Dietary Melatonin Supplementation Improved Intestinal Health and Immune Function of Pacific White Shrimp (Litopenaeus vannamei) Under High Alkali Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Experimental Shrimp and Feeding Management
2.3. Paraffin-Embedded Tissue Sections of Intestinal
2.4. Determination of Oxidative Stress-Related Indicators in Intestinal
2.5. Identification of Genes Involved in Apoptosis and Immunity in Intestinal Samples
2.6. Gut Microbiota Analysis
2.7. Statistical Analyses
3. Results
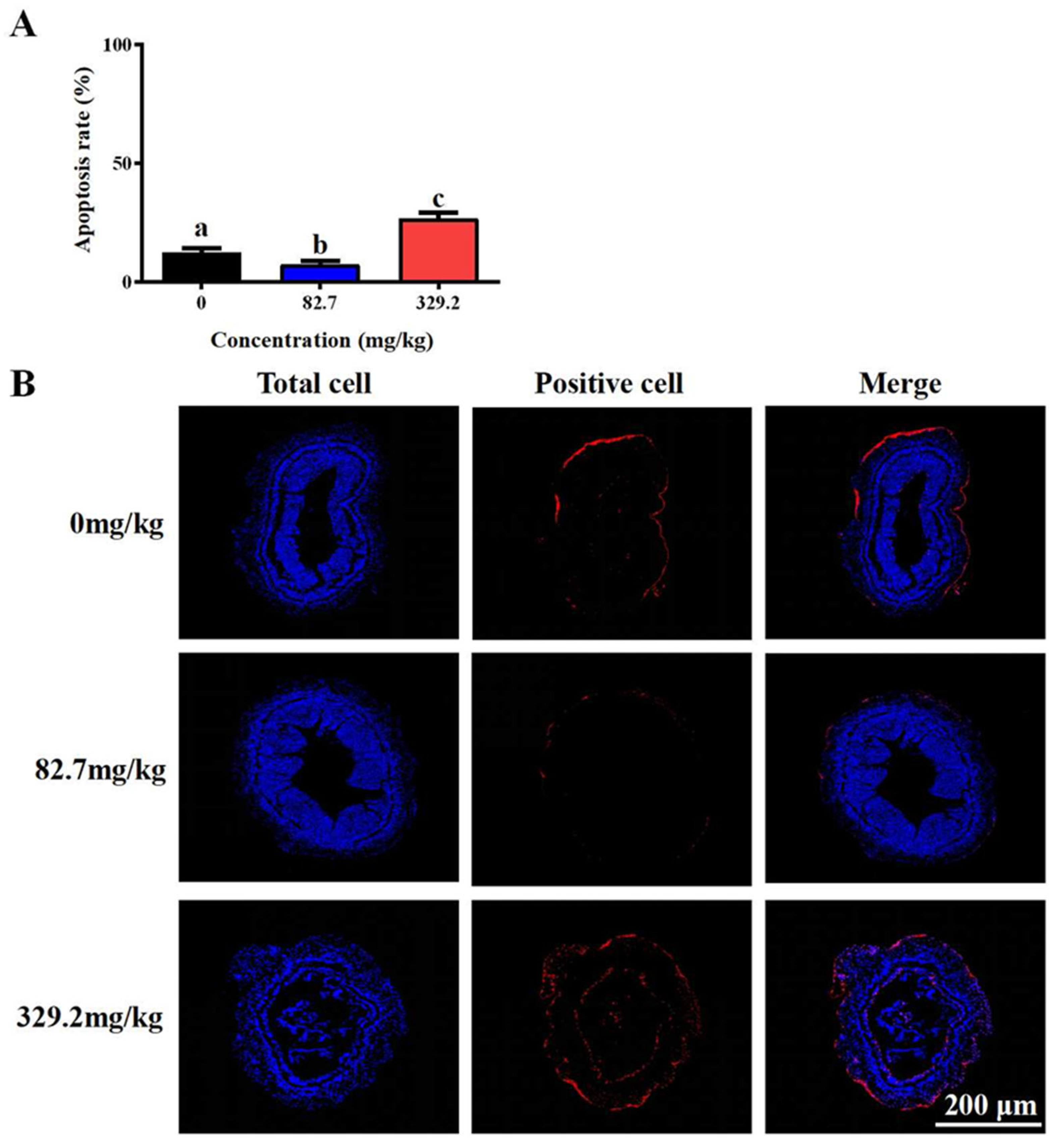
3.1. Survival Rate and Morphology of Intestinal
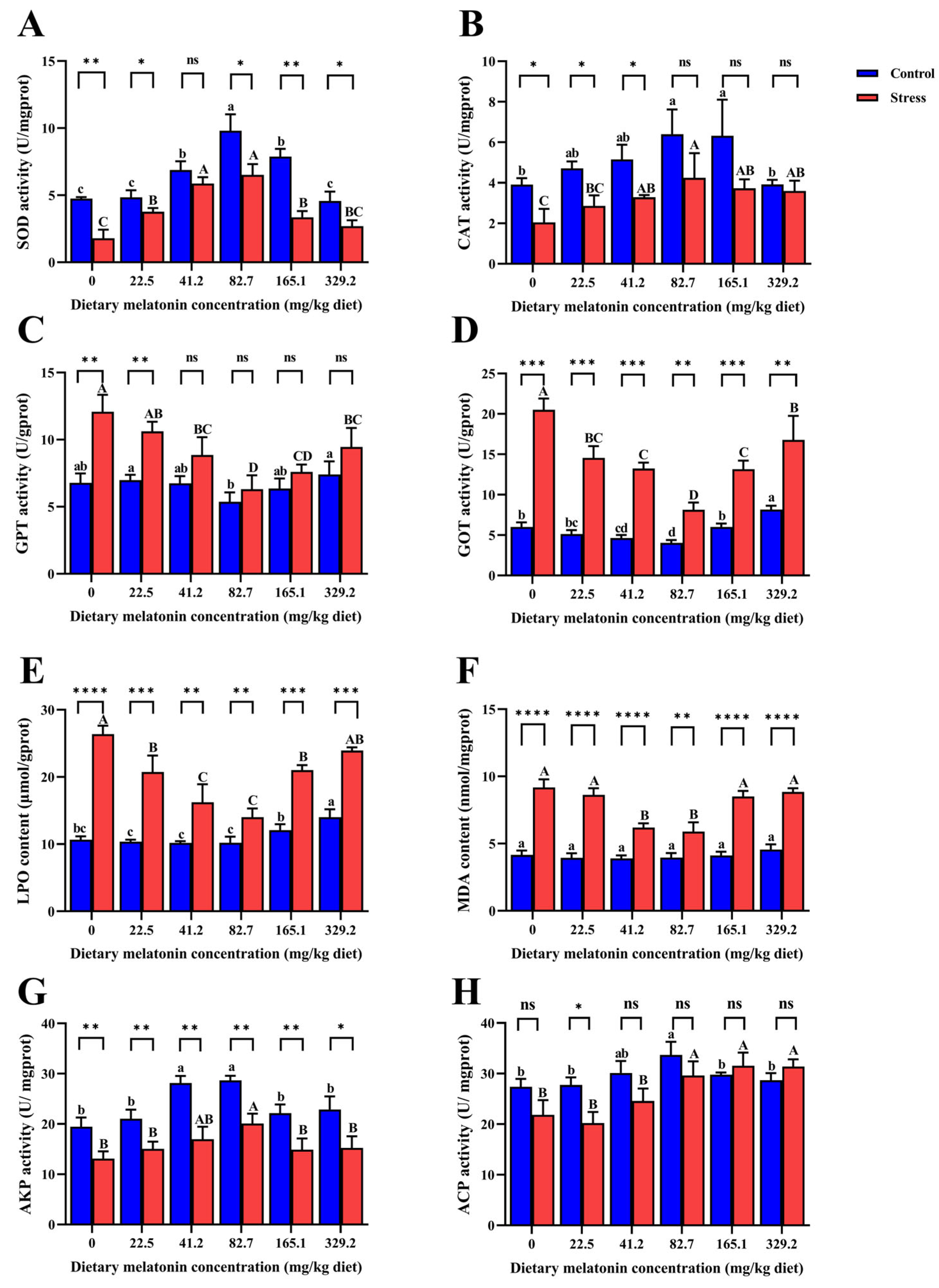
3.2. Oxidative Stress-Related Indicators
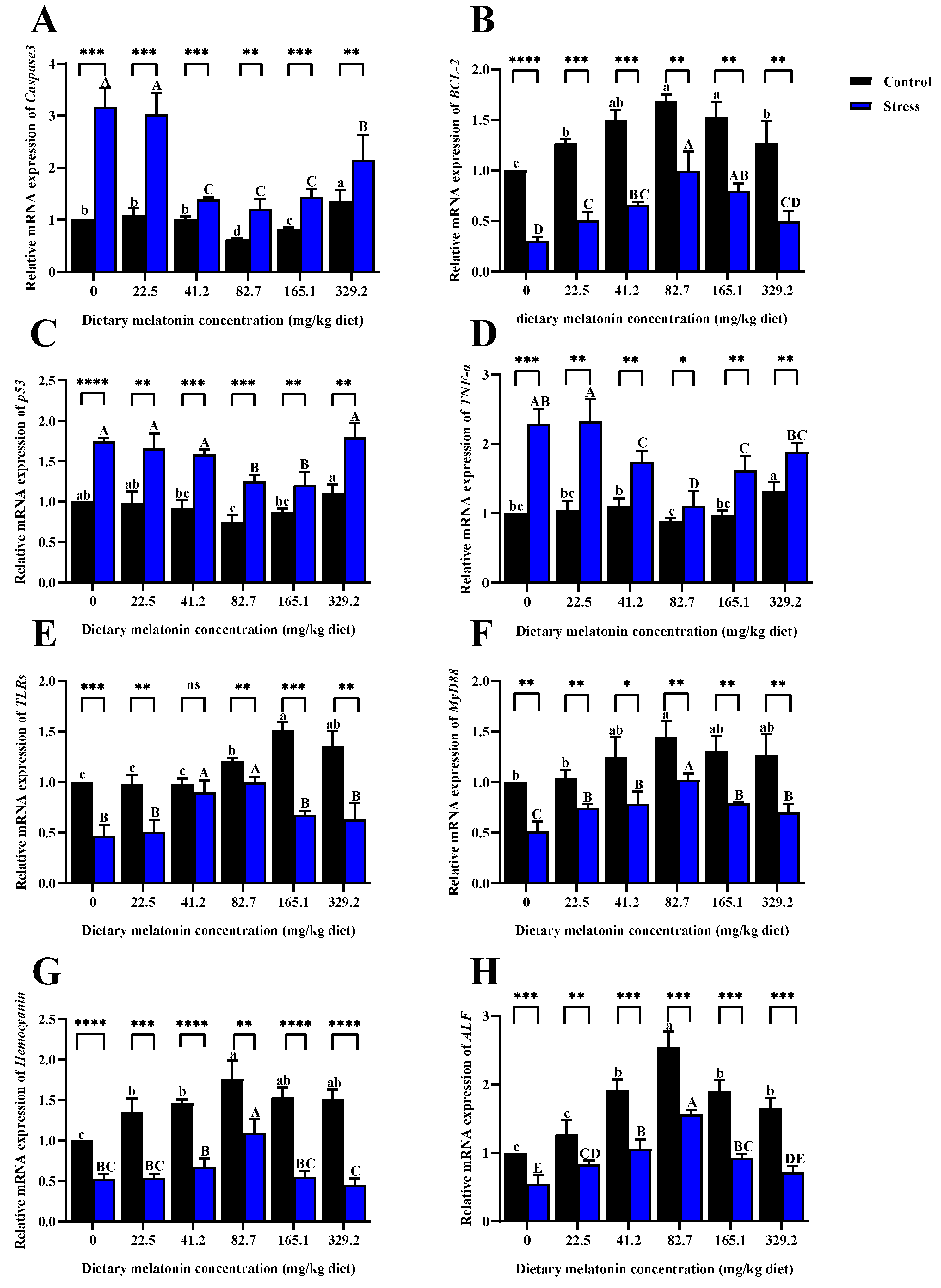
3.3. Gene Expressions
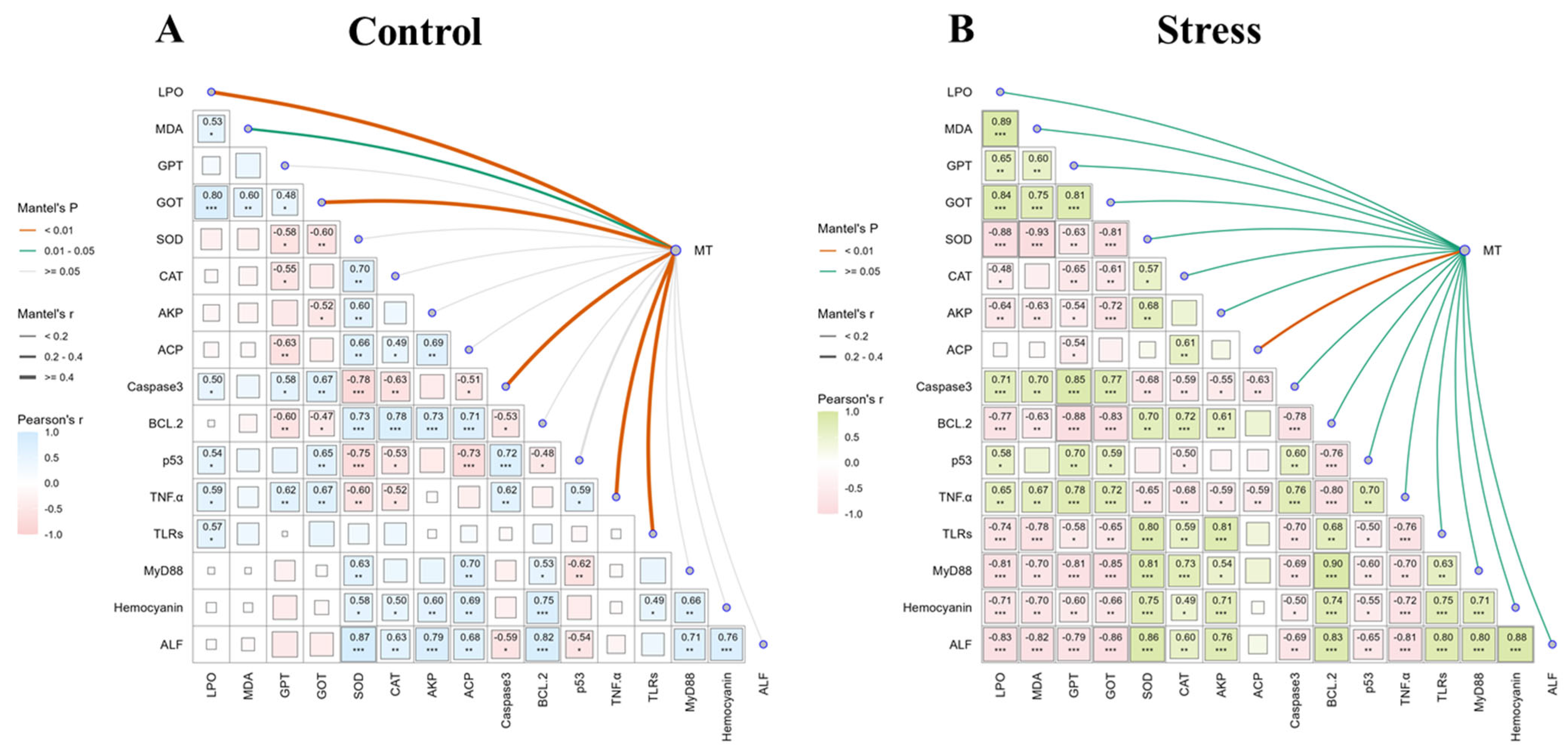
3.4. Correlation Analysis
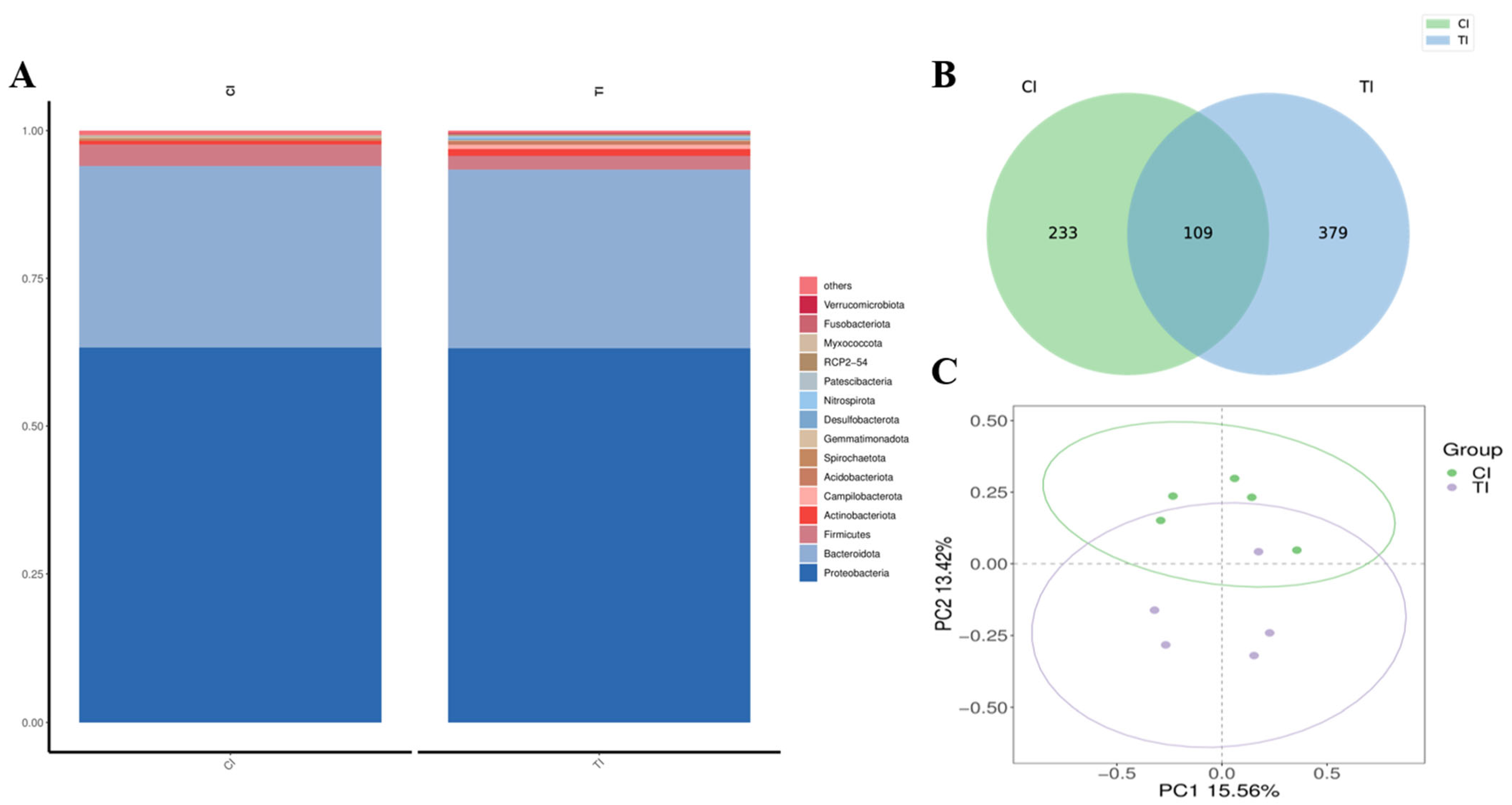
3.5. Sequencing of 16S rRNA and Annotation and Evaluation of Species
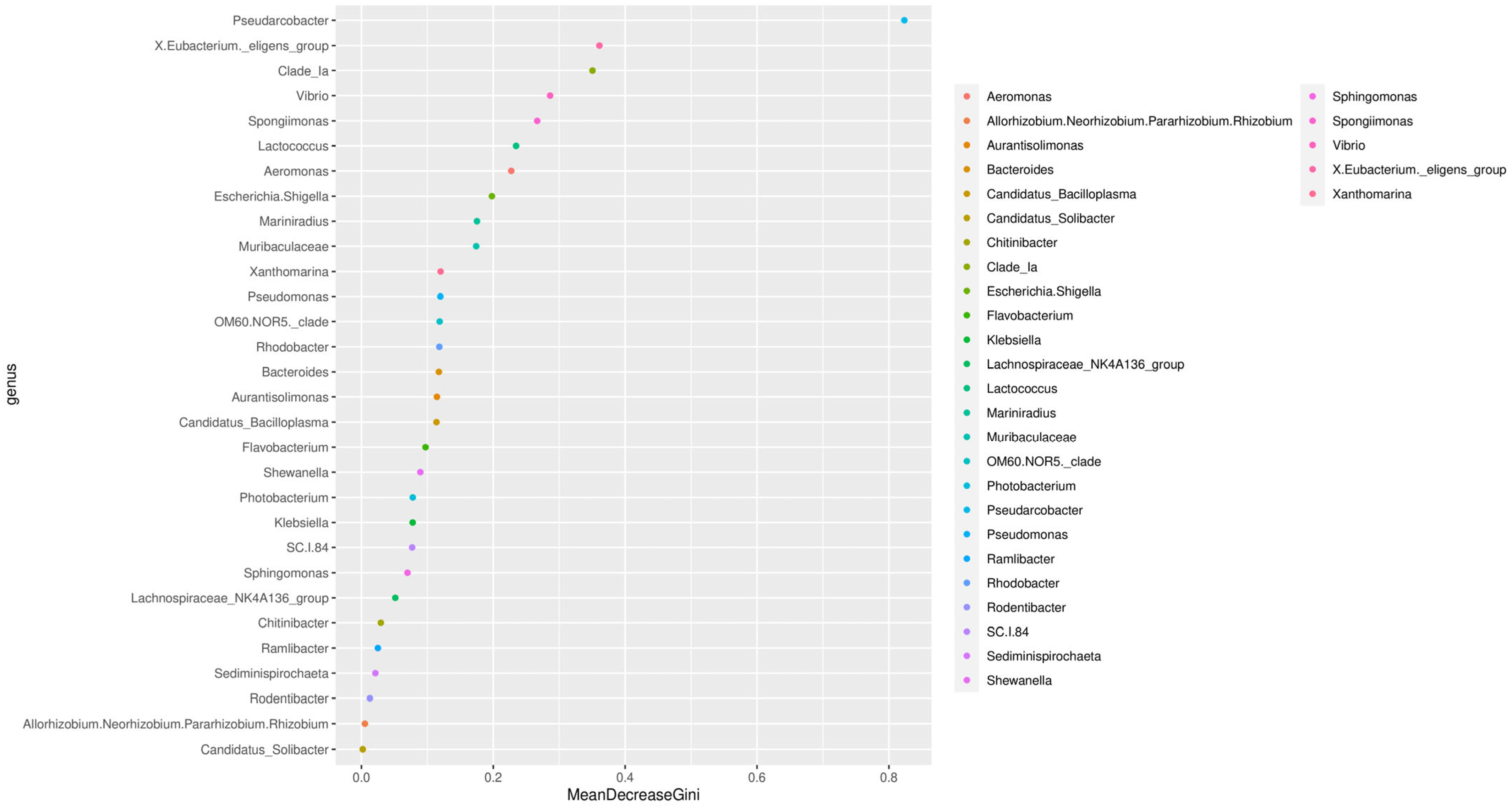
3.6. Analysis of Species and Genera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.J.; Hegde, M.L.; Anitha, S.; Musicco, M.; Zucca, F.A.; Turro, N.J.; Zecca, L. Amyloid beta and neuromelanin–toxic or protective molecules? The cellular context makes the difference. Prog. Neurobiol. 2006, 78, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Ángeles Esteban, M.; Cuesta, A.; Chaves-Pozo, E.; Meseguer, J. Influence of melatonin on the immune system of fish: A review. Int. J. Mol. Sci. 2013, 14, 7979–7999. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Sutradhar, S.; Das, P.; Mukherjee, S. Gut melatonin: A potent candidate in the diversified journey of melatonin research. Gen. Comp. Endocrinol. 2021, 303, 113693. [Google Scholar] [CrossRef]
- Tilden, A.R.; Brauch, R.; Ball, R.; Janze, A.M.; Ghaffari, A.H.; Sweeney, C.T.; Yurek, J.C.; Cooper, R.L. Modulatory effects of melatonin on behavior, hemolymph metabolites, and neurotransmitter release in crayfish. Brain Res. 2003, 992, 252–262. [Google Scholar] [CrossRef]
- Pape, C.; Teschke, M.; Meyer, B. Melatonin and its possible role in mediating seasonal metabolic changes of Antarctic krill, Euphausia superba. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 426–434. [Google Scholar] [CrossRef]
- Sainath, S.B.; Reddy, P.S. Evidence for the involvement of selected biogenic amines (serotonin and melatonin) in the regulation of molting of the edible crab, Oziotelphusa senex senex Fabricius. Aquaculture 2010, 302, 261–264. [Google Scholar] [CrossRef]
- Maciel, F.E.; Geihs, M.A.; Cruz, B.P.; Vargas, M.A.; Allodi, S.; Marins, L.F.; Nery, L.E.M. Melatonin as a signaling molecule for metabolism regulation in response to hypoxia in the crab Neohelice granulata. Int. J. Mol. Sci. 2014, 15, 22405–22420. [Google Scholar] [CrossRef]
