Local Therapy Can Enhance the Prognosis of Certain Patients with Pathologically Diagnosed Neuroendocrine Prostate Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Univariate Analysis Using the Cox Proportional Hazards Model
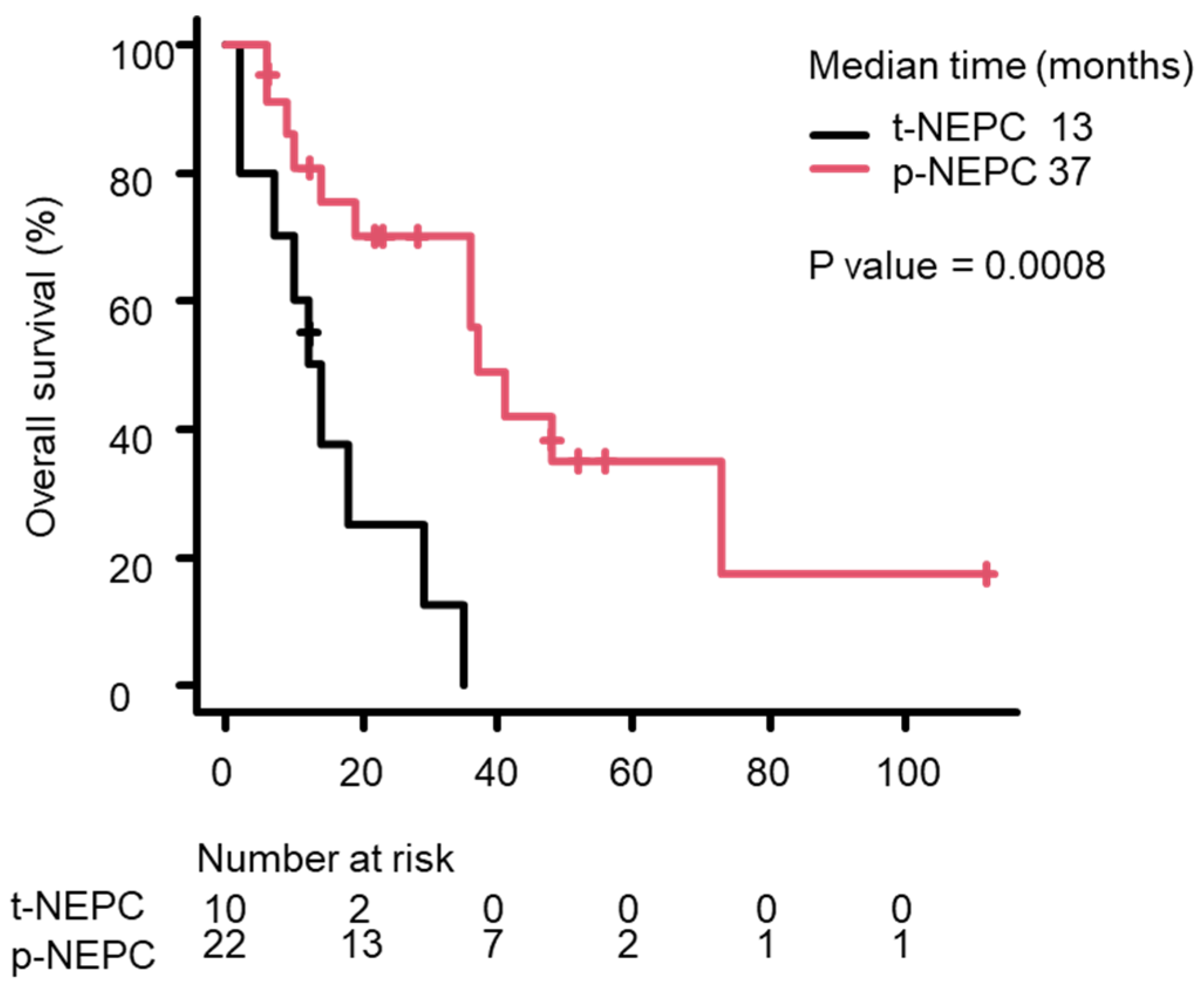
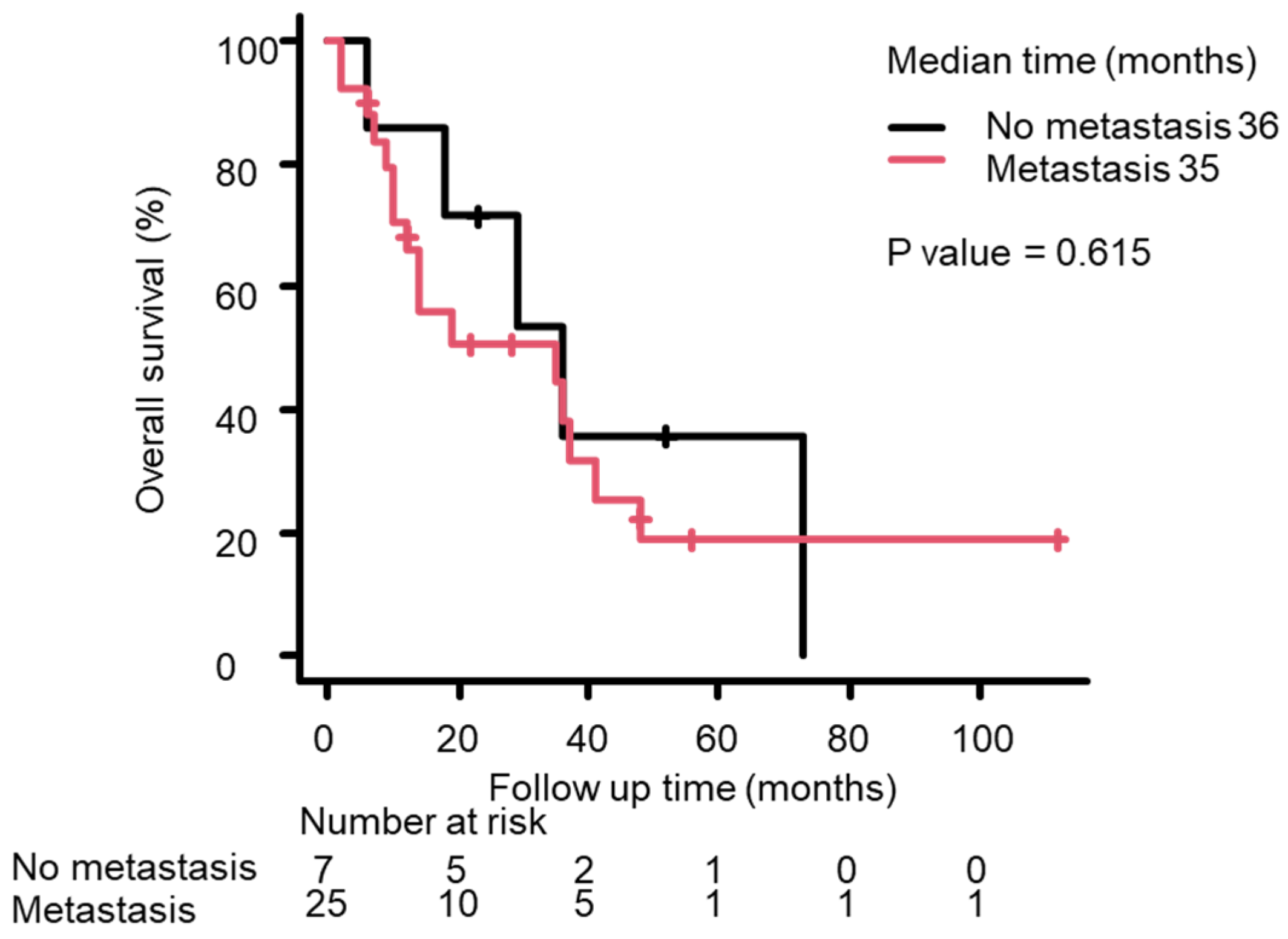
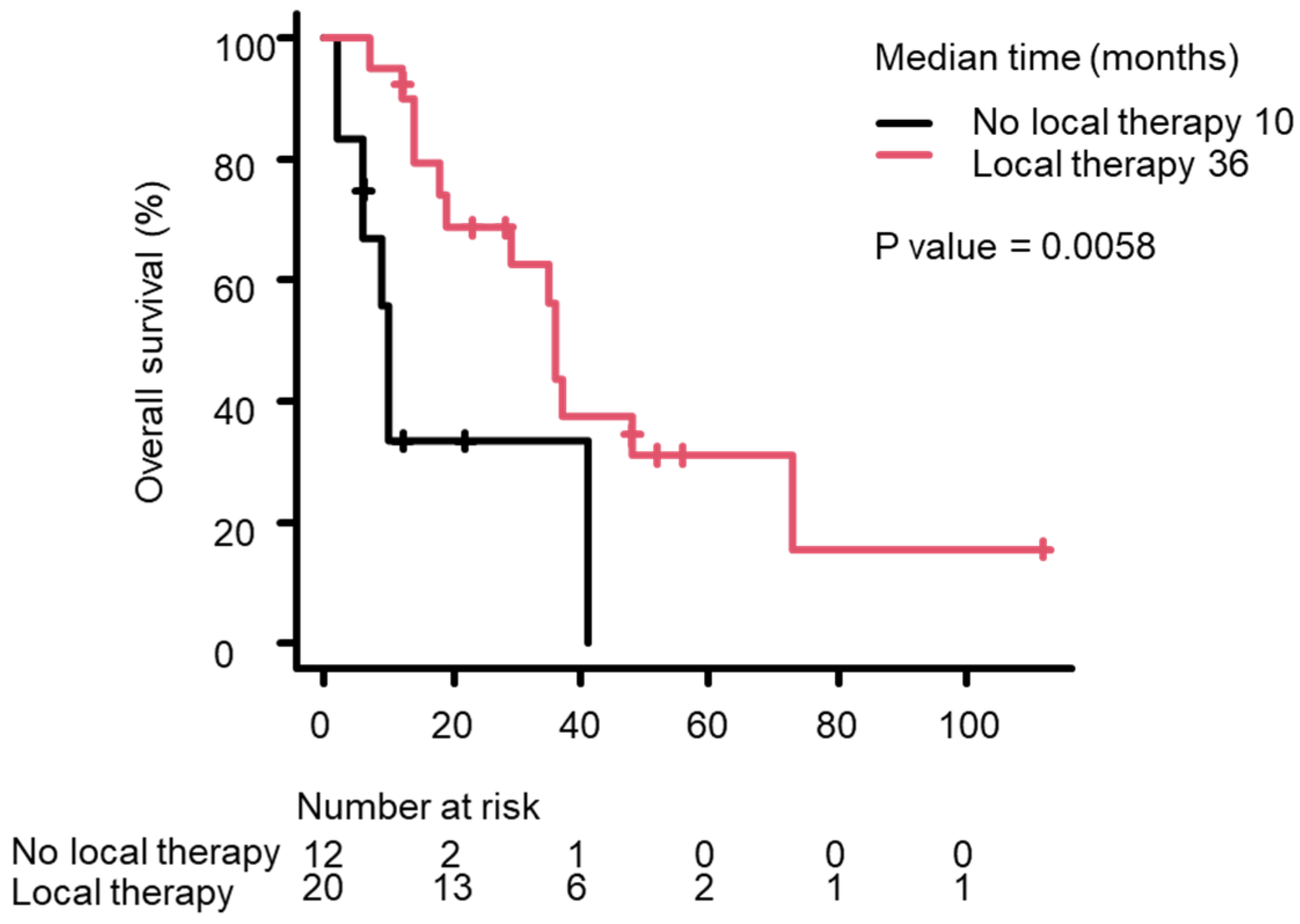
3.3. Survival Analysis Using the Kaplan–Meier Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NEPC | Neuroendocrine prostate cancer |
| p-NEPC | Primary neuroendocrine prostate cancer |
| t-NEPC | Treatment-related neuroendocrine prostate cancer |
| OS | Overall survival |
| ADT | Androgen deprivation therapy |
| HRs | Hazard ratios |
| PSA | Prostate-specific antigen |
| ProGRP | Pro-gastrin-releasing peptide |
| NSE | Neuron-specific enolase |
| CI | Confidence interval |
| SEER | Surveillance epidemiology and end results |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Rickman, D.S.; Park, K.; Chae, S.S.; Sboner, A.; MacDonald, T.Y.; Wang, Y.; Sheikh, K.L.; Terry, S.; Tagawa, S.T.; et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011, 1, 487–495. [Google Scholar] [CrossRef]
- Aggarwal, R.; Huang, J.; Alumkal, J.J.; Zhang, L.; Feng, F.Y.; Thomas, G.V.; Weinstein, A.S.; Friedl, V.; Zhang, C.; Witte, O.N.; et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J. Clin. Oncol. 2018, 36, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, S.; Inoue, T.; Ogawa, O.; Gleave, M.E. Clinical and molecular features of treatment-related neuroendocrine prostate cancer. Int. J. Urol. 2018, 25, 345–351. [Google Scholar] [CrossRef]
