Multifactorial Risk Stratification in Patients with Heart Failure, Chronic Kidney Disease, and Atrial Fibrillation: A Comprehensive Analysis
Abstract
1. Introduction
2. Pathophysiological Interplay of Heart Failure, Chronic Kidney Disease, Atrial Fibrillation, and Hypertension
2.1. Shared Pathophysiological Mechanisms
2.2. Impact of Hypertension on the Triad of HF, CKD, and AF
2.3. Hemodynamic and Metabolic Dysregulation
3. Risk Stratification in Patients with HF, CKD, and AF
3.1. Established Risk Scores for Clinical Assessment
3.2. Limitations of Current Risk Scores in Multimorbid Patients
3.3. Emerging Approaches to Risk Stratification
4. The Role of Renal Function in Cardiovascular Risk Stratification
4.1. CKD Progression and Cardiovascular Outcomes
4.2. Renal Biomarkers and Risk Prediction
4.3. Challenges in Anticoagulation Decision Making in CKD
4.3.1. Balancing Thromboembolic vs. Bleeding Risk in CKD Patients with AF
4.3.2. Use of Direct Oral Anticoagulants (DOACs) vs. Vitamin K Antagonists (VKAs)
5. Stroke Prevention in AF: Standard Guidelines
5.1. Anticoagulation Challenges in HF and CKD
5.2. Personalized Approaches to Anticoagulation
6. Impact of Hypertension on Clinical Outcomes in Multimorbid Patients
6.1. Blood Pressure Control in Patients with HF, CKD, and AF
6.2. Role of Antihypertensive Agents in Disease Progression
6.3. Individualized Hypertension Management Strategies
7. Comorbidities and Their Influence on Risk Stratification
7.1. Common Comorbidities in HF, CKD, and AF Patients
7.2. Polypharmacy and Drug–Drug Interactions
7.3. Multidisciplinary Management Approaches
8. Clinical Outcomes and Future Directions
8.1. Major Adverse Cardiovascular Events (MACE) and Mortality Trends
8.2. Gaps in Current Research and Future Perspectives
8.3. Personalized Medicine in HF, CKD, and AF
9. Subjects and Methods
9.1. Study Design and Population
- Demographics: age, gender, and comorbidity burden.
- Sample size and power considerations.
- Clinical parameters: HF phenotypes (HFpEF vs. HFrEF), NYHA classification, renal function (eGFR), and hypertension severity.
- Comorbidities: presence of neurological, psychiatric, pulmonary (COPD, chronic obstructive pulmonary disease), and metabolic (diabetes) disorders.
- AF risk scores: CHA2DS2-VASc, HAS-BLED, and ATRIA.
- Anticoagulation therapy: whether patients were receiving DOACs (direct oral anticoagulants) or VKAs (vitamin K antagonists).
- Hospitalization outcomes: length of stay and in-hospital mortality.
9.2. Statistical Analysis
- The correlation between renal function (eGFR) and HF severity (NYHA classification).
- The association between thromboembolic and bleeding risk scores (CHADS-VASC, HAS-BLED, and ATRIA) and in-hospital mortality.
- The differences in hospital stay duration between HFpEF and HFrEF patients.
- The impact of anticoagulation (direct oral anticoagulants [DOACs] vs. vitamin K antagonists [VKAs]) on bleeding risk (HAS-BLED score).
- The effect of previous stroke history on hospital stay duration.
- The relationship between the number of comorbidities and NYHA classification.
- Spearman correlation was used to assess the relationship between the following:
- o
- HTA severity and HF severity (NYHA classification).
- o
- Neurological, psychiatric, and metabolic comorbidities and AF risk scores (CHA2DS2-VASc, HAS-BLED, and ATRIA).
- Independent t-tests compared the following:
- o
- CHA2DS2-VASc, HAS-BLED, and ATRIA scores between survivors and non-survivors.
- o
- Hospital stay duration between HFpEF and HFrEF patients.
- o
- HAS-BLED scores between DOAC and VKA users.
- o
- Hospital stay duration in patients with and without prior stroke.
- Multivariate regression analysis was performed to identify independent predictors of high-risk scores for stroke and bleeding.
- The multivariate regression model included the following variables: hypertension severity, renal function (eGFR), the presence of diabetes, COPD, neurological disorders, and type of anticoagulation (DOACs vs. VKAs). Multicollinearity was assessed using the variance inflation factor (VIF), and all included variables had a VIF < 2, indicating low multicollinearity. The model fit was evaluated using the coefficient of determination (R2), which was 0.28, indicating that approximately 28% of the variance in the risk scores could be explained by the included variables.
10. Results
- Demographics: Almost half of the patients (48%) had multiple hospitalizations, indicating a population with advanced chronic diseases.