- Maciel, F.E.; Geihs, M.A.; Vargas, M.A.; Cruz, B.P.; Ramos, B.P.; Vakkuri, O.; Meyer-Rochow, V.B.; Maia Nery, L.E.; Allodi, S. Daily variation of melatonin content in the optic lobes of the crab Neohelice granulata. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 162–166. [Google Scholar] [CrossRef]
- Chen, S.; Migaud, H.; Shi, C.; Song, C.; Wang, C.; Ye, Y.; Ren, Z.; Wang, H.; Mu, C. Light intensity impacts on growth, molting and oxidative stress of juvenile mud crab Scylla paramamosain. Aquaculture 2021, 545, 737159. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress: A review. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, W.; Du, X.; Ye, Y.; Tian, J.; Li, Y.; Jiang, Q.; Zhao, Y. Effects of dietary melatonin on growth performance, antioxidant capacity, and nonspecific immunity in crayfish, Cherax destructor. Fish Shellfish Immunol. 2023, 138, 108846. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Y.; Li, S.; Feng, J.; Liu, X.; Che, X.; Jiang, Q.; Chen, X. Transcriptomic analysis of the antioxidant responses and immunomodulatory effects of dietary melatonin in red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2023, 142, 109173. [Google Scholar] [CrossRef]
- Yang, X.; Song, X.; Zhang, C.; Pang, Y.; Song, Y.; Cheng, Y.; Nie, L.; Zong, X. Effects of dietary melatonin on hematological immunity, antioxidant defense and antibacterial ability in the Chinese mitten crab, Eriocheir sinensis. Aquaculture 2020, 529, 735578. [Google Scholar] [CrossRef]
- Shang, X.; Geng, L.; Yang, J.; Zhang, Y.; Xu, W. Transcriptome analysis reveals the mechanism of alkalinity exposure on spleen oxidative stress, inflammation and immune function of Luciobarbus capito. Ecotoxicol. Environ. Saf. 2021, 225, 112748. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, D.; Li, J.; Li, C.; Zheng, X.; Wang, L. Phosphorus Nutrition in Songpu Mirror Carp (Cyprinus carpio Songpu) During Chronic Carbonate Alkalinity Stress: Effects on Growth, Intestinal Immunity, Physical Barrier Function, and Intestinal Microflora. Front. Immunol. 2022, 13, 900793. [Google Scholar] [CrossRef]
- Song, Z.; Li, K.; Li, K. Integrated characterizations of intestinal bacteria and transcriptomics revealed the acute stress response to carbonate alkalinity in white shrimp Penaeus vannamei. Fish Shellfish Immunol. 2024, 146, 109420. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Guo, J.; Mao, X.; Fan, B. Acute stress response in gill of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquaculture 2024, 586, 740766. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Guo, J.; Mao, X.; Fan, B. Acute stress response in hepatopancreas of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquac. Rep. 2024, 35, 101981. