Musculoskeletal Impairments and Dysfunction in Individuals with Head and Neck Cancer Following Surgery with Neck Dissection—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Criteria for Studies
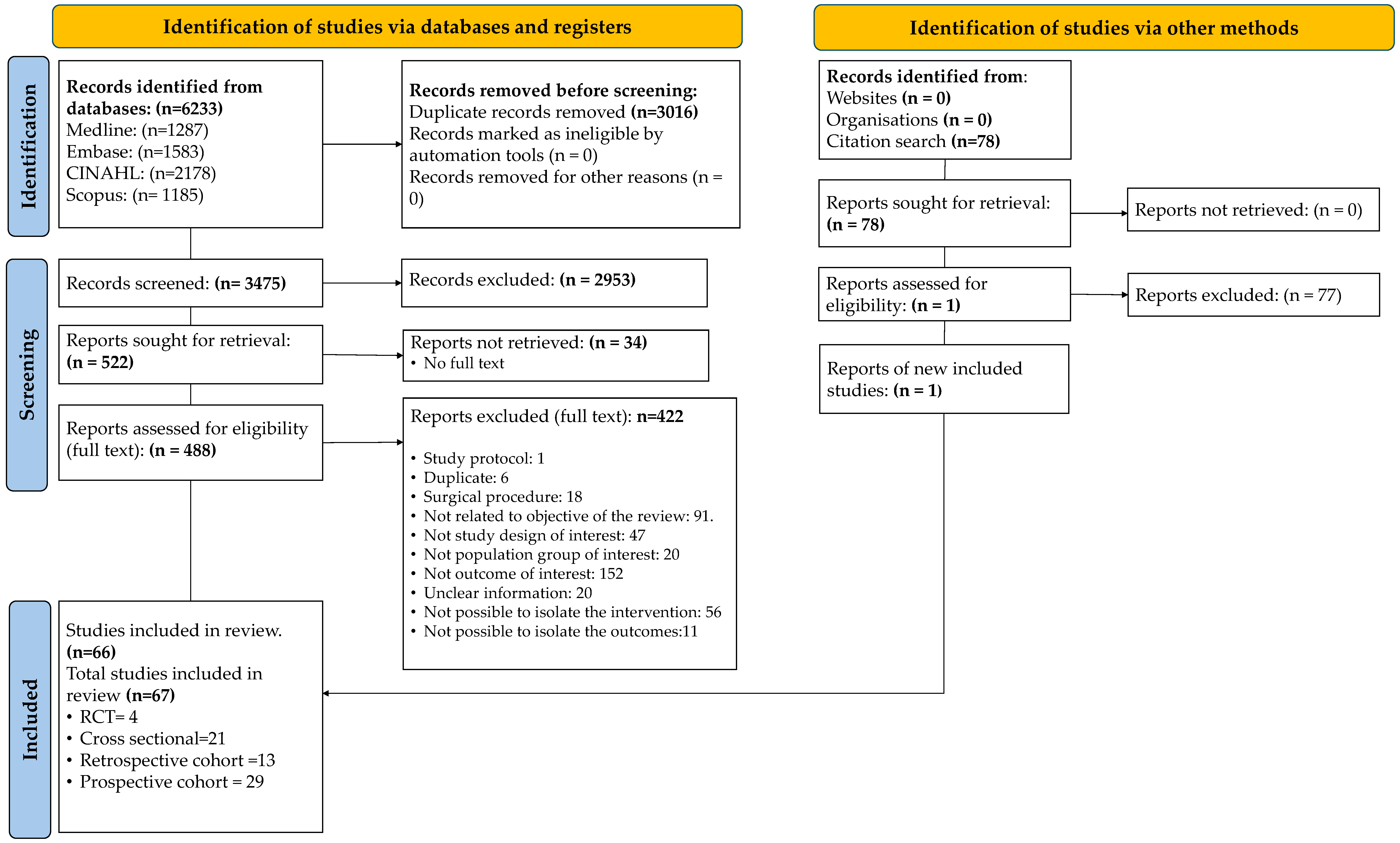
2.4. Selection of the Studies
2.5. Data Extraction
2.6. Risk of Bias Assessment
- Randomized controlled trials
- Non-randomized studies
2.7. Evaluation of the Overall Evidence
2.8. Data Synthesis
3. Results
3.1. Study Selection
3.2. Study Characteristics
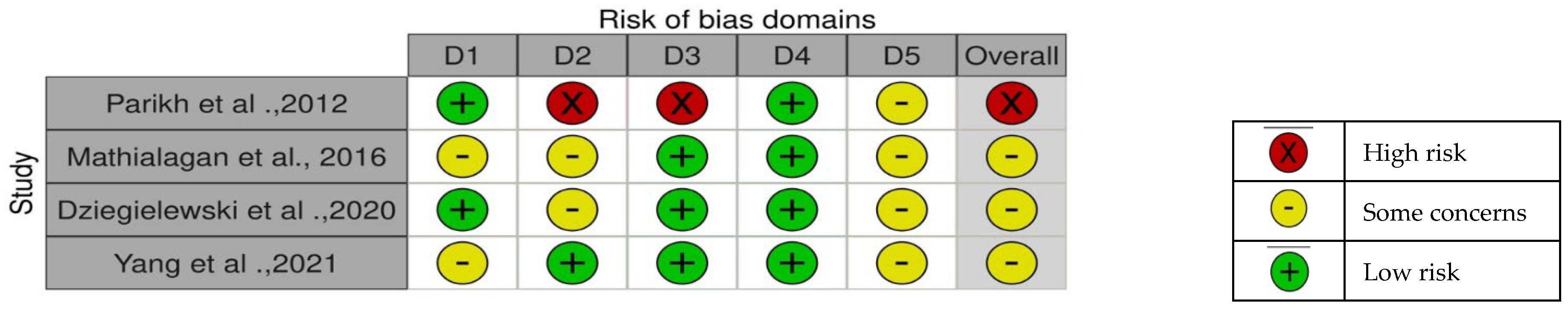
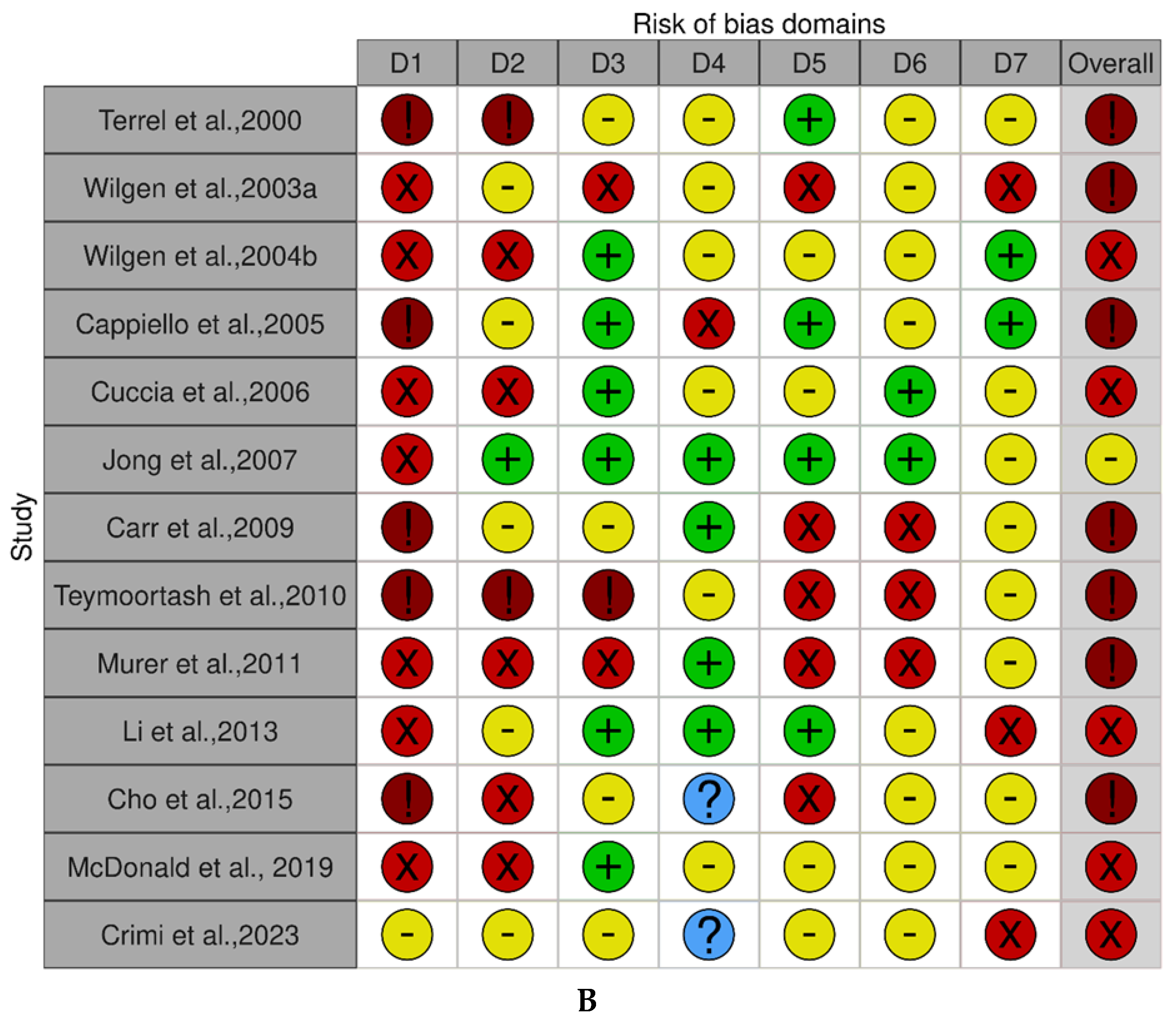
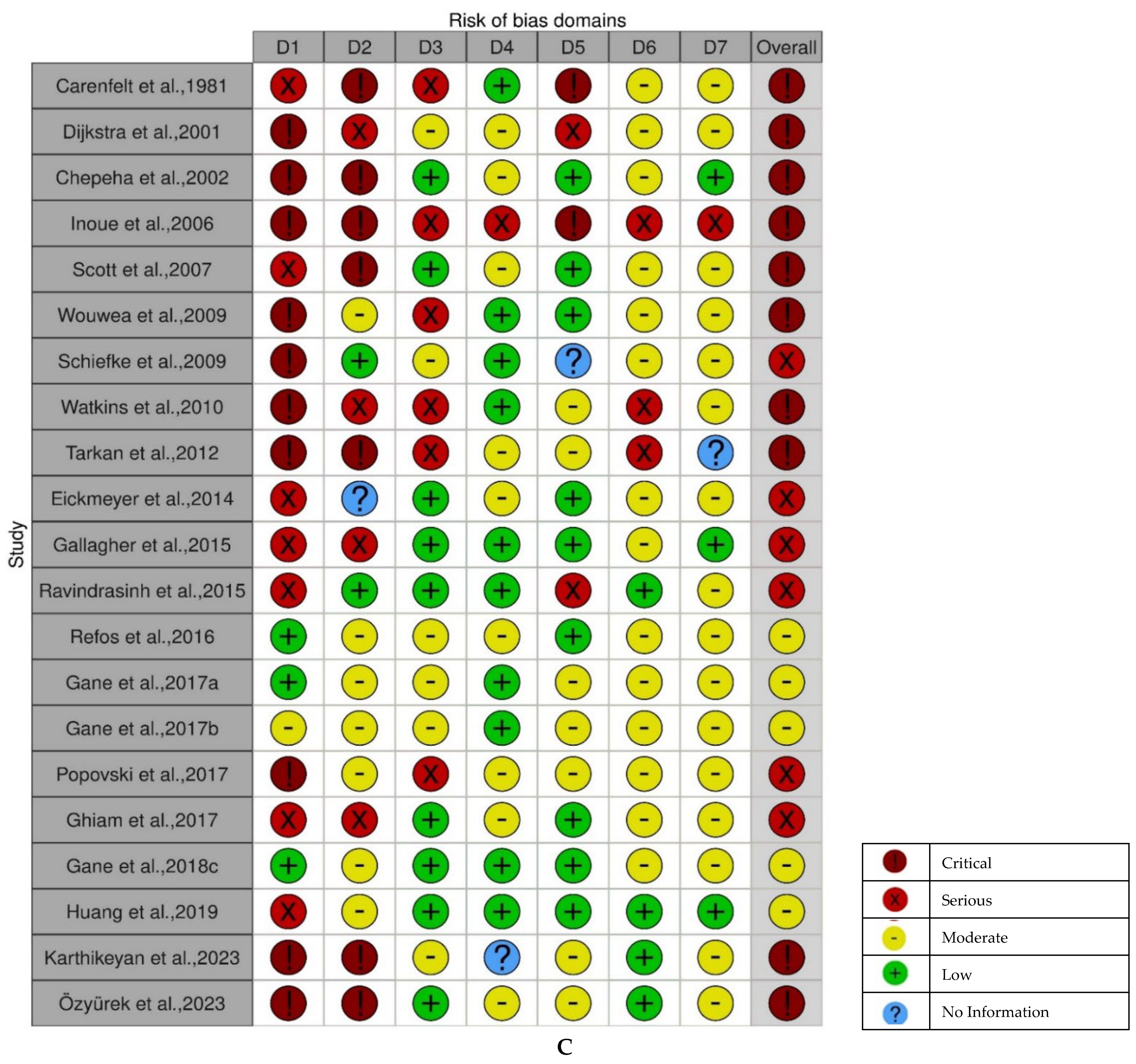
3.3. Risk of Bias Within and Across the Studies
- Randomized controlled trials
- Cohort studies
- Cross-sectional studies
3.4. Quality of Evidence
3.5. Synthesis of the Results
3.5.1. Pain Intensity Outcomes
- 1.
- Shoulder pain
- Radical Neck Dissection (RND) vs. others.
- RND (sacrificed SAN) vs. MRND (preserved SAN)
- RND vs. SND
- Modified Radical Neck Dissection (MRND) vs. others
- MRND vs. SND
- MRND vs. SOND
- Selective Neck Dissection (SND) vs. others
- SND vs. non-surgical side
- SND vs. SND (with or without other therapies)
- SND with radiotherapy vs. SND without radiotherapy
- SND with sacrificed cervical plexus vs. SND without sacrificed cervical plexus
- SND (with electrocautery (EC) vs. SND (with harmonic scalpel (HS)
- SND vs. Functional neck dissection (FND)
- Supraomohyoid neck dissection (SOND) alone
- Mixed Neck Dissection (ND): SND and MRND (preserved SAN) alone
- 2.