- Clinical parameters:
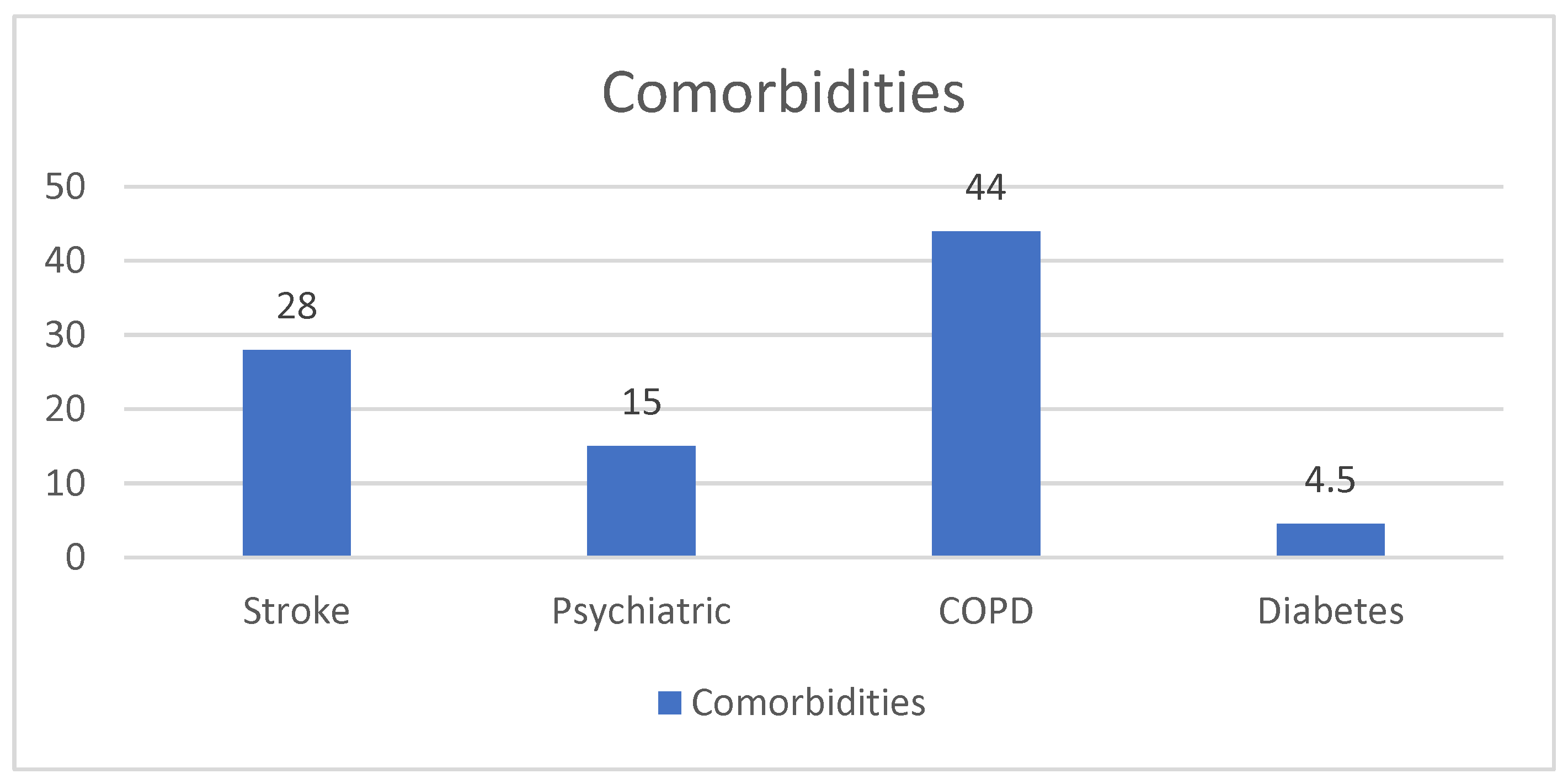
- Comorbidities:
- Anticoagulation therapy: A total of 69, 5% (121) of patients were on DOACs, while 30, 4% (53) of patients were on VKAs.
- AF risk scores:
- Hospitalization outcomes:
- Statistical Analysis
10.1. Hypertension Severity and HF Symptom Burden (NYHA Classification)
10.2. Neurological Conditions vs. NYHA Score
10.3. Psychiatric Conditions vs. NYHA Score
10.4. Other Comorbidities (COPD and Diabetes) vs. NYHA Score
- Hypertension: there is a small negative association, which may indicate that hypertension severity is not a primary driver of symptomatic HF severity (NYHA).
- Neurological, psychiatric, and metabolic comorbidities do not seem to strongly influence HF symptom burden (NYHA class).
- This suggests that other factors (e.g., cardiac output, ejection fraction, and renal function) might play a larger role in determining functional limitation in HF patients.
10.5. Comorbidities and AF Risk Scores
10.5.1. Neurological Conditions (e.g., Cortical Atrophy, Cognitive Decline, and Parkinson’s)
10.5.2. Psychiatric Conditions (e.g., Depression, Anxiety, and Bipolar Disorder)
10.5.3. COPD and Diabetes
10.6. Hospitalization Outcomes
11. Discussion
11.1. Comorbidities and AF Risk Scores
11.2. Hypertension Severity and HF Symptom Burden
11.3. Hospitalization Outcomes
11.4. Implications for Clinical Practice
- Clinicians may overestimate stroke risk in patients with cognitive decline while underestimating it in those with metabolic disorders.