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Effects of saline-alkali stress on the tissue structure, antioxidation, immunocompetence and metabolomics of Eriocheir sinensis. Sci. Total Environ. 2023, 871, 162109. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Li, J.; Liu, P.; Fei, F.; Liu, B.; Li, J. The effect of astaxanthin on the alkalinity stress resistance of Exopalaemon carinicauda. Sci. Total Environ. 2024, 917, 170415. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, H.; Wang, Z.; Zhu, J.; Wang, W.; Wang, J.; Wang, Y.; Wang, C. Rumen-protected lysine supplementation improved amino acid balance, nitrogen utilization and altered hindgut microbiota of dairy cows. Anim. Nutr. 2023, 15, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Medina-Félix, D.; Garibay-Valdez, E.; Vargas-Albores, F.; Martínez-Porchas, M. Fish disease and intestinal microbiota: A close and indivisible relationship. Rev. Aquac. 2023, 15, 820–839. [Google Scholar] [CrossRef]
- Yan, Y.; Li, B.; Gao, Q.; Wu, M.; Ma, H.; Bai, J.; Ma, C.; Xie, X.; Gong, Y.; Xu, L.; et al. Intestine-Decipher Engineered Capsules Protect Against Sepsis-induced Intestinal Injury via Broad-spectrum Anti-inflammation and Parthanatos Inhibition. Adv. Sci. 2025, 12, 2412799. [Google Scholar] [CrossRef]
- Guo, K.; Ruan, G.; Fan, W.; Wang, Q.; Fang, L.; Luo, J.; Liu, Y. Immune response to acute heat stress in the intestine of the red swamp crayfish, Procambarus clarkii. Fish Shellfish Immunol. 2020, 100, 146–151. [Google Scholar] [CrossRef]
- Zhou, Z.; Ding, Z.; Huiyuan, L.V. Effects of Dietary Short-chain Fructooligosaccharides on Intestinal Microflora, Survival, and Growth Performance of Juvenile White Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2007, 38, 296–301. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Wang, K.; Chen, J.; Jin, K.; Peng, K.; Chen, X.; Liu, Z.; Ouyang, J.; Wang, Y.; et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front. Pharmacol. 2023, 14, 1166022. [Google Scholar] [CrossRef]
- Pan, S.; Hong, F.; Li, L.; Guo, Y.; Qiao, X.; Zhang, J.; Xu, P.; Zhai, Y. Melatonin Attenuates Dextran Sodium Sulfate Induced Colitis in Obese Mice. Pharmaceuticals 2021, 14, 822. [Google Scholar] [CrossRef]
- Velarde, E.; Alonso-Gómez, Á.L.; Azpeleta, C.; Isorna, E.; De Pedro, N.; Delgado, M.J. Melatonin effects on gut motility are independent of the relaxation mediated by the nitrergic system in the goldfish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Zhao, P.; Cao, Q.; Ding, Y.; Xu, S. Protective effect of melatonin on imidacloprid-induced pyroptosis and ferroptosis by mediating peptidoglycan in the gut of the common carp (Cyprinus carpio). Pestic. Biochem. Physiol. 2024, 202, 105935. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Song, X.; Wu, M.; Pang, Y.; Shi, A.; Shi, X.; Niu, C.; Cheng, Y.; Yang, X. The protective effects of melatonin on survival, immune response, digestive enzymes activities and intestinal microbiota diversity in Chinese mitten crab (Eriocheir sinensis) exposed to glyphosate. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2020, 238, 108845. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Taghizadieh, M.; Mehdizadehfar, E.; Hasani, A.; Khalili Fard, J.; Feizi, H.; Hamishehkar, H.; Ansarin, M.; Yekani, M.; Memar, M.Y. Gut microbiota in neurological diseases: Melatonin plays an important regulatory role. Biomed. Pharmacother. 2024, 174, 116487. [Google Scholar] [CrossRef]
- Wyban, J. Selective Breeding of Penaeus vannamei: Impact on World Aquaculture and Lessons for Future. J. Coast. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Yuan, H.; Xie, M.; Chen, J.; Hu, N.; Wang, H.; Tan, B.; Shi, L.; Zhang, S. Combined intestinal microbiota and transcriptomic analysis to investigate the effect of different stocking densities on the ability of Pacific white shrimp (Litopenaeus vannamei) to utilize Chlorella sorokiniana. Anim. Nutr. 2024, 18, 203–219. [Google Scholar] [CrossRef]
- Tendencia, E.A.; de la Peña, L.D. Antibiotic resistance of bacteria from shrimp ponds. Aquaculture 2001, 195, 193–204. [Google Scholar] [CrossRef]
- Mardones, O.; Devia, E.; Labbé, B.S.; Oyarzún, R.; Vargas-Chacoff, L.; Muñoz, J.L.P. Effect of l-tryptophan and melatonin supplementation on the serotonin gastrointestinal content and digestive enzymatic activity for Salmo salar and Oncorhynchus kisutch. Aquaculture 2018, 482, 203–210. [Google Scholar] [CrossRef]
- Liu, X.-L.; Xi, Q.-Y.; Yang, L.; Li, H.-Y.; Jiang, Q.-Y.; Shu, G.; Wang, S.-B.; Gao, P.; Zhu, X.-T.; Zhang, Y.-L. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, X.; Shi, A.; Song, Y.; Niu, C.; Yu, X.; Shi, X.; Pang, Y.; Ma, X.; Cheng, Y. The effects of ammonia-N stress on immune parameters, antioxidant capacity, digestive function, and intestinal microflora of Chinese mitten crab, Eriocheir sinensis, and the protective effect of dietary supplement of melatonin. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 250, 109127. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, S.; Zhu, B.; Yang, Y.; Du, X.; Li, Y.; Zhao, Y. Effects of dietary melatonin on growth performance, nutrient composition, and lipid metabolism of Pacific white shrimp (Penaeus vannamei). Aquaculture 2024, 578, 740095. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, S.; Ye, Y.; Du, X.; Liu, X.; Jiang, Q.; Che, X. Effects of dietary melatonin on antioxidant and immune function of the Pacific white shrimp (Litopenaeus vannamei), as determined by transcriptomic analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shi, X.; Liu, Z.; Sun, J.; Sun, T.; Lei, M. Histological, Physiological and Transcriptomic Analysis Reveal the Acute Alkalinity Stress of the Gill and Hepatopancreas of Litopenaeus vannamei. Mar. Biotechnol. 2023, 25, 588–602. [Google Scholar] [CrossRef]