- Neck pain
- Radical Neck Dissection (sacrificed SAN) vs. Modified Radical Neck Dissection (preserved SAN).
- Modified Radical Neck Dissection (MRND) vs. Selective Neck Dissection (SND)
- Selective Neck Dissection (SND) vs. others
- SND (with radiotherapy) vs. SND (without radiotherapy)
- SND vs. non-surgical group
- Mixed Neck Dissection: SND and MRND (sacrificed cervical branches) vs. preserved cervical branches
3.5.2. Range of Motion Outcomes
- 1.
- Shoulder range of motion.
- Radical Neck Dissection (RND) vs. others
- RND vs. MRND
- RND vs. SOND
- RND vs. SND
- Modified Radical Neck Dissection (MRND) vs. Others
- MRND vs. non-surgical side
- MRND alone
- MRND vs. SND
- MRND with pectoralis major myocutaneous flap (PMMC) vs. without PMMC
- Selective Neck Dissection vs. others
- SND vs. non-surgical side
- SND alone
- SND IIb vs. others
- SND IIb vs. SND IIa
- SND IIb spared bilaterally vs. SND IIb spared unilaterally
- SND IIb and V dissection vs. SND IIb preserved
- SND (level V) vs. SND (level II–IV)
- SND vs. FND
- Mixed Neck Dissection vs. others
- MRND and SND with cervical root branches removed vs. MRND and SND with preserved cervical root branches
- MRND and SND with preserved SAN alone
- MRND and SND alone
- 2.
- Cervical range of motion
- Modified Radical Neck Dissection vs. others
- MRND vs. SND
- Flexion and extension
- Lateral flexion
- MRND vs. SOND
- Selective Neck Dissection vs. others
- SND vs. non-surgical side
- SND (level 2b spared bilaterally) vs. SND (level 2b spared unilaterally)
- SOND vs. sentinel node biopsy (SNB)
- Mixed Neck Dissection vs. Others
- MRND and SND with removed cervical root branches vs. preserved cervical root branches
- 3.
- Jaw range of motion
- Modified Radical Neck Dissection vs. Selective Neck Dissection
3.5.3. Muscle Strength Outcomes
- 1.
- Shoulder muscle strength
- Radical Neck Dissection vs. others
- RND with sacrificed SAN vs. RND with preserved SAN
- RND vs. MRND
- RND vs. SND
- MRND with pectoralis major myocutaneous flap (PMMF) vs. MRND without PMMF
- Selective Neck Dissection vs. others
- SND (level IIb dissected) vs. SND (level IIb preserved)
- SND (level 2b spared bilaterally) vs. SND (level 2b spared unilaterally)
- SND (level II–V) vs. SND (level II–IV)
- Mixed Neck Dissection vs. Others
- Mixed ND (SND and MRND) with preserved SAN alone
- 2.
- Neck muscle strength
- Selective Neck Dissection vs. others
- SND (level 2b spared bilaterally) vs. SND (level 2b spared unilaterally)
- SND (preserved SAN) vs. MRND (preserved SAN)
- 3.
- Respiratory muscle strength
- Mixed Neck Dissection: RND, MRND, and SND (stage I–VI neck dissection)
3.5.4. Disability Questionnaire Outcomes
- 1.
- Shoulder disability
- Radical Neck Dissection vs. others
- RND vs. MRND
- RND vs. MRND/SND
- Modified Radical Neck Dissection vs. Others
- MRND (nerve monitored) vs. (MRND non-monitored)
- MRND vs. SND
- MRND vs. SOND
- MRND with a pectoralis major myocutaneous (PMMC) flap vs. MRND without PMMC flap
- Selective Neck Dissection alone or vs. others
- SND alone
- SND vs. non-surgery
- SND vs. FND
- SND vs. SND with radiotherapy/chemoradiation
- SND unilateral level V vs. bilateral level III to IV dissections
- Supraomohyoid Neck Dissection (SOND) vs. others
- SOND vs. Sentinel Node Biopsy (SNB)
- SOND vs. MISOND (minimally invasive supraomohyoid neck dissection)
- SOND vs. extended SOND and lateral neck dissection
- SOND vs. mixed ND (SND/MRND/RND)
- Mixed Neck Dissection vs. others
- Mixed ND (SND and MRND (preserved SAN) alone
- Mixed ND with PMMC vs. without PMMC
- 2.
- Shoulder and neck disability
- Radical Neck Dissection vs. Functional Neck Dissection
- Modified Radical Neck Dissection vs. Selective Neck Dissection
- Selective Neck Dissection (levels 2a–4 included dissection of level 2b) vs. Selective Neck Dissection (levels 2a–4 without dissection of level 2b)
- 3.
- Neck disability
- Modified Radical Neck Dissection vs. Selective Neck Dissection
- Mixed Neck Dissection (spared CN XI) vs. without ND
4. Discussion
4.1. Shoulder Dysfunction
4.2. Neck Dysfunction
4.3. Other Neuromusculoskeletal Dysfunctions
4.4. Methodological and Quality of Evidence
4.5. Strengths and Limitations of This Review
4.6. Clinical Relevance and Rehabilitation
4.7. Clinical and Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HNC | Head and neck cancer |
| HPV | Human papillomavirus |
| QOL | Quality of life |
| ND | Neck dissection |
| RND | Radical neck dissection |
| MRND | Modified radical neck dissection |
| SND | Selective neck dissection |
| SOND | Supraomohyoid neck dissection |
| END | Extended neck dissection |
| FND | Functional neck dissection |
| MISOND | Minimally invasive supraomohyoid neck dissection |
| SAN | Spinal accessory nerve |
| HRQOL | Health related quality of life |
| VAS | Visual analogue scale |
| UW-QOL | University of Washington Quality of Life questionnaire |
| EC | Electrocautery |
| HS | Harmonic scalpel |
| AAT | Arm abduction test |
| ADLs | Activities of daily living |
| PMMC | Pectoralis major myocutaneous flap |
| ROM | Range of motion |
| MMT | Manual muscle testing |
| SDQ | Shoulder Disability Questionnaire |
| SPADI | Shoulder Pain and Disability Index |
| CAS | Clinical Assessment Score |
| SFPS | Shoulder Function and Performance Score |
| DASH | Disability of the Arm, Shoulder and Hand |
| WORC | Western Ontario Rotator Cuff |
| GARS | Groningen Activity Restriction Scale |
| NDI | Neck Disability Index |
| NPDS | Neck Pain and Disability Scale |
| NPNPQ | Northwick Park Neck Pain Questionnaire |
| PMMF | Pectoralis major myocutaneous flap |
References
- Macfarlane, T.V.; Wirth, T.; Ranasinghe, S.; Ah-See, K.W.; Renny, N.; Hurman, D. Head and Neck Cancer Pain: Systematic Review of Prevalence and Associated Factors. J. Oral Maxillofac. Res. 2012, 3, e1. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Dutta, S.; Chaturvedi, P.; Nair, S. Head and Neck Cancers in Developing Countries. Rambam Maimonides Med. J. 2014, 5, e0009. [Google Scholar] [CrossRef] [PubMed]