- Psychiatric conditions, despite being under-represented in scoring systems, could still affect clinical outcomes through adherence and follow-up challenges.
11.5. Limitations
11.6. Future Directions
- Validate these findings in larger, multicenter cohorts.
- Explore psychosocial and frailty markers as potential modifiers of stroke and bleeding risk.
- Investigate long-term outcomes, such as rehospitalization rates, mortality, and quality of life.
- Assess whether incorporating novel biomarkers or machine learning algorithms could enhance risk prediction in complex multimorbid populations.
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehm, M.; Rothenbacher, D.; Iacoviello, L.; Costanzo, S.; Tunstall-Pedoe, H.; Fitton, C.A.; Söderberg, S.; Hultdin, J.; Salomaa, V.; Jousilahti, P.; et al. Chronic kidney disease and risk of atrial fibrillation and heart failure in general population-based cohorts: The BiomarCaRE project. ESC Heart Fail. 2022, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Boriani, G.; Vitolo, M.; Mei, D.A. CHA2DS2-VA instead of CHA2DS2-VASc for stroke risk stratification in patients with atrial fibrillation: Not just a matter of sex. Europace 2024, 26, euae281. [Google Scholar] [CrossRef]
- Morrone, D.; Kroep, S.; Ricci, F.; Renda, G.; Patti, G.; Kirchhof, P.; Chuang, L.H.; van Hout, B.; De Caterina, R. Mortality Prediction of the CHA2DS2-VASc Score, the HAS-BLED Score, and Their Combination in Anticoagulated Patients with Atrial Fibrillation. J. Clin. Med. 2020, 9, 3987. [Google Scholar] [CrossRef]
- Golla, M.S.G.; Hajouli, S.; Ludhwani, D. Heart failure and ejection fraction. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Law, J.P.; Pickup, L.; Pavlovic, D.; Townend, J.N.; Ferro, C.J. Hypertension and cardiomyopathy associated with chronic kidney disease: Epidemiology, pathogenesis and treatment considerations. J. Hum. Hypertens. 2023, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, H.-P. Acute Cardiorenal Syndrome: Epidemiology, Pathophysiology, Assessment, and Treatment. Rev. Cardiovasc. Med. 2023, 24, 40. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; Massy, Z.A.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular complications in chronic kidney disease: A review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef]
- Kahan, T.; Bergfeldt, L. Left ventricular hypertrophy in hypertension: Its arrhythmogenic potential. Heart 2005, 91, 250–256. [Google Scholar] [CrossRef]
- Kim, S.M.; Jeong, Y.; Kim, Y.L.; Kang, M.; Kang, E.; Ryu, H.; Kim, Y.; Han, S.S.; Ahn, C.; Oh, K. Association of Chronic Kidney Disease with Atrial Fibrillation in the General Adult Population: A Nationwide Population-Based Study. J. Am. Heart Assoc. 2023, 12, e028496. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; Gröne, H.-J.; Assmus, B.; Bauer, P.; Gall, H.; Seeger, W.; Ghofrani, A.; Ronco, C.; Birk, H. Congestive nephropathy: A neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. 2021, 8, 183–203. [Google Scholar] [CrossRef]
- Wu, Y.; Kong, X.-J.; Ji, Y.-Y.; Fan, J.; Ji, C.-C.; Chen, X.-M.; Ma, Y.-D.; Tang, A.-L.; Cheng, Y.-J.; Wu, S.-H. Serum electrolyte concentrations and risk of atrial fibrillation: An observational and mendelian randomization study. BMC Genom. 2024, 25, 280. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.theguardian.com/society/2024/dec/28/algorithm-could-help-prevent-thousands-of-strokes-in-uk-each-year (accessed on 17 January 2025).