- Wang, B.; Feng, L.; Chen, G.F.; Jiang, W.D.; Liu, Y.; Kuang, S.Y.; Jiang, J.; Tang, L.; Wu, P.; Tang, W.N.; et al. Jian carp (Cyprinus carpio var. Jian) intestinal immune responses, antioxidant status and tight junction protein mRNA expression are modulated via Nrf2 and PKC in response to dietary arginine deficiency. Fish Shellfish Immunol. 2016, 51, 116–124. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Feng, L.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Li, S.; Tang, L.; Zhou, X.Q. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 2015, 167, 91–99. [Google Scholar] [CrossRef]
- Campa-Córdova, A.I.; Hernández-Saavedra, N.Y.; De Philippis, R.; Ascencio, F. Generation of superoxide anion and SOD activity in haemocytes and muscle of American white shrimp (Litopenaeus vannamei) as a response to β-glucan and sulphated polysaccharide. Fish Shellfish Immunol. 2002, 12, 353–366. [Google Scholar] [CrossRef]
- Xu, Z.; Regenstein, J.M.; Xie, D.; Lu, W.; Ren, X.; Yuan, J.; Mao, L. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish Immunol. 2018, 72, 564–571. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, R.; Liu, Z.; Sun, J.; Li, L.; Zhao, G.; Lu, J. Combined analysis of mRNA and miRNA reveals the mechanism of pacific white shrimp (Litopenaeus vannamei) under acute alkalinity stress. PLoS ONE 2023, 18, e0290157. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Metabolomics analysis reveals the response mechanism to carbonate alkalinity toxicity in the gills of Eriocheir sinensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109487. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Zhang, J.; Liu, Q.; Ding, X. Morphologic, digestive enzymes and immunological responses of intestine from Litopenaeus vannamei after lipopolysaccharide injection. J. Invertebr. Pathol. 2018, 153, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Geihs, M.A.; Vargas, M.A.; Maciel, F.E.; Caldas, S.S.; Cruz, B.P.; Primel, E.G.; Monserrat, J.M.; Nery, L.E.M. Effect of melatonin in the antioxidant defense system in the locomotor muscles of the estuarine crab Neohelice granulata (Decapoda, Brachyura). Gen. Comp. Endocrinol. 2010, 166, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Q.; Pang, Y.; Song, X.; Zhou, N.; Wang, J.; He, L.; Lv, J.; Song, Y.; Cheng, Y.; et al. The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin. Sci. Total Environ. 2019, 653, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, L.-T.; Zang, W.-L.; Deng, P.-P.; Zou, W.-L.; Ding, F. Effect of Ca~(2+), Mg~(2+) and salinity on survival, growth and shrimp taste of Litopenaeus vannamei. J. Fish. China 2012, 36, 914. [Google Scholar] [CrossRef]