- Conteduca, V.; Oromendia, C.; Eng, K.W.; Bareja, R.; Sigouros, M.; Molina, A.; Faltas, B.M.; Sboner, A.; Mosquera, J.M.; Elemento, O.; et al. Clinical features of neuroendocrine prostate cancer. Eur. J. Cancer 2019, 121, 7–18. [Google Scholar] [CrossRef]
- Antwi, S.; Everson, T.M. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: A population-based, propensity score analysis. Cancer Epidemiol. 2014, 38, 435–441. [Google Scholar] [CrossRef]
- Culp, S.H.; Schellhammer, P.F.; Williams, M.B. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A seer-based study. Eur. Urol. 2014, 65, 1058–1066. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Asmis, T.R.; Reaume, M.N.; Dahrouge, S.; Malone, S. Genitourinary small cell carcinoma: A retrospective review of treatment and survival patterns at the Ottawa Hospital Regional Cancer Center. BJU Int. 2006, 97, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Nadal, R.; Schweizer, M.; Kryvenko, O.N.; Epstein, J.I.; Eisenberger, M.A. Small cell carcinoma of the prostate. Nat. Rev. Urol. 2014, 11, 213–219. [Google Scholar] [CrossRef]
- Zaffuto, E.; Pompe, R.; Zanaty, M.; Bondarenko, H.D.; Leyh-Bannurah, S.-R.; Moschini, M.; Dell’Oglio, P.; Gandaglia, G.; Fossati, N.; Stabile, A.; et al. Contemporary Incidence and Cancer Control Outcomes of Primary Neuroendocrine Prostate Cancer: A SEER Database Analysis. Clin. Genitourin. Cancer 2017, 15, e793–e800. [Google Scholar] [CrossRef]
- Fléchon, A.; Pouessel, D.; Ferlay, C.; Perol, D.; Beuzeboc, P.; Gravis, G.; Joly, F.; Oudard, S.; Deplanque, G.; Zanetta, S.; et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: Results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann. Oncol. 2011, 22, 2476–2481. [Google Scholar] [CrossRef]