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [PubMed]
- Lane, D.A.; Lip, G.Y.H. Use of the CHA2DS2-VASc and HAS-BLED Scores to Aid Decision Making for Thromboprophylaxis in Nonvalvular Atrial Fibrillation. Circulation 2012, 126, 860–865. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Iatridi, F.; Carrero, J.J.; Gall, E.C.; Kanbay, M.; Luyckx, V.; Shroff, R.; Ferro, C.J. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease in Children and Adults: A commentary from the European Renal Best Practice (ERBP). Nephrol. Dial. Transplant. 2025, 40, 273–482. [Google Scholar] [CrossRef]
- Parsons, C.; Cha, S.; Shen, W.K.; Chamberlain, A.M.; Luis, S.A.; Keddis, M.; Shamoun, F. Usefulness of the Addition of Renal Function to the CHA2DS2-VASc Score as a Predictor of Thromboembolism and Mortality in Patients Without Atrial Fibrillation. Am. J. Cardiol. 2018, 122, 597–603. [Google Scholar] [CrossRef]
- Goudis, C.; Daios, S.; Korantzopoulos, P.; Liu, T. Does CHA2DS2-VASc score predict mortality in chronic kidney disease? Intern Emerg. Med. 2021, 16, 1737–1742. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Chen C-h Yang, H.-C. AI-enhanced integration of genetic and medical imaging data for risk assessment of Type 2 diabetes. Nat. Commun. 2024, 15, 4230. [Google Scholar] [CrossRef]
- Oluleye, O.W.; Folsom, A.R.; Nambi, V.; Lutsey, P.L.; Ballantyne, C.M.; Troponin, T. B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Ann. Epidemiol. 2013, 23, 66–73. [Google Scholar] [CrossRef]
- Inserra, F.; Forcada, P.; Castellaro, A.; Castellaro, C. Chronic Kidney Disease and Arterial Stiffness: A Two-Way Path. Front. Med. 2021, 8, 765924. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Xie, H.; Rahman, M.; Anderson, A.H.; Isakova, T.; Ojo, A.; Zhang, X.; Nessel, L.; Hamano, T.; Grunwald, J.E.; et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 2014, 25, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xia, P.; Tan, Z.; Song, T.; Mei, K.; Wang, J.; Ma, J.; Jiang, Y.; Zhang, J.; Zhao, Y.; et al. Fibroblast growth factor-23 and the risk of cardiovascular diseases and mortality in the general population: A systematic review and dose-response meta-analysis. Front. Cardiovasc. Med. 2022, 9, 989574. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.B.; Lip, G.Y.; Kamper, A.L.; Hommel, K.; Køber, L.; Lane, D.A.; Lindhardsen, J.; Gislason, G.H.; Torp-Pedersen, C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2012, 367, 625–635. [Google Scholar] [CrossRef]
- Hart, R.G.; Tonarelli, S.B.; Pearce, L.A. Avoiding central nervous system bleeding during antithrombotic therapy: Recent data and ideas. Stroke 2005, 36, 1588–1593. [Google Scholar] [CrossRef]
- Kumar, S.; Lim, E.; Covic, A.; Verhamme, P.; Gale Chris, P.; Camm, A.J.; Goldsmith, D. Anticoagulation in Concomitant Chronic Kidney Disease and Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 2204–2215. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Zhou, J.; Zhang, J. Efficacy and safety of direct oral anticoagulants in patients with atrial fibrillation combined with chronic kidney disease: A systematic review and meta-analysis. Thromb. J. 2024, 22, 40. [Google Scholar] [CrossRef]
- Hammett, C.; Badve, S.V.; Kerr, P.G.; Tran, H.A.; Dundon, B.K.; Lo, S.; Wong, A.; Joseph, J.E.; Deague, J.; Perkovic, V. Oral Anticoagulant Use in Patients with Atrial Fibrillation and Chronic Kidney Disease: A Review of the Evidence with Recommendations for Australian Clinical Practice. Heart Lung Circ. 2022, 31, 1604–1611. [Google Scholar] [CrossRef]
- Chan, K.E.; Giugliano, R.P.; Patel, M.R.; Abramson, S.; Jardine, M.; Zhao, S.; Perkovic, V.; Maddux, F.W.; Piccini, J.P. Nonvitamin K Anticoagulant Agents in Patients with Advanced Chronic Kidney Disease or on Dialysis with AF. J. Am. Coll. Cardiol. 2016, 67, 2888–2899. [Google Scholar] [CrossRef]