- Xue, J.; Xu, Y.; Jin, L.; Liu, G.; Sun, Y.; Li, S.; Zhang, J. Effects of traditional Chinese medicine on immune responses in abalone, Haliotis discus hannai Ino. Fish Shellfish Immunol. 2008, 24, 752–758. [Google Scholar] [CrossRef]
- Hossain, M.S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Sony, N.M. Dietary effects of adenosine monophosphate to enhance growth, digestibility, innate immune responses and stress resistance of juvenile red sea bream, Pagrus major. Fish Shellfish Immunol. 2016, 56, 523–533. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.H.; Chu, J.; Luo, L.Z. Improvement of innate immune responses and defense activity in mitten crab (Eriocheir sinensis) by oral administration of beta-glucan. Biotechnol. Lett. 2008, 30, 1721–1725. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, R.; Zhao, D.; Wang, L.; Sun, M.; Wang, M.; Song, L. Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2016, 54, 523–528. [Google Scholar] [CrossRef]
- Prives, C. Signaling to p53: Breaking the MDM2–p53 Circuit. Cell 1998, 95, 5–8. [Google Scholar] [CrossRef]
- Ko, L.J.; Prives, C. p53: Puzzle and paradigm. Genes Dev. 1996, 10, 1054–1072. [Google Scholar] [CrossRef]
- Houston, A.; O’Connell, J. The Fas signalling pathway and its role in the pathogenesis of cancer. Curr. Opin. Pharmacol. 2004, 4, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Deepika, A.; Sreedharan, K.; Paria, A.; Makesh, M.; Rajendran, K.V. Toll-pathway in tiger shrimp (Penaeus monodon) responds to white spot syndrome virus infection: Evidence through molecular characterisation and expression profiles of MyD88, TRAF6 and TLR genes. Fish Shellfish Immunol. 2014, 41, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Dechamma, M.M.; Rajeish, M.; Maiti, B.; Mani, M.K.; Karunasagar, I. Expression of Toll-like receptors (TLR), in lymphoid organ of black tiger shrimp (Penaeus monodon) in response to Vibrio harveyi infection. Aquac. Rep. 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Mekata, T.; Sudhakaran, R.; Okugawa, S.; Inada, M.; Kono, T.; Sakai, M.; Itami, T. A novel gene of tumor necrosis factor ligand superfamily from kuruma shrimp, Marsupenaeus japonicus. Fish Shellfish Immunol. 2010, 28, 571–578. [Google Scholar] [CrossRef]
- Gu, H.-J.; Sun, Q.-L.; Jiang, S.; Zhang, J.; Sun, L. First characterization of an anti-lipopolysaccharide factor (ALF) from hydrothermal vent shrimp: Insights into the immune function of deep-sea crustacean ALF. Dev. Comp. Immunol. 2018, 84, 382–395. [Google Scholar] [CrossRef]
- Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Esquifino, A.I.; Perumal, S.R.P.; Miller, S.C. Melatonin, immune function and aging. Immun. Ageing 2005, 2, 17. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Moslehi, M.; Moazamiyanfar, R.; Dakkali, M.S.; Rezaei, S.; Rastegar-Pouyani, N.; Jafarzadeh, E.; Mouludi, K.; Khodamoradi, E.; Taeb, S.; Najafi, M. Modulation of the immune system by melatonin; implications for cancer therapy. Int. Immunopharmacol. 2022, 108, 108890. [Google Scholar] [CrossRef]
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5, 494. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Ding, X.; Xiong, D.; Zhang, J. Response of intestine microbiota, digestion, and immunity in Pacific white shrimp Litopenaeus vannamei to dietary succinate. Aquaculture 2020, 517, 734762. [Google Scholar] [CrossRef]
- Hirano, T.; Yokoyama, M.; Ikejima, M.; Shiraishi, H.; Hakamata, W.; Nishio, T. Impact of chitin-derived β-N-acetyl-d-glucosaminyl-(1,4)-d-glucosamine on chitinase upregulation in Shewanella baltica. FEMS Microbiol. Lett. 2024, 371, fnae064. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ren, J.; Xue, Y.; Gao, J.; Fu, Q.; Shao, P.; Zhu, H.; Zhang, M.; Ding, F. Fatty acid synthesis promoted by PA1895-1897 operon delays quorum sensing activation in Pseudomonas aeruginosa. AMB Express 2024, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wen, M.; Xiang, L.; Shen, H.; Jiang, G.; Cheng, J.; Hu, Y.; Qian, J. Segmental variations in intestinal microbiota composition and functional capacity along the digestive tract of Litopenaeus vannamei. Aquac. Rep. 2024, 34, 101922. [Google Scholar] [CrossRef]
- Zhu, H.; Qiang, J.; He, J.; Tao, Y.; Bao, J.; Xu, P. Physiological parameters and gut microbiome associated with different dietary lipid levels in hybrid yellow catfish (Tachysurus fulvidraco♀ × Pseudobagrus vachellii♂). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100777. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Baekelandt, S.; Cornet, V.; Mandiki, S.N.M.; Lambert, J.; Dubois, M.; Kestemont, P. Ex vivo approach supports both direct and indirect actions of melatonin on immunity in pike-perch Sander lucioperca. Fish Shellfish Immunol. 2021, 112, 143–150. [Google Scholar] [CrossRef]


| Ingredients | g kg−1 Diet |
|---|---|
| Casein | 450 |
| Wheat flour | 260 |
| Gelatin | 80 |
| Fish oil | 40 |
| Soybean oil | 20 |
| Soyabean lecithin | 10 |
| Vitamin premix a | 20 |
| Mineral premix b | 20 |
| Choline chloride | 5 |
| Cholesterol | 5 |
| Carboxy methyl cellulose | 20 |
| Alpha-cellulose | 70 |
| Total | 1000 |
| Proximate composition | |
| Crude protein | 37.27 |
| Crude lipid | 10.12 |
| Crude ash | 3.45 |
| Moisture | 10.28 |
| Melatonin basic content c (mg/kg) | ND |
| Primer Name | Primer Sequence (5′–3′) | NCBI Database, Gene Accession Number |