- Hirano, D.; Okada, Y.; Minei, S.; Takimoto, Y.; Nemoto, N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur. Urol. 2004, 45, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.N.; Daliani, D.D.; Thall, P.F.; Tu, S.-M.; Wang, X.; Reyes, A.; Troncoso, P.; Logothetis, C.J. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J. Clin. Oncol. 2002, 20, 3072–3080. [Google Scholar] [CrossRef]
- Culine, S.; El Demery, M.; Lamy, P.-J.; Iborra, F.; Avancès, C.; Pinguet, F. Docetaxel and cisplatin in patients with metastatic androgen independent prostate cancer and circulating neuroendocrine markers. J. Urol. 2007, 178, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Boevé, L.M.; Hulshof, M.C.; Vis, A.N.; Zwinderman, A.H.; Twisk, J.W.; Witjes, W.P.; Delaere, K.P.; van Moorselaar, R.J.A.; Verhagen, P.C.; van Andel, G. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur. Urol. 2019, 75, 410–418. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Terada, N.; Mizowaki, T.; Saito, T.; Yokomizo, A.; Kohei, N.; Tabata, K.; Shiota, M.; Takahashi, A.; Shimazui, T.; Goto, T.; et al. Potential effectiveness of local radiotherapy for extending survival and reducing symptomatic local events in patients with de novo metastatic prostate cancer. BJUI Compass 2020, 1, 165–173. [Google Scholar] [CrossRef]
- Shiota, M.; Terada, N.; Kimura, T.; Kitamura, H.; Kamoto, T.; Eto, M.; Japanese Urological Oncology Group (JUOG). Differential cancer-specific survival with curative radiotherapy to theprostate for metastatic prostate cancer according to estimated survival by risk group. Int. J. Urol. 