [79] | |||
| Venezuelan equine encephalitis virus (VEEV) | Togaviridae | ~11–12 kb, +ssRNA | Cell culture Complement fixation test (CFT) | [64] |
| Rift Valley fever virus (RVFV) | Phenuiviridae | ~11.9 kb, −ssRNA | Virus isolation in animal models Histopathology of liver tissue Complement fixation test (CFT) Hemagglutination inhibition test (HI) Electron microscopy | [61] |
| Snowshoe hare virus (SSHV) | Peribunyaviridae | ~12–13 kb, −ssRNA | Cell culture. Serological test | [68] |
| Unclassified bunyaviruses | Anteriormente Bunyaviridae | ~12–13 kb, −ssRNA | Cell culture. Plaque reduction neutralization test (PRNT) | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Martínez, A.; Laredo-Tiscareño, S.V.; Adame-Gallegos, J.R.; Luna-Santillana, E.d.J.d.; Rodríguez-Alarcón, C.A.; García-Rejón, J.E.; Casas-Martínez, M.; Garza-Hernández, J.A. Viruses in Simuliidae: An Updated Systematic Review of Arboviral Diversity and Vector Potential. Life 2025, 15, 807. https://doi.org/10.3390/life15050807
Rivera-Martínez A, Laredo-Tiscareño SV, Adame-Gallegos JR, Luna-Santillana EdJd, Rodríguez-Alarcón CA, García-Rejón JE, Casas-Martínez M, Garza-Hernández JA. Viruses in Simuliidae: An Updated Systematic Review of Arboviral Diversity and Vector Potential. Life. 2025; 15(5):807. https://doi.org/10.3390/life15050807
Chicago/Turabian StyleRivera-Martínez, Alejandra, S. Viridiana Laredo-Tiscareño, Jaime R. Adame-Gallegos, Erick de Jesús de Luna-Santillana, Carlos A. Rodríguez-Alarcón, Julián E. García-Rejón, Mauricio Casas-Martínez, and Javier A. Garza-Hernández. 2025. "Viruses in Simuliidae: An Updated Systematic Review of Arboviral Diversity and Vector Potential" Life 15, no. 5: 807. https://doi.org/10.3390/life15050807
APA StyleRivera-Martínez, A., Laredo-Tiscareño, S. V., Adame-Gallegos, J. R., Luna-Santillana, E. d. J. d., Rodríguez-Alarcón, C. A., García-Rejón, J. E., Casas-Martínez, M., & Garza-Hernández, J. A. (2025). Viruses in Simuliidae: An Updated Systematic Review of Arboviral Diversity and Vector Potential. Life, 15(5), 807. https://doi.org/10.3390/life15050807