- Mourad, M.; Jetmore, T.; Jategaonkar, A.A.; Moubayed, S.; Moshier, E.; Urken, M.L. Epidemiological Trends of Head and Neck Cancer in the United States: A SEER Population Study. J. Oral Maxillofac. Surg. 2017, 75, 2562–2572. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Zorzi, M.; Del Mistro, A.; Da Mosto, M.C.; Tirelli, G.; Buzzoni, C.; Rugge, M.; Polesel, J.; Guzzinati, S.; for the AIRTUM Working Group. The evolution of the epidemiological landscape of head and neck cancer in Italy: Is there evidence for an increase in the incidence of potentially HPV-related carcinomas? PLoS ONE 2018, 13, e0192621. [Google Scholar] [CrossRef]
- Radosevich, J.A. Head & Neck Cancer: Current Perspectives, Advances, and Challenges; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Fonsêca, T.C.; Jural, L.A.; Marañón-Vásquez, G.A.; Magno, M.B.; Roza, A.L.O.C.; Ferreira, D.M.T.P.; Maia, L.C.; Romañach, M.J.; Agostini, M.; Abrahão, A.C. Global prevalence of human papillomavirus-related oral and oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Clin. Oral Investig. 2023, 28, 62. [Google Scholar] [CrossRef]
- Mifsud, M.; Eskander, A.; Irish, J.; Gullane, P.; Gilbert, R.; Brown, D.; de Almeida, J.R.; Urbach, D.R.; Goldstein, D.P. Evolving trends in head and neck cancer epidemiology: Ontario, Canada 1993–2010. Head Neck 2017, 39, 1770–1778. [Google Scholar] [CrossRef]
- Gane, E.; Michaleff, Z.; Cottrell, M.; McPhail, S.; Hatton, A.; Panizza, B.; O’Leary, S. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: A systematic review. Eur. J. Surg. Oncol. 2017, 43, 1199–1218. [Google Scholar] [CrossRef]
- Larsen, M.H.; Lorenzen, M.M.; Bakholdt, V.; Sørensen, J.A. The prevalence of nerve injuries following neck dissections—A systematic review and meta-analysis. Dan. Med. J. 2020, 67, A08190464. [Google Scholar]
- Nilsen, M.L.; Lyu, L.; Belsky, M.A.; Mady, L.J.; Zandberg, D.P.; Clump, D.A.; Skinner, H.D.; Das Peddada, S.; George, S.; Johnson, J.T. Impact of Neck Disability on Health-Related Quality of Life among Head and Neck Cancer Survivors. Otolaryngol. Neck Surg. 2020, 162, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hammermüller, C.; Hinz, A.; Dietz, A.; Wichmann, G.; Pirlich, M.; Berger, T.; Zimmermann, K.; Neumuth, T.; Mehnert-Theuerkauf, A.; Wiegand, S.; et al. Depression, anxiety, fatigue, and quality of life in a large sample of patients suffering from head and neck cancer in comparison with the general population. BMC Cancer 2021, 21, 94. [Google Scholar] [CrossRef]
- Pickens, C. Effectiveness of Prophylactic Lingual Strengthening Exercises for Patients with Head and Neck Cancer; ProQuest: Ann Arbor, MI, USA, 2019; pp. 1–76. [Google Scholar]
- Gabrysz-Forget, F.; Tabet, P.; Rahal, A.; Bissada, E.; Christopoulos, A.; Ayad, T. Free versus pedicled flaps for reconstruction of head and neck cancer defects: A systematic review. J. Otolaryngol. Head Neck Surg. 2019, 48, 13. [Google Scholar] [CrossRef]
- Watters, A.L.; Cope, S.; Keller, M.N.; Padilla, M.; Enciso, R. Prevalence of trismus in patients with head and neck cancer: A systematic review with meta-analysis. Head Neck 2019, 41, 3408–3421. [Google Scholar] [CrossRef]
- Bensadoun, R.J.; Riesenbeck, D.; Lockhart, P.B.; Elting, L.S.; Spijkervet, F.K.L.; Brennan, M.T. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support. Care Cancer 2010, 18, 1033–1038. [Google Scholar] [CrossRef]
- Chen, S.C. Oral Dysfunction in Patients with Head and Neck Cancer: A Systematic Review. J. Nurs. Res. 2019, 27, e58. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020. Available online: https://www.gradepro.org/ (accessed on 18 December 2024).
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J. GRADE guidelines—An introduction to the 10th–13th articles in the series. J. Clin. Epidemiol. 2013, 66, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Sultan, S.; Glasziou, P.; Akl, E.A.; Alonso-Coello, P.; Atkins, A.; Kunz, R.; Brozek, J.; Montori, V.; et al. GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol. 2011, 64, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Parida, P.K.; Bhoi, S.K.; Sahoo, J.; Samal, D.K.; Dash, A.; Mittal, Y.; Chithambaram, K.S.; Swarup, A.; Chenniappan, S.; et al. Shoulder Dysfunction and Quality of Life Following Modified Radical and Selective Neck Dissection: A Prospective Comparative Study. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 3245–3255. [Google Scholar] [CrossRef]
- Sakai, A.; Nonaka, T.; Furuya, H.; Ebisumoto, K.; Sugimoto, R.; Maki, D.; Iijima, H.; Hanayama, K.; Okami, K. Shoulder function after neck dissection with level IIb preservation: A prospective observational study. Acta Otolaryngol. 2023, 143, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Patekar, S.; Ishwarya, M.; Padmakshan, S.; Bradoo, R. Shoulder Dysfunction Post Spinal Accessory Nerve Preserving Neck Dissections: Our Experience. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 675–679. [Google Scholar] [CrossRef]
- Sharma, N.; George, N.A.; Sebastian, P. Neurovascular Complications After Neck Dissection: A Prospective Analysis at a Tertiary Care Centre in South India. Indian J. Surg. Oncol. 2020, 11, 746–751. [Google Scholar] [CrossRef]
- Reddy, G.R.K.; Hulikal, N.; Lakshmi, A.Y.; Vengamma, B. Nerve and vein preserving neck dissections for oral cancers: A prospective evaluation of spinal accessory nerve function and internal jugular vein patency following treatment. Acta Otorhinolaryngol. Ital. 2018, 38, 7–12. [Google Scholar] [CrossRef]
- Chan, J.Y.W.; Wong, S.T.S.; Chan, R.C.L.; Wei, W.I. Shoulder Dysfunction after Selective Neck Dissection in Recurrent Nasopharyngeal Carcinoma. Otolaryngol. Head Neck Surg. 2015, 153, 379–384. [Google Scholar] [CrossRef]
- Sobol, S.; Jensen, C.; Sawyer, W.; Costiloe, P.; Thong, N. Objective Comparison of Physical Dysfunction After Neck Dissection. Head Neck 1985, 150, 503–509. [Google Scholar] [CrossRef]
- Celik, B.; Coskun, H.; Kumas, F.F.; Irdesel, J.; Zarifoglu, M.; Erisen, L.; Onart, S. Accessory nerve function after level 2b–preserving selective neck dissection. Head Neck 2009, 31, 1496–1501. [Google Scholar] [CrossRef]