2023, 30, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Metzger, A.L.; Abel, S.; Wegner, R.E.; Fuhrer, R.; Mao, S.; Miller, R.; Beriwal, S.; Horne, Z.D. Patterns of care and outcomes in small cell carcinoma of the prostate: A national cancer database analysis. Prostate 2019, 79, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Median (Lowest–Highest) or Number (Percentage) |
|---|---|
| Median age (year) | 68 (46–85) |
| Median PSA level (ng/mL) | 6.1 (0.01–325) |
| Median NSE level (ng/mL) | 19.2 (7.3–268.3) |
| Median ProGRP level (pg/mL) | 59.4 (34.1–829) |
| [Primary (p) or treatment-related (t) NEPC] | |
| p-NEPC | 22 (69%) |
| t-NEPC | 10 (31%) |
| [Pathological diagnosis] | |
| Small cell carcinoma | 17 (53%) |
| Mixed NE carcinoma–adenocarcinoma | 9 (28%) |
| Adenocarinoma with NE differentiation | 6 (19%) |
| [Metastatic lesion] | |
| Any | 25 (78%) |
| Lymph node | 21 (66%) |
| Bone | 19 (59%) |
| Lung | 5 (16%) |
| Liver | 3 (9%) |
| Adrenal | 1 (3%) |
| [Treatment] | |
| Radical prostatectomy | 3 (9%) |
| Local radiotherapy | 18 (56%) |
| Androgen deprivation therapy | 29 (91%) |
| Chemotherapy | 22 (69%) |
| n | HR (95% CI) | p-Value | |
|---|---|---|---|
| Age | |||
| ≦69.5 | 16 | ||
| >69.5 | 16 | 1.236 (0.522–2.93) | 0.623 |
| PSA | |||
| ≦6.1 | 16 | ||
| >6.1 | 16 | 0.8179 (0.338–1.98) | 0.66 |
| NSE | |||
| ≦19.2 | 13 | ||
| >19.2 | 13 | 1.138 (0.954–1.357) | 0.15 |
| ProGRP | |||
| ≦59.1 | 10 | ||
| >59.1 | 10 | 3.402 (0.835–13.86) | 0.087 |
| Pathological diagnosis | |||
| Mixed NE carcinoma–adenocarcinoma/ Adenocarinoma with NE differentiation | 15 | ||
| Small cell carcinoma | 17 | 1.648 (0.691–3.928) | 0.26 |