- Siontis, K.C.; Zhang, X.; Eckard, A.; Bhave, N.; Schaubel, D.E.; He, K.; Tilea, A.; Stack, A.G.; Balkrishnan, R.; Yao, X. Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation 2018, 138, 1519–1529. [Google Scholar] [CrossRef]
- Aursulesei, V.; Costache, I.I. Anticoagulation in chronic kidney disease: From guidelines to clinical practice. Clin. Cardiol. 2019, 42, 774–782. [Google Scholar] [CrossRef]
- Pivatto Junior, F.; Scheffel, R.S.; Ries, L.; Wolkind, R.R.; Marobin, R.; Barkan, S.S.; Amon, L.C.; Biolo, A. SAMe-TT2R2 Score in the Outpatient Anticoagulation Clinic to Predict Time in Therapeutic Range and Adverse Events. Arq. Bras. Cardiol. 2017, 108, 290–296. [Google Scholar] [CrossRef]
- Zheng, W.; Li, S.; Jiang, C.; Hao, W.; Ai, H.; Wang, X.; Ma, C.; Nie, S. Effect of Intensive Blood Pressure Control and Comorbidity Status on the Prognosis of Patients with Hypertension: Insights from SPRINT. J. Am. Heart Assoc. 2025, 14, e036719. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Taha, M.; Asmar, M. Polypharmacy and severe potential drug-drug interactions among older adults with cardiovascular disease in the United States. BMC Geriatr. 2021, 21, 233. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M.; de Koning, E.M.; Fontana, V.; Thompson, A.; Pirmohamed, M. Multimorbidity, polypharmacy, and drug-drug-gene interactions following a non-ST elevation acute coronary syndrome: Analysis of a multicentre observational study. BMC Med. 2020, 18, 367. [Google Scholar] [CrossRef]
- Forman Daniel, E.; Maurer Mathew, S.; Boyd, C.; Brindis, R.; Salive Marcel, E.; Horne Frances, M.; Bell, S.P.; Fulmer, T.; Reuben, D.B.; Zieman, S.; et al. Multimorbidity in Older Adults with Cardiovascular Disease. J. Am. Coll. Cardiol. 2018, 71, 2149–2161. [Google Scholar] [CrossRef]