- Anehosur, V.; Vadera, H.; Bhat, A.; Satyanarayana, S.; Kumar, N. Does Pectoralis Major Myocutaneous Flap Cause the Shoulder Morbidity: A Clinical Comparative Study. Indian J. Otolaryngol. Head Neck Surg. 2020, 74, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Sato, Y.; Abe, J.; Kumagai, J.; Morita, S.; Saijo, S.; Yamazaki, T.; Asada, Y.; Matsuura, K. Shoulder function after neck dissection: Assessment via a shoulder-specific quality-of-life questionnaire and active shoulder abduction. Auris Nasus Larynx 2021, 48, 138–147. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, S.; Wang, D.; Xu, N.; Jin, S.F.; Wang, C.C. Does pectoralis major flap harvesting induce upper extremity dysfunction? J. Int. Med. Res. 2015, 43, 555–559. [Google Scholar] [CrossRef]
- Prasad, B.R.; Sharma, S.M.; Thomas, S.; Sabastian, P.; Aashal, S. Assessment of shoulder function after functional neck dissection and selective neck dissection (Levels I, II, III) in patients with carcinoma of tongue: A comparative study. J. Maxillofac. Oral Surg. 2009, 8, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.B.; Feng, Z.; Zhang, J.G.; Peng, X.; Cai, Z.G.; Mao, C.; Zhang, Y.; Yu, G.Y.; Li, J.N.; Niu, L.X. Supraomohyoid neck dissection and modified radical neck dissection for clinically node-negative oral squamous cell carcinoma: A prospective study of prognosis, complications and quality of life. J. Cranio Maxillofac. Surg. 2014, 42, 1885–1890. [Google Scholar] [CrossRef]
- Laverick, S.; Lowe, D.; Brown, J.S.; Vaughan, E.D. The Impact of Neck Dissection on Health-Related Quality of Life. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Güldiken, Y.; Orhan, K.S.; Demirel, T.; Ural, H.I.; Yücel, E.A.; Deǧer, K. Assessment of shoulder impairment after functional neck dissection: Long term results. Auris Nasus Larynx 2005, 32, 387–391. [Google Scholar] [CrossRef]
- Erisen, L.; Basel, B.; Irdesel, J.; Zarifoglu, M.; Coskun, H.; Basut, O.; Tezel, I.; Hizalan, I.; Onart, S. Shoulder function after accessory nerve-sparing neck dissections. Head Neck 2004, 26, 967–971. [Google Scholar] [CrossRef]
- Garzaro, M.; Riva, G.; Raimondo, L.; Aghemo, L.; Giordano, C.; Pecorari, G. A study of neck and shoulder morbidity following neck dissection: The benefits of cervical plexus preservation. Ear Nose Throat J. 2015, 94, 330–334. [Google Scholar]
- Agarwal, S.K.; Munjal, M.; Koul, R.; Agarwal, R. Prospective evaluation of the quality of life of oral tongue cancer patients before and after the treatment. Ann. Palliat. Med. 2014, 3, 238–243. [Google Scholar]
- Santana, A.F.S.G.; Caruso, P.; Santana, P.V.; Porto, G.C.L.M.; Kowalski, L.P.; Vartanian, J.G. Inspiratory muscle weakness, diaphragm immobility and diaphragm atrophy after neck dissection. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1227–1234. [Google Scholar] [CrossRef]
- Short, S.O.; Kaplan, J.N.; Laramore, G.E.; Cummings, C.W. Shoulder pain and function after neck dissection with or without preservation of the spinal accessory nerve. Am. J. Surg. 1984, 148, 478–482. [Google Scholar] [CrossRef]
- Cheng, P.-T.; Lin, Y.-H.; Hao, S.-P.; Yeh, A.R.-M. Objective comparison of shoulder dysfunction after three neck dissection techniques. Ann. Otol. Rhinol. Laryngol. 2000, 109, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Orhan, K.S.; Demirel, T.; Baslo, B.; Orhan, E.K.; A Yücel, E.; Güldiken, Y.; Değer, K. Spinal accessory nerve function after neck dissections. J. Laryngol. Otol. 2007, 121, 44–48. [Google Scholar] [CrossRef]
- Selcuk, A.; Selcuk, B.; Bahar, S.; Dere, H. Shoulder function in various types of neck dissection. Role of spinal accessory nerve and cervical plexus preservation. Tumori 2008, 94, 36–39. [Google Scholar] [CrossRef]
- Dilber, M.; Kasapoglu, F.; Erisen, L.; Basut, O.; Tezel, I. The relationship between shoulder pain and damage to the cervical plexus following neck dissection. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Oz, B.; Memis, A. Development of musculoskeletal complaints and functional disabilities in patients with laryngeal carcinoma after neck dissection sparing spinal accessory nerve. Eur. J. Cancer Care 2009, 18, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Merve, A.; Mitra, I.; Swindell, R.; Homer, J.J. Shoulder morbidity after pectoralis major flap reconstruction for head and neck cancer. Head Neck 2009, 31, 1470–1476. [Google Scholar] [CrossRef]
- Ahlberg, A.; Nikolaidis, P.; Engström, T.; Gunnarsson, K.; Johansson, H.; Sharp, L.; Laurell, G. Morbidity of supraomohyoidal and modified radical neck dissection combined with radiotherapy for head and neck cancer. A prospective longitudinal study. Head Neck 2012, 34, 66–72. [Google Scholar] [CrossRef]
- Speksnijder, C.M.; Van Der Bilt, A.; Slappendel, M.; De Wijer, A.; Merkx, M.A.W.; Koole, R. Neck and shoulder function in patients treated for oral malignancies: A 1-year prospective cohort study. Head Neck 2012, 35, 1303–1313. [Google Scholar] [CrossRef]
- Lanišnik, B.; Žitnik, L.; Levart, P.; Žargi, M.; Rodi, Z. The impact on post-operative shoulder function of intraoperative nerve monitoring of cranial nerve XI during modified radical neck dissection. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 4445–4451. [Google Scholar] [CrossRef]
- Özyürek, S.; Kalkan, A.C.; Doğan, E.; Bülbül, H.M.; Kamar, M.A.; Balci, A.; İkiz, A.Ö.; Keskinoğlu, P.; Genç, A. Decreased muscle strength and scapular muscle endurance associated with shoulder function after neck dissection. J. Back Musculoskelet. Rehabil. 2023, 36, 347–355. [Google Scholar] [CrossRef]
- Karthikeyan, G.R.; Venkatramanaiah, C.; Balasubramaniyam, B.; Aiyathurai, M.; Velu, D.; Indrapriyadharshini, K. Quality of life and shoulder function among oral cancer patients treated with selective neck dissection: A cross-sectional study. Indian J. Cancer 2023, 60, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Ricciardi, W.; Cadoni, G.; Arzani, D.; Petrelli, L.; Paludetti, G.; Brennan, P.; Luce, D.; Stucker, I.; Matsuo, K.; et al. Adult height and head and neck cancer: A pooled analysis within the INHANCE Consortium. Head Neck 2014, 36, 1391. [Google Scholar] [CrossRef]
- Refos, J.W.J.; Witte, B.I.; de Goede, C.J.T.; de Bree, R. Shoulder morbidity after pectoralis major flap reconstruction. Head Neck 2016, 38, 1221–1228. [Google Scholar] [CrossRef]