|---|---|---|
| Caspase-3 F | AGTTAGTACAAACAGATTGGAGCG | KC660103.1 |
| Caspase-3 R | TTGTGGACAGACAGTATGAGGC | |
| BCL-2 F | CCTTGCTTGACACAGTCGGA | MH559339.1 |
| BCL-2 R | CAGACAAGGTCGTGAGGTGG | |
| p53 F | CCAAGCAGCAATGTGTCAG | KX179650.1 |
| P53 R | CTTGTTGCGATCTTTGTTGC | |
| TNF-α F | CTCAGCCATCTCCTTCTTG | JN180639.1 |
| TNF-α R | TGTTCTCCTCGTTCTTCAC | |
| TLRs F | CCAGCTTAGAAGACCGGCAA | KT372179.1 |
| TLRs R | GTTGTCCGAGCAGAAGTCCA | |
| MyD88 F | GCTGTTCCACCGCCATTT | JX073568.1 |
| MyD88 R | GCATCATAGTGCTGTAGTCCAAGA | |
| Hemocyanin F | GTCTTAGTGGTTCTTGGGCTTGTC | KJ151291.1 |
| Hemocyanin R | GGTCTCCGTCCTGAATGTCTCC | |
| ALF F | AGAGGATCGTTGGGTTGTGG | KJ000049.1 |
| ALF R | AATTCTAGCGTCGTCCTCCG | |
| β-actin F | CAGGTCGTGACTTGACCGAT | KY780290 |
| β-actin R | CGTCAGGGAGCTCGTAAGAC |
| Dietary Melatonin Level (mg/kg) | Parameters | ||||
|---|---|---|---|---|---|
| SR (%) | Initial Weight (g) | Initial Length (cm) | Terminal Weight (g) | Terminal Length (cm) | |
| 0 | 41.67 ± 6.24 c | 3.86 ± 0.63 | 6.08 ± 0.32 | 3.87 ± 0.46 | 6.12 ± 0.26 |
| 22.5 | 48.33 ± 6.24 bc | 3.86 ± 0.63 | 6.08 ± 0.32 | 3.95 ± 0.49 | 6.15 ± 0.21 |
| 41.2 | 60 ± 4.08 ab | 3.86 ± 0.63 | 6.08 ± 0.32 | 3.96 ± 0.88 | 6.11 ± 0.37 |
| 82.7 | 68.33 ± 4.71 a | 3.86 ± 0.63 | 6.08 ± 0.32 | 3.93 ± 0.58 | 6.19 ± 0.28 |
| 165.1 | 58.33 ± 6.24 ab | 3.86 ± 0.63 | 6.08 ± 0.32 | 3.96 ± 0.48 | 6.12 ± 0.3 |
| 329.2 | 51.67 ± 2.36 bc | 3.86 ± 0.63 | 6.08 ± 0.32 | 3.9 ± 0.3 | 6.11 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ye, Y.; Yuan, H.; Yao, Z.; Li, Y.; Sun, Z.; Wei, Y.; Zhao, Y.; Lai, Q. Dietary Melatonin Supplementation Improved Intestinal Health and Immune Function of Pacific White Shrimp (Litopenaeus vannamei) Under High Alkali Stress. Life 2025, 15, 772. https://doi.org/10.3390/life15050772
Li Y, Ye Y, Yuan H, Yao Z, Li Y, Sun Z, Wei Y, Zhao Y, Lai Q. Dietary Melatonin Supplementation Improved Intestinal Health and Immune Function of Pacific White Shrimp (Litopenaeus vannamei) Under High Alkali Stress. Life. 2025; 15(5):772. https://doi.org/10.3390/life15050772
Chicago/Turabian StyleLi, Yiming, Yucong Ye, Haojuan Yuan, Zongli Yao, Yan Li, Zhen Sun, Yuxing Wei, Yunlong Zhao, and Qifang Lai. 2025. "Dietary Melatonin Supplementation Improved Intestinal Health and Immune Function of Pacific White Shrimp (Litopenaeus vannamei) Under High Alkali Stress" Life 15, no. 5: 772. https://doi.org/10.3390/life15050772
APA StyleLi, Y., Ye, Y., Yuan, H., Yao, Z., Li, Y., Sun, Z., Wei, Y., Zhao, Y., & Lai, Q. (2025). Dietary Melatonin Supplementation Improved Intestinal Health and Immune Function of Pacific White Shrimp (Litopenaeus vannamei) Under High Alkali Stress. Life, 15(5), 772. https://doi.org/10.3390/life15050772