- Mariani, M.V.; Lavalle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’Amato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Piro, A.; et al. SGLT2i reduce arrhythmic events in heart failure patients with cardiac implantable electronic devices. ESC Heart Fail. 2025, 12, 2125–2133. [Google Scholar] [CrossRef]
- Suhov, L.; Apostol, A.; Dăniluc, L.; Haj Ali, L.; Sandu, O.E.; Bogdan, C.; Andor, M. Implications of Heart Failure Treatment on Atrial Fibrillation Onset: A Retrospective Study. Medicina 2025, 61, 414. [Google Scholar] [CrossRef]
- Serna, M.J.; Rivera-Caravaca, J.M.; López-Gálvez, R.; Soler-Espejo, E.; Lip, G.Y.H.; Marín, F.; Roldán, V. Dynamic assessment of CHA2DS2-VASc and HAS-BLED scores for predicting ischemic stroke and major bleeding in atrial fibrillation patients. Rev. Española De Cardiol. (Engl. Ed.) 2024, 77, 835–842. [Google Scholar] [CrossRef]
- Gomez, K.A.; Tromp, J.; Figarska, S.M.; Beldhuis, I.E.; Cotter, G.; Davison, B.A.; Felker, G.M.; Gimpelewicz, C.; Greenberg, B.H.; Lam, C.S.P.; et al. Distinct Comorbidity Clusters in Patients With Acute Heart Failure: Data From RELAX-AHF-2. J. Am. Coll. Cardiol. Heart Fail. 2024, 12, 1762–1774. [Google Scholar]
- Fox, K.A.A.; Virdone, S.; Pieper, K.S.; Bassand, J.P.; Camm, A.J.; Fitzmaurice, D.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; Kayani, G.; et al. GARFIELD-AF risk score for mortality, stroke, and bleeding within 2 years in patients with atrial fibrillation. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Lindbäck, J.; Alexander, J.H.; Hanna, M.; Held, C.; Hylek, E.M.; Lopes, R.D.; Oldgren, J.; Siegbahn, A.; Stewart, R.A. The ABC (age, biomarkers, clinical history) stroke risk score: A biomarker-based risk score for predicting stroke in atrial fibrillation. Eur. Heart J. 2016, 37, 1582–1590. [Google Scholar] [CrossRef]

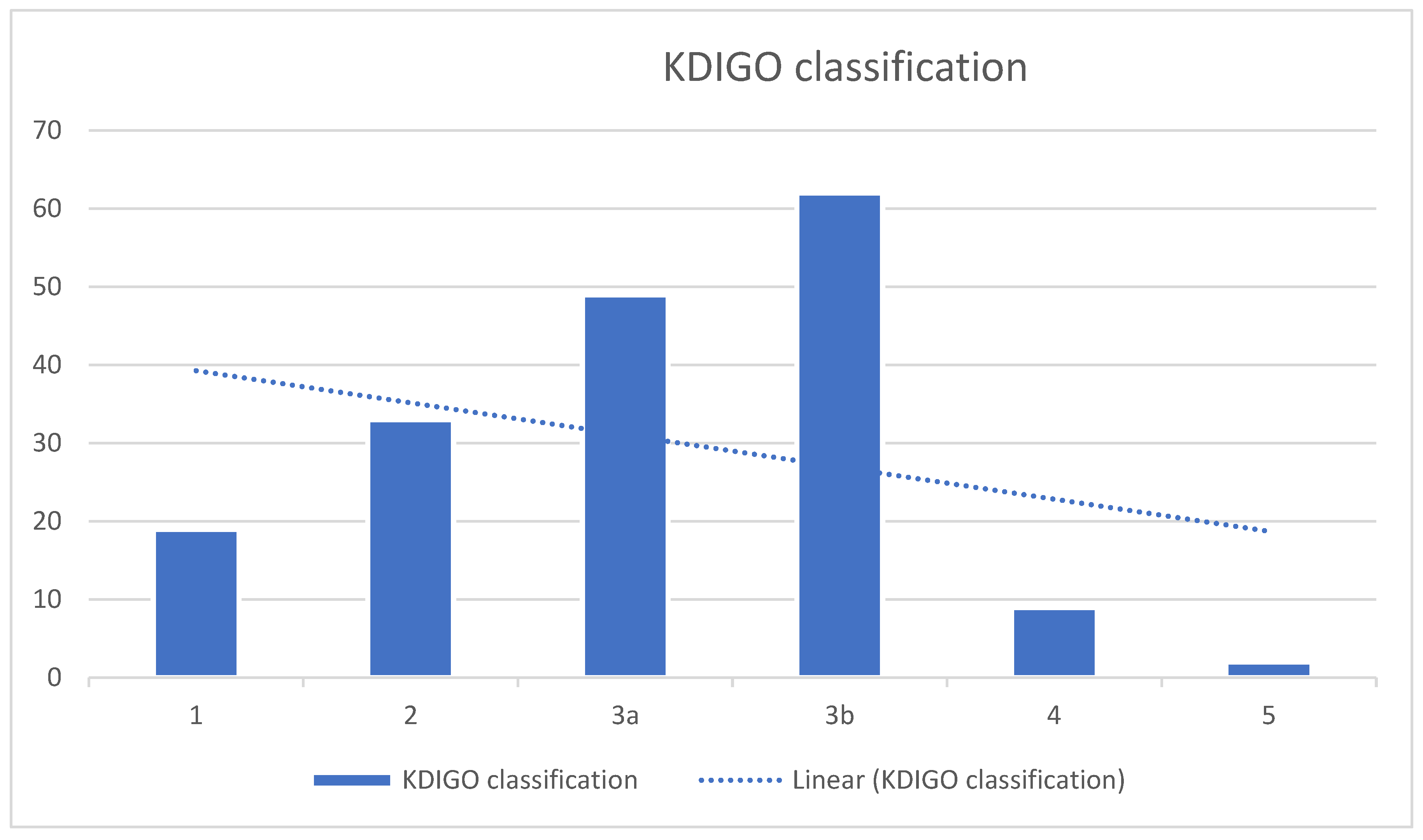
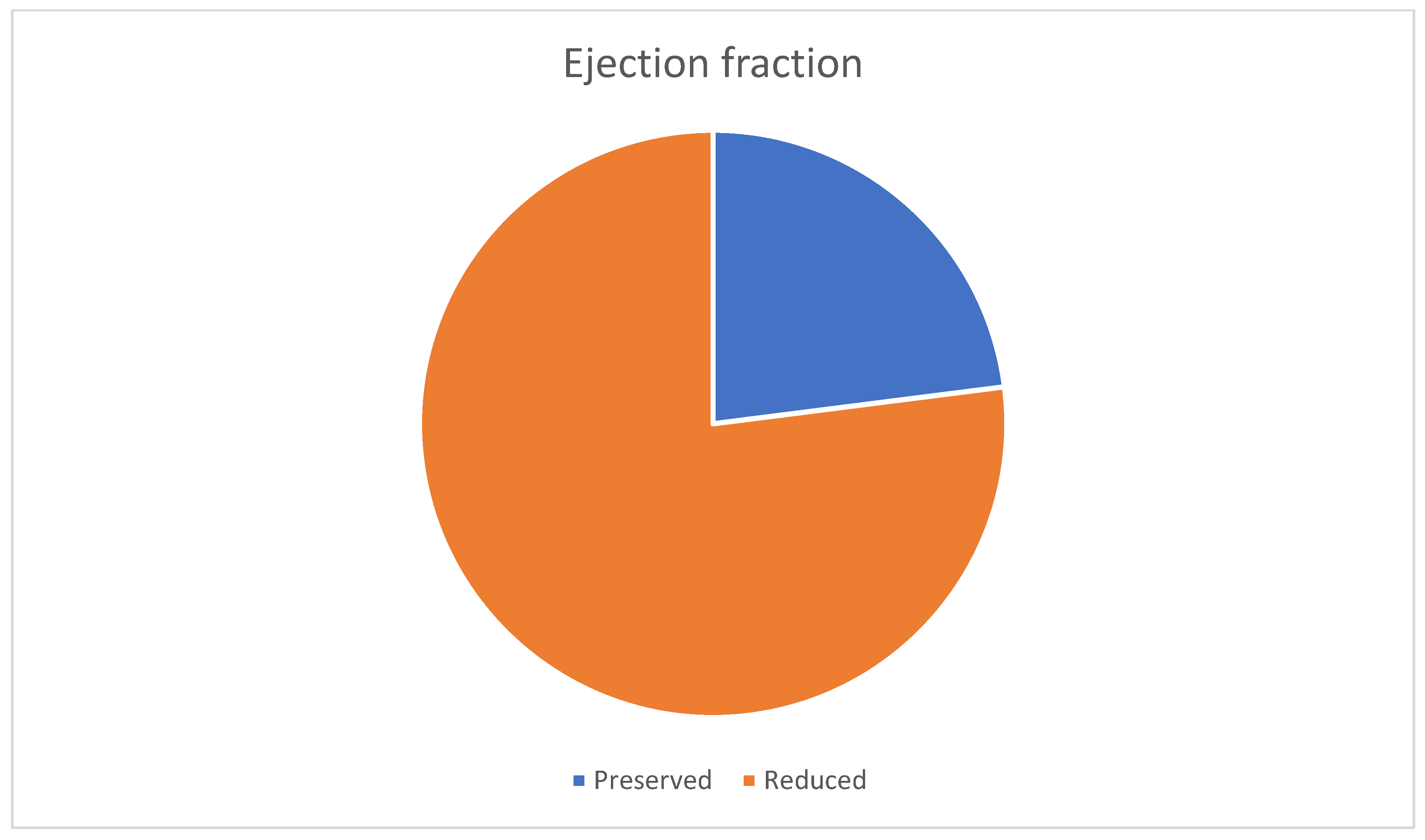
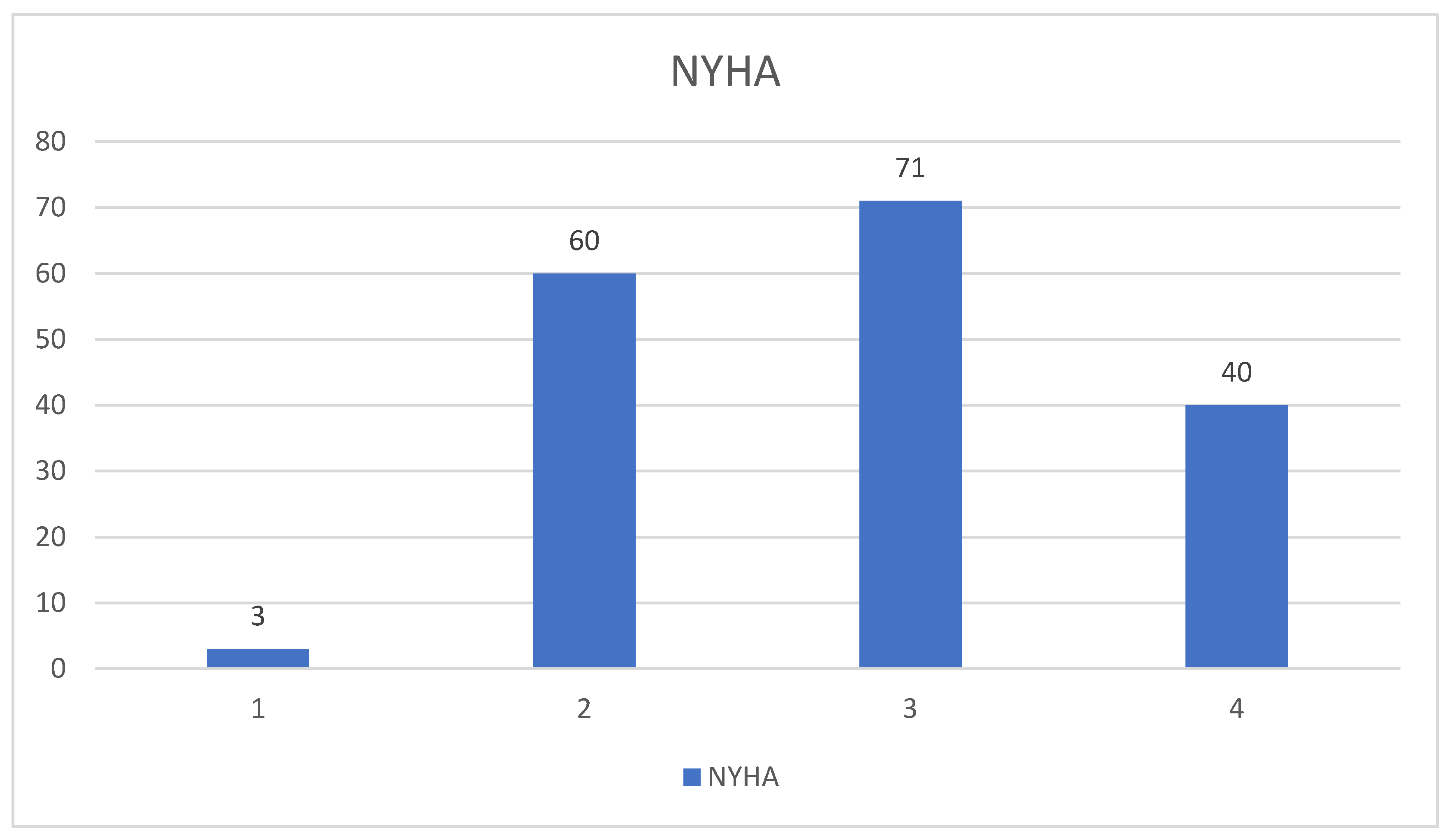
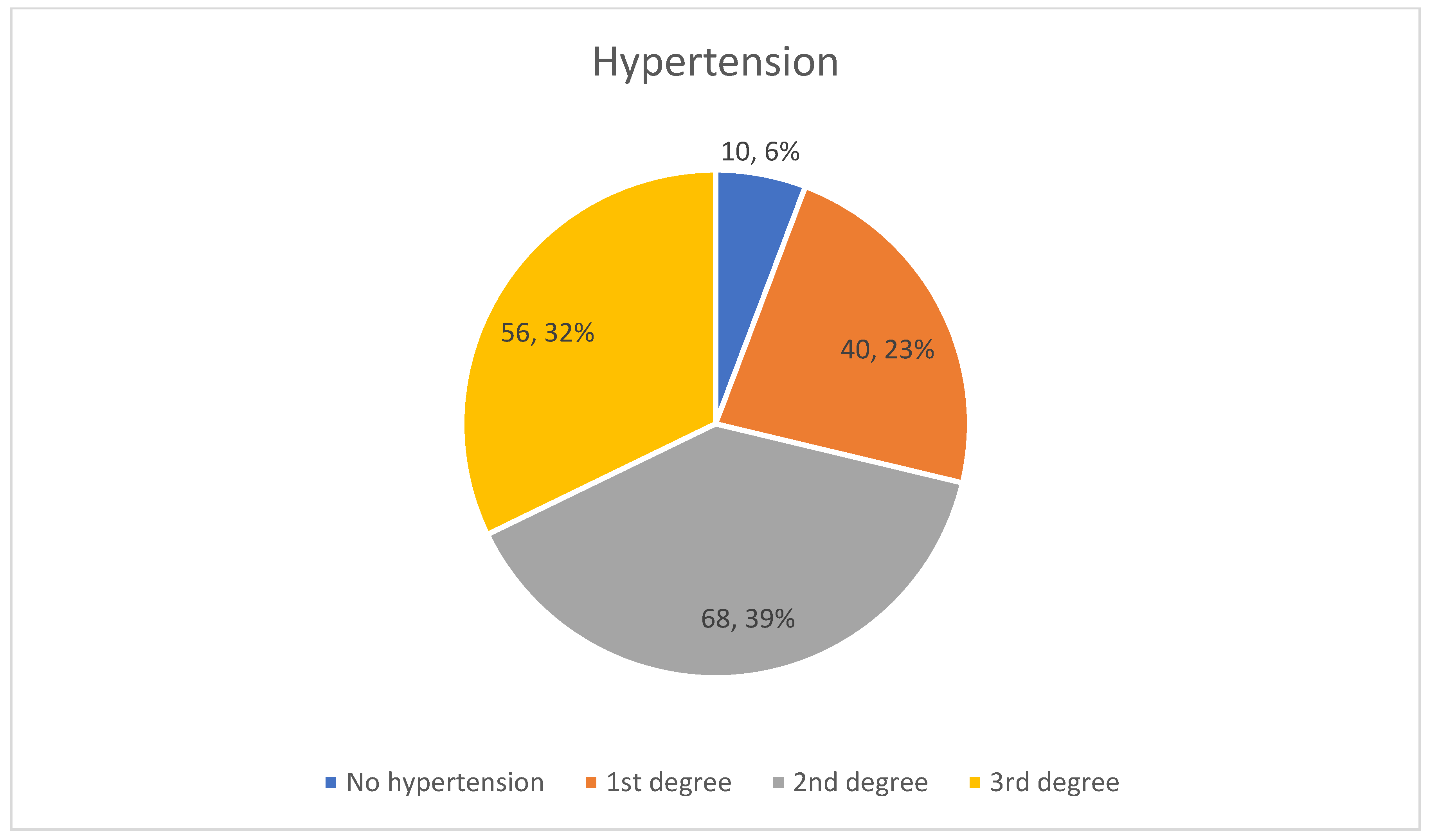
| Variable | Male (n = 96) | Female (n = 78) | Total (n = 174) |