- Popovski, V.; Benedetti, A.; Popovic-Monevska, D.; Grcev, A.; Stamatoski, A.; Zhivadinovik, J. Preservazione del nervo accessorio spinale nelle dissezioni del collo: Outcomes chirurgici e funzionali. Acta Otorhinolaryngol. Ital. 2017, 37, 368–374. [Google Scholar] [CrossRef]
- Gane, E.M.; O’Leary, S.P.; Hatton, A.L.; Panizza, B.J.; McPhail, S.M. Neck and Upper Limb Dysfunction in Patients following Neck Dissection: Looking beyond the Shoulder. Otolaryngol. Head Neck Surg. 2017, 157, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Lotwala, V.; Anajwala, P. Minimally invasive supraomohyoid neck dissection by total endoscopic technique for oral squamous carcinoma. Surg. Endosc. 2016, 30, 2315–2320. [Google Scholar] [CrossRef] [PubMed]
- Schiefke, F.; Akdemir, M.; Weber, A.; Akdemir, D.; Singer, S.; Frerich, B. Function, Postoperative Morbidity, And Quality of Life After Cervical Sentinel Node Biopsy And After Selective Neck Dissection. Head Neck 2009, 31, 503–512. [Google Scholar] [CrossRef]
- Watkins, J.P.; Williams, G.B.; Mascioli, A.A.; Wan, J.Y.; Samant, S. Shoulder function in patients undergoing selective neck dissection with or without radiotion and chemotherapy. Head Neck 2011, 33, 615–619. [Google Scholar] [CrossRef]
- Eickmeyer, S.M.; Walczak, C.K.; Myers, K.B.; Lindstrom, D.R.; Layde, P.; Campbell, B.H. Quality of life, shoulder range of motion, and spinal accessory nerve status in 5-year survivors of head andneck cancer. PMR 2014, 6, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Ghiam, M.K.; Mannion, K.; Dietrich, M.S.; Stevens, K.L.; Gilbert, J.; Murphy, B.A. Assessment of musculoskeletal impairment in head and neck cancer patients. Support. Care Cancer 2017, 25, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.K.; Sacco, A.G.; Lee, J.S.J.; Taylor, R.; Chanowski, E.J.; Bradford, C.R.; Prince, M.E.; Moyer, J.S.; Wolf, G.T.; Worden, F.P.; et al. Association between multimodality neck treatment and work and leisure impairment: A disease-specific measure to assess both impairment and rehabilitation after neck dissection. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Carenfelt, C.; Eliasson, K. Radical neck dissection and permanent sequelae associated with spinal accessory nerve injuries. Acta Otolaryngol. 1981, 91, 155–160. [Google Scholar] [CrossRef]
- Chepeha, D.B.; Taylor, R.J.; Chepeha, J.C.; Teknos, T.N.; Bradford, C.R.; Sharma, P.K.; Terrell, J.E.; Wolf, G.T. Functional assessment using Constant’s Shoulder Scale after modified radical and selective neck dissection. Head Neck 2002, 24, 432–436. [Google Scholar] [CrossRef]
- Dijkstra, P.U.; van Wilgen, P.C.; Buijs, R.P.; Brendeke, W.; de Goede, C.J.T.; Kerst, A.; Koolstra, M.; Marinus, J.; Schoppink, E.M.; Stuiver, M.M.; et al. Incidence of shoulder pain after neck dissection: A clinical explorative study for risk factors. Head Neck 2001, 23, 947–953. [Google Scholar] [CrossRef]
- Inoue, H.; Nibu, K.-I.; Saito, M.; Otsuki, N.; Ishida, H.; Onitsuka, T.; Fujii, T.; Kawabata, K.; Saikawa, M. Quality of life after neck dissection. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 662–666. [Google Scholar] [CrossRef]
- Scott, B.; Lowe, D.; Rogers, S.N. The impact of selective neck dissection on shoulder and cervical spine movements. Physiotherapy 2007, 93, 102–109. [Google Scholar] [CrossRef]
- van Wouwe, M.; de Bree, R.; Kuik, D.J.; de Goede, C.J.; Verdonck-de Leeuw, I.M.; Doornaert, P.; Leemans, C.R. Shoulder morbidity after non-surgical treatment of the neck. Radiother. Oncol. 2009, 90, 196–201. [Google Scholar] [CrossRef]
- Tarkan, Ö.; Tuncer, Ü.; BozdemIr, H.; Sarpel, T.; ÖzdemIr, S.; SürmelIoǧlu, Ö. Clinical and electrophysiological evaluation of shoulder functions in spinal accessory nerve-preserving neck dissection. Turkish J. Med. Sci. 2012, 42, 852–860. [Google Scholar] [CrossRef]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. Predictors of health-related quality of life in patients treated with neck dissection for head and neck cancer. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 4183–4193. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lee, Y.-Y.; Tso, H.-H.; Chen, P.-C.; Chen, Y.-C.; Chien, C.-Y.; Chung, Y.-J.; Leong, C.-P. The Sonography and Physical Findings on Shoulder after Selective Neck Dissection in Patients with Head and Neck Cancer: A Pilot Study. Biomed Res. Int. 2019, 2019, 2528492. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. Neck and Shoulder Motor Function following Neck Dissection: A Comparison with Healthy Control Subjects. Otolaryngol. Head Neck Surg. 2019, 160, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Crimi, S.; Battaglia, S.; Maugeri, C.; Mirabella, S.; Fiorillo, L.; Cervino, G.; Bianchi, A. Does Age Affect the Rate of Spinal Nerve Injury after Selective Neck Dissection? Age as a Prognostic Factor of Spinal Nerve Injury after Selective Neck Dissection. J. Pers. Med. 2023, 13, 1082. [Google Scholar] [CrossRef] [PubMed]
- van Wilgen, C.P.; Dijkstra, P.U.; van der Laan, B.F.A.M.; Plukker, J.T.M.; Roodenburg, J.L.N. Shoulder complaints after nerve sparing neck dissections. Int. J. Oral Maxillofac. Surg. 2004, 33, 253–257. [Google Scholar] [CrossRef]
- van Wilgen, C.P.; Dijkstra, P.U.; Nauta, J.M.; Vermey, A.; Roodenburg, J.L.N. Shoulder pain and disability in daily life, following supraomohyoid neck dissection: A pilot study. J. Cranio-Maxillofac. Surg. 2003, 31, 183–186. [Google Scholar] [CrossRef]
- Carr, S.D.; Bowyer, D.; Cox, G. Upper limb dysfunction following selective neck dissection: A retrospective questionnaire study. Head Neck 2009, 31, 789–792. [Google Scholar] [CrossRef]
- Terrell, J.E.; Welsh, D.E.; Bradford, C.R.; Chepeha, D.B.; Esclamado, R.M.; Hogikyan, N.D.; Wolf, G.T. Pain, quality of life, and spinal accessory nerve status after neck dissection. Laryngoscope 2000, 110, 620–626. [Google Scholar] [CrossRef]
- Cappiello, J.; Piazza, C.; Giudice, M.; De Maria, G.; Nicolai, P. Shoulder disability after different selective neck dissections (levels II-IV versus levels II-V): A comparative study. Laryngoscope 2005, 115, 259–263. [Google Scholar] [CrossRef]
- Cuccia, G.; Shelley, O.P.; D’Alcontres, F.S.; Giannitrapani, M.; Soutar, D.S.; Camilleri, I.G. Evidence of significant sternocleidomastoid atrophy following modified radical neck dissection type III. Plast. Reconstr. Surg. 2006, 117, 227–232. [Google Scholar] [CrossRef]