| Metastasis/no metastasis | 25/7 | 1.292 (0.469–3.559) | 0.62 |
| Local therapy/no local therapy | 21/11 | 0.284 (0.109–0.738) | 0.01 * |
| Chemotherapy/no chemotherapy | 22/10 | 2.435 (0.873–6.795) | 0.089 |
| pNEPC/t-NEPC | 22/12 | 0.196 (0.068–0.566) | 0.003 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, S.; Terada, N.; Soumiya, S.; Goto, T.; Negoro, H.; Mukai, S.; Ogawa, O.; Akamatsu, S.; Kobayashi, T.; Sawada, A.; et al. Local Therapy Can Enhance the Prognosis of Certain Patients with Pathologically Diagnosed Neuroendocrine Prostate Carcinoma. Life 2025, 15, 797. https://doi.org/10.3390/life15050797
Kimura S, Terada N, Soumiya S, Goto T, Negoro H, Mukai S, Ogawa O, Akamatsu S, Kobayashi T, Sawada A, et al. Local Therapy Can Enhance the Prognosis of Certain Patients with Pathologically Diagnosed Neuroendocrine Prostate Carcinoma. Life. 2025; 15(5):797. https://doi.org/10.3390/life15050797
Chicago/Turabian StyleKimura, Shoichi, Naoki Terada, Shinnya Soumiya, Takayuki Goto, Hiromitsu Negoro, Shoichiro Mukai, Osamu Ogawa, Shusuke Akamatsu, Takashi Kobayashi, Atsuro Sawada, and et al. 2025. "Local Therapy Can Enhance the Prognosis of Certain Patients with Pathologically Diagnosed Neuroendocrine Prostate Carcinoma" Life 15, no. 5: 797. https://doi.org/10.3390/life15050797
APA StyleKimura, S., Terada, N., Soumiya, S., Goto, T., Negoro, H., Mukai, S., Ogawa, O., Akamatsu, S., Kobayashi, T., Sawada, A., & Kamoto, T. (2025). Local Therapy Can Enhance the Prognosis of Certain Patients with Pathologically Diagnosed Neuroendocrine Prostate Carcinoma. Life, 15(5), 797. https://doi.org/10.3390/life15050797