|---|---|---|---|
| Mean age (years) | 73 (±12.5) | 76 (±13) | 75 (±12) |
| Mean BMI (kg/m2) | 27, 73 | 29, 53 | 28, 53 |
| Hypertension (%) | 94 | 93, 5 | 94, 25 |
| Diabetes (%) | 24 | 35 | 28, 7 |
| Tobacco use (%) | 72, 9 | 74, 35 | 77 |
| Mean ejection fraction (%) | 43, 65 | 42, 88 | 43, 31 |
| Mean eGFR (mL/min/1.73 m2) | 43, 96 | 42, 78 | 43, 44 |
| Comorbidities (COPD, stroke, and psychiatric) | 55, 2 | 44, 8 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacob, M.S.; Kundnani, N.R.; Sharma, A.; Meche, V.; Ciobotaru, P.; Bedreag, O.; Sandesc, D.; Dragan, S.R.; Papurica, M.; Stanga, L.C. Multifactorial Risk Stratification in Patients with Heart Failure, Chronic Kidney Disease, and Atrial Fibrillation: A Comprehensive Analysis. Life 2025, 15, 786. https://doi.org/10.3390/life15050786
Iacob MS, Kundnani NR, Sharma A, Meche V, Ciobotaru P, Bedreag O, Sandesc D, Dragan SR, Papurica M, Stanga LC. Multifactorial Risk Stratification in Patients with Heart Failure, Chronic Kidney Disease, and Atrial Fibrillation: A Comprehensive Analysis. Life. 2025; 15(5):786. https://doi.org/10.3390/life15050786
Chicago/Turabian StyleIacob, Mihai Sorin, Nilima Rajpal Kundnani, Abhinav Sharma, Vlad Meche, Paul Ciobotaru, Ovidiu Bedreag, Dorel Sandesc, Simona Ruxanda Dragan, Marius Papurica, and Livia Claudia Stanga. 2025. "Multifactorial Risk Stratification in Patients with Heart Failure, Chronic Kidney Disease, and Atrial Fibrillation: A Comprehensive Analysis" Life 15, no. 5: 786. https://doi.org/10.3390/life15050786
APA StyleIacob, M. S., Kundnani, N. R., Sharma, A., Meche, V., Ciobotaru, P., Bedreag, O., Sandesc, D., Dragan, S. R., Papurica, M., & Stanga, L. C. (2025). Multifactorial Risk Stratification in Patients with Heart Failure, Chronic Kidney Disease, and Atrial Fibrillation: A Comprehensive Analysis. Life, 15(5), 786. https://doi.org/10.3390/life15050786