- Roh, J.-L.; Yoon, Y.-H.; Kim, S.Y.; Park, C.I. Cervical sensory preservation during neck dissection. Oral Oncol. 2007, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Teymoortash, A.; Hoch, S.; Eivazi, B.; Werner, J.A. Postoperative morbidity after different types of selective neck dissection. Laryngoscope 2010, 120, 924–929. [Google Scholar] [CrossRef]
- Murer, K.; Huber, G.F.; Haile, S.R.; Stoeckli, S.J. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the N0 neck in patients with oral squamous cell carcinoma. Head Neck 2010, 33, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Yang, K. Evaluation of the efficacy of a novel radical neck dissection preserving the external jugular vein, greater auricular nerve, and deep branches of the cervical nerve. Onco. Targets. Ther. 2013, 6, 361–367. [Google Scholar] [PubMed]
- Cho, J.-G.; Lee, N.; Park, M.-W.; Baek, S.-K.; Kwon, S.-Y.; Jung, K.-Y.; Woo, J.-S. Measurement of the trapezius muscle volume: A new assessment strategy of shoulder dysfunction after neck dissection for the treatment of head and neck cancers. Head Neck 2015, 37, 619–623. [Google Scholar] [CrossRef]
- McDonald, C.; Lowe, D.; Bekiroglu, F.; Schache, A.; Shaw, R.; Rogers, S.N. Health-related quality of life in patients with T1N0 oral squamous cell carcinoma: Selective neck dissection compared with wait and watch surveillance. Br. J. Oral Maxillofac. Surg. 2019, 57, 649–654. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Y.; Liu, P. Application of supraomohyoid neck dissection via retroauricular hairline incision in patients with oral cancer. Auris Nasus Larynx 2022, 49, 126–132. [Google Scholar] [CrossRef]
- Dziegielewski, P.T.; McNeely, M.L.; Ashworth, N.; O’connell, D.A.; Barber, B.; Courneya, K.S.; Debenham, B.J.; Seikaly, H. 2b or Not 2b? Shoulder Function After Level 2b Neck Dissection: A Double-Blind Randomized Controlled Clinical Trial. Cancer 2020, 126, 1492–1501. [Google Scholar] [CrossRef]
- Parikh, S.; Tedmanb, B.S.; Lowea, D.; Rogers, S.N. A double blind randomised trial of IIb or not IIb neck dissections on electromyography, clinical examination, and questionnaire-based outcomes: A feasibility study. Br. J. Oral Maxillofac. Surg. 2012, 50, 394–403. [Google Scholar] [CrossRef]
- Mathialagan, A.; Verma, R.K.; Panda, N.K. Comparison of spinal accessory dysfunction following neck dissection with harmonic scalpel and electrocautery—A randomized study. Oral Oncol. 2016, 61, 142–145. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. The relationship between physical impairments, quality of life and disability of the neck and upper limb in patients following neck dissection. J. Cancer Surviv. 2018, 12, 619–631. [Google Scholar] [CrossRef]
- Dugena, O.; Islam, S.; Hayter, J. The rate of phrenic nerve injury following neck dissection for head and neck cancer and its impact on length of hospital stay. Int. J. Oral Maxillofac. Surg. 2017, 46, 283. [Google Scholar] [CrossRef]
- Goldstein, D.P.; Ringash, J.; Bissada, E.; Jaquet, Y.; Irish, J.; Chepeha, D.; Davis, A.M. Scoping review of the literature on shoulder impairments and disability after neck dissection. Head Neck 2014, 36, 299–308. [Google Scholar] [CrossRef]
- Gosselin, L.E.; Villemure-Poliquin, N.; Audet, N. Quality of Life After Head and Neck Cancer Surgery and Free Flap Reconstruction: A Systematic Review. J. Otolaryngol. Head Neck Surg. 2024, 53, 19160216241248666. [Google Scholar] [CrossRef]
- Falade, I.O.; Murphy, A.I.; Switalla, K.M.; Yin, R.R.; Rose, J.A. Functional donor-site morbidity following reconstruction with pectoralis major flaps: A systematic review. JPRAS Open 2024, 39, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.; Hewitta, L.; Yabea, T.E.; Mitchell, J.; Ashforda, B. Quality of Life Impacts Following Surgery for Advanced Head and Neck Cancer. World J. Oncol. 2023, 14, 150–157. [Google Scholar] [CrossRef]
- Taylor, J.C.; Terrell, J.E.; Ronis, D.L.; Fowler, K.E.; Bishop, C.; Lambert, M.T.; Myers, L.L.; Duffy, S.A.; The University of Michigan Head and Neck Cancer Team. Disability in patients with head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 764–769. [Google Scholar] [CrossRef]
- Harris, A.; Lyu, L.; Wasserman-Winko, T.; George, S.; Johnson, J.T.; Nilsen, M.L. Neck Disability and Swallowing Function in Posttreatment Head and Neck Cancer Patients. Otolaryngol. Head Neck Surg. 2020, 163, 763–770. [Google Scholar] [CrossRef]
- Vartanian, J.G.; Carvalho, A.L.; Toyota, J.; Kowalski, I.S.G.; Kowalski, L.P. Socioeconomic effects of and risk factors for disability in long-term survivors of head and neck cancer. Arch. Otolaryngol. Neck Surg. 2006, 132, 32–35. [Google Scholar] [CrossRef]
- Graca, F.A.; Rai, M.; Hunt, L.C.; Stephan, A.; Wang, Y.-D.; Gordon, B.; Wang, R.; Quarato, G.; Xu, B.; Fan, Y.; et al. The myokine Fibcd1 is an endogenous determinant of myofiber size and mitigates cancer-induced myofiber atrophy. Nat. Commun. 2022, 13, 2370. [Google Scholar] [CrossRef] [PubMed]
- Guzel, S.; Umay, E.; Gundogdu, I.; Bahtiyarca, Z.T.; Cankurtaran, D. Effects of diaphragm thickness on rehabilitation outcomes in post-ICU patients with spinal cord and brain injury. Eur. J. Trauma Emerg. Surg. 2022, 48, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Olivo, S.; Dennett, L.; Arienti, C.; Dahchi, M.; Arokoski, J.; Heinemann, A.W.; Malmivaara, A. Blinding in Rehabilitation Research: Empirical Evidence on the Association between Blinding and Treatment Effect Estimates. Am. J. Phys. Med. Rehabil. 2020, 99, 198–209. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Fuentes, C.J.; da Costa, B.R.; Ha, C.; Saltaji, H.; Cummings, G. Blinding in Physical Therapy Trials and its Association with Treatment Effects: A Meta-Epidemiological Study. Am. J. Phys. Med. Rehabil. 2017, 96, 34–44. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 statement: Updated guideline for reporting randomised trials. BMJ 2025, 389, e081123. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Parke, S.; Leite, V.F.; Capozzi, L.; Gutierrez, C.; Woodrow, L.; Langelier, D. Rehabilitation Interventions in Head and Neck Cancer: A Scoping Review. Am. J. Phys. Med. Rehabil. 2024, 103, S62–S71. [Google Scholar] [CrossRef]
- Almeida, K.A.M.; Rocha, A.P.; Carvas, N.; Pinto, A.C.P.N. Rehabilitation interventions for shoulder dysfunction in patients with head and neck cancer: Systematic review and meta-analysis. Phys. Ther. 2020, 100, 1997–2008. [Google Scholar] [CrossRef]
- McNeely, M.L.; Chan, K.M.; Spychka, R.A.; Nedeljak, J.; Debenham, B.; Jha, N.; Seikaly, H. Building a Bridge to Community: A Pragmatic Randomized Trial Examining a Combined Physical Therapy and Resistance Exercise Intervention for People after Head and Neck Cancer. Cancers 2024, 16, 1758. [Google Scholar] [CrossRef]
- Nedeljak, J.; Armijo-Olivo, S.; Hernandez, I.A.; Nayar, S.; McNeely, M.L. A Scoping Review of Physiotherapeutic Interventions for Trismus in Head and Neck Cancer: Where Is the Manual Therapy? Physiother. Can. 2021, 74, 173–183. [Google Scholar] [CrossRef]
- Wang, Y.H.; Huang, Y.A.; Chen, I.H.; Hou, W.H.; Kang, Y.N. Exercise for Trismus Prevention in Patients with Head and Neck Cancer: A Network Meta-Analysis of Randomized Controlled Trials. Healthcare 2022, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Nayiga, B.K.; Abrams, S.W.; Rhayel, A.; Edward, H.; Tang, A.; Kho, M.E.; Sebestien, H.; Smith-Turchyn, J. Exploring the use of rehabilitation in individuals with head and neck cancer undergoing treatment: A scoping review. Disabil. Rehabil. 2024, 46, 6302–6322. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Mansilla, B.; Galiano-Castillo, N.; Lozano-Lozano, M.; Fernández-Lao, C.; Lopez-Garzon, M.; Arroyo-Morales, M. Effect of physical therapy modalities on quality of life of head and neck cancer survivors: A systematic review with meta-analysis. J. Clin. Med. 2021, 10, 4696. [Google Scholar] [CrossRef] [PubMed]

| Component | Details |
|---|---|
| Population (P) | Individuals diagnosed with head and neck cancer who underwent surgical treatment, including neck dissection. |
| Intervention (I) | Studies reporting musculoskeletal impairments and dysfunctions following surgery with neck dissection |
| Comparison (C) | Not mandatory; some studies may include comparisons (e.g., affected vs. unaffected side, pre- vs. post-surgery, or control group), but comparison was not required for inclusion |
| Outcomes (O) | Musculoskeletal impairments and dysfunctions such as shoulder pain and dysfunction (e.g., limited range of motion, weakness), neck pain and dysfunction (e.g., limited range of motion, weakness), functional limitations related to activities of daily living |
| Study Design (S) | RCTs, observational studies, including cohort and cross-sectional); published in English. |
| Country | n (%) | Publication Date | n(%) | |
|---|---|---|---|---|
| India Turkey USA United Kingdom Netherland China Italy Canada Japan Australia German Brazil Korea Taiwan Sweden Others (Switzerland, Hongkong, Ireland, Macedonia, Slovenia | 9 (13) 8 (12) 6 (9) 6 (9) 6 (9) 4 (6) 4 (6) 3 (4.5) 3 (4.5) 3 (4.5) 2 (3) 2 (3) 2 (3) 2 (3) 2 (3) 5 (7.5) | After 2016 2010–2015 2000–2009 1985–1999 Study Design RCT Cross-sectional Retrospective Cohort Prospective Cohort Gender Mixed | 23 (34) 17 (26) 24 (36) 3 (4) n (%) 4 (6) 21 (32) 13 (19) 29 (43) 67 (100) | |
| Head and Neck Cancer (HNC) Diagnosis | ||||
| Mixed HNC Oral, Tongue, and Oropharynx Larynx | 43(65) 14 (21) 4 (6) | Nasopharyngeal Not reported | 1(2) 4(6) | |
| Types of Neck Dissection | ||||
| Radical Neck Dissection (RND) Modified Radical Neck Dissection (MRND) Selective Neck Dissection (SND) Elective/Functional Neck Dissection (END/FND) | 10 17 27 3 | Mixed Neck Dissection (preserved/removed Cervical nerve root) SND/MRND with Reconstruction | 8 1 | |
| Musculoskeletal Impairments | ||||
| Pain Shoulder Neck Myofascial muscle pain Range of Motion Shoulder Joint Cervical Joint Jaw Strength Shoulder Muscles Neck Muscles Respiratory Muscles | 11/67 5/67 1/67 26/67 9/67 1/67 10/67 2/67 1/67 | Muscle Activation Trapezius Muscle Sternocleidomastoid (SCM) Muscle volume Trapezius Musculoskeletal disability Shoulder disability Shoulder and neck disability Neck disability Other Posture | 6/67 2/67 1/67 32/67 5/67 3/67 1/67 | |
| Outcome measure tools | ||||
| Pain VAS (11/67) HNQOL (2/67) UWQOL (1/67) Range of Motion Goniometer (22/67) Inclinometer (7/67) Tape measurement (1/67) Ruler (1/67) Posture (1/67) Muscle Activation EMG (6/67) | Muscle Volume CT scan (1/67) Ultrasound (1/67) Strength Dynamometer (4/67) Manual muscle test (7/67) Isokinetic (1/67) Micro RPM (1/67) Musculoskeletal disability Neck Disability - NPNP (1/67) - NPDS (1/67) - NDI (2/67) | Shoulder disability
Shoulder and Neck Disability
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad, N.; Oliveira-Souza, A.I.S.d.; Ntoukas, S.M.; Castro-Carletti, E.M.d.; Munajat, M.; Dennett, L.; Courneya, K.S.; Armijo-Olivo, S.; McNeely, M.L. Musculoskeletal Impairments and Dysfunction in Individuals with Head and Neck Cancer Following Surgery with Neck Dissection—A Systematic Review. Life 2025, 15, 800. https://doi.org/10.3390/life15050800
Mohamad N, Oliveira-Souza AISd, Ntoukas SM, Castro-Carletti EMd, Munajat M, Dennett L, Courneya KS, Armijo-Olivo S, McNeely ML. Musculoskeletal Impairments and Dysfunction in Individuals with Head and Neck Cancer Following Surgery with Neck Dissection—A Systematic Review. Life. 2025; 15(5):800. https://doi.org/10.3390/life15050800
Chicago/Turabian StyleMohamad, Norazlin, Ana Izabela Sobral de Oliveira-Souza, Stephanie M. Ntoukas, Ester Moreira de Castro-Carletti, Munayati Munajat, Liz Dennett, Kerry S. Courneya, Susan Armijo-Olivo, and Margaret L. McNeely. 2025. "Musculoskeletal Impairments and Dysfunction in Individuals with Head and Neck Cancer Following Surgery with Neck Dissection—A Systematic Review" Life 15, no. 5: 800. https://doi.org/10.3390/life15050800
APA StyleMohamad, N., Oliveira-Souza, A. I. S. d., Ntoukas, S. M., Castro-Carletti, E. M. d., Munajat, M., Dennett, L., Courneya, K. S., Armijo-Olivo, S., & McNeely, M. L. (2025). Musculoskeletal Impairments and Dysfunction in Individuals with Head and Neck Cancer Following Surgery with Neck Dissection—A Systematic Review. Life, 15(5), 800. https://doi.org/10.3390/life15050800






