The Impact of Surgery on Quality of Life in Hidradenitis Suppurativa: Results from a Prospective Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
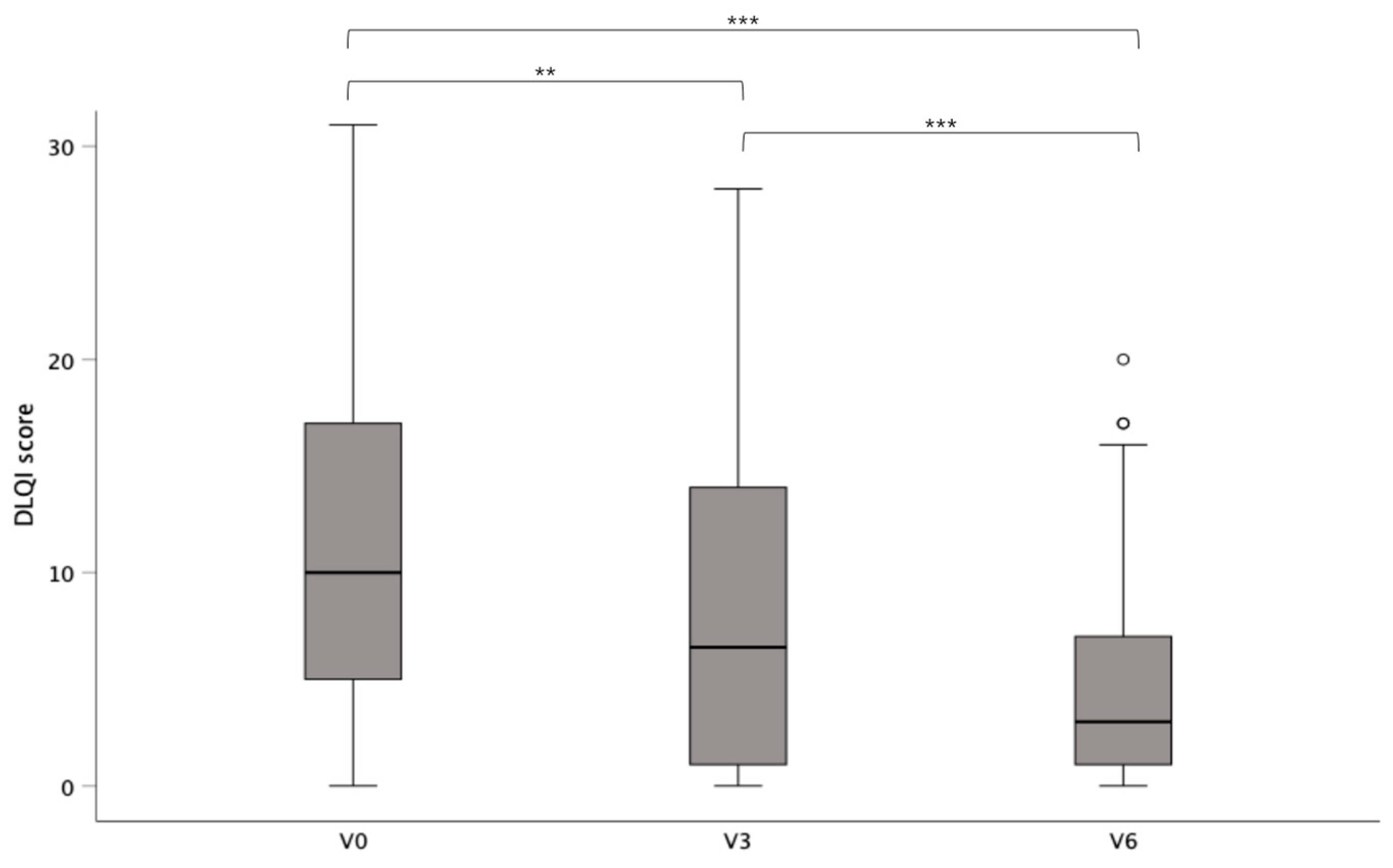
3.1. Impact of Surgery on Quality of Life
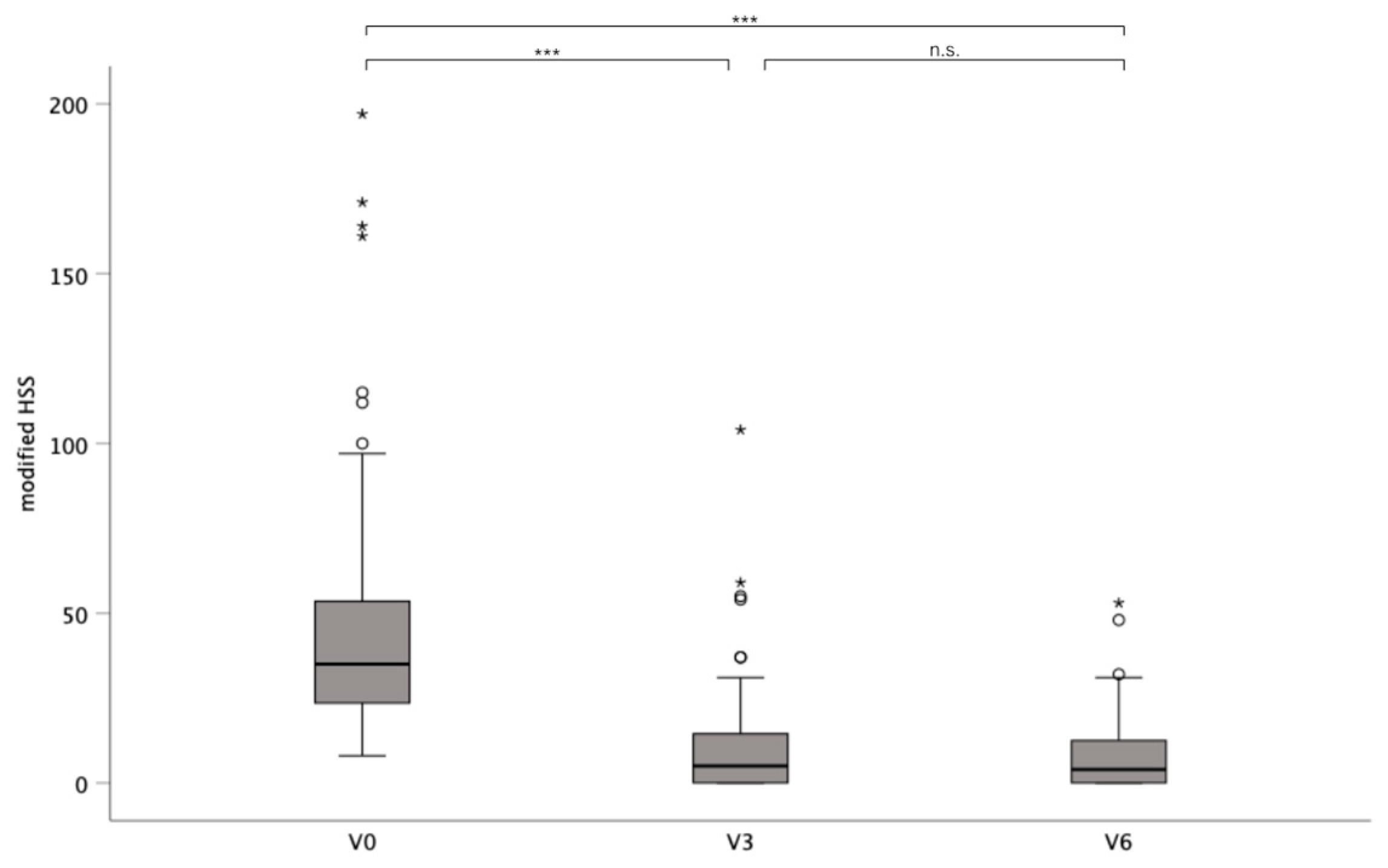
3.2. Impact of Surgery on Disease Severity
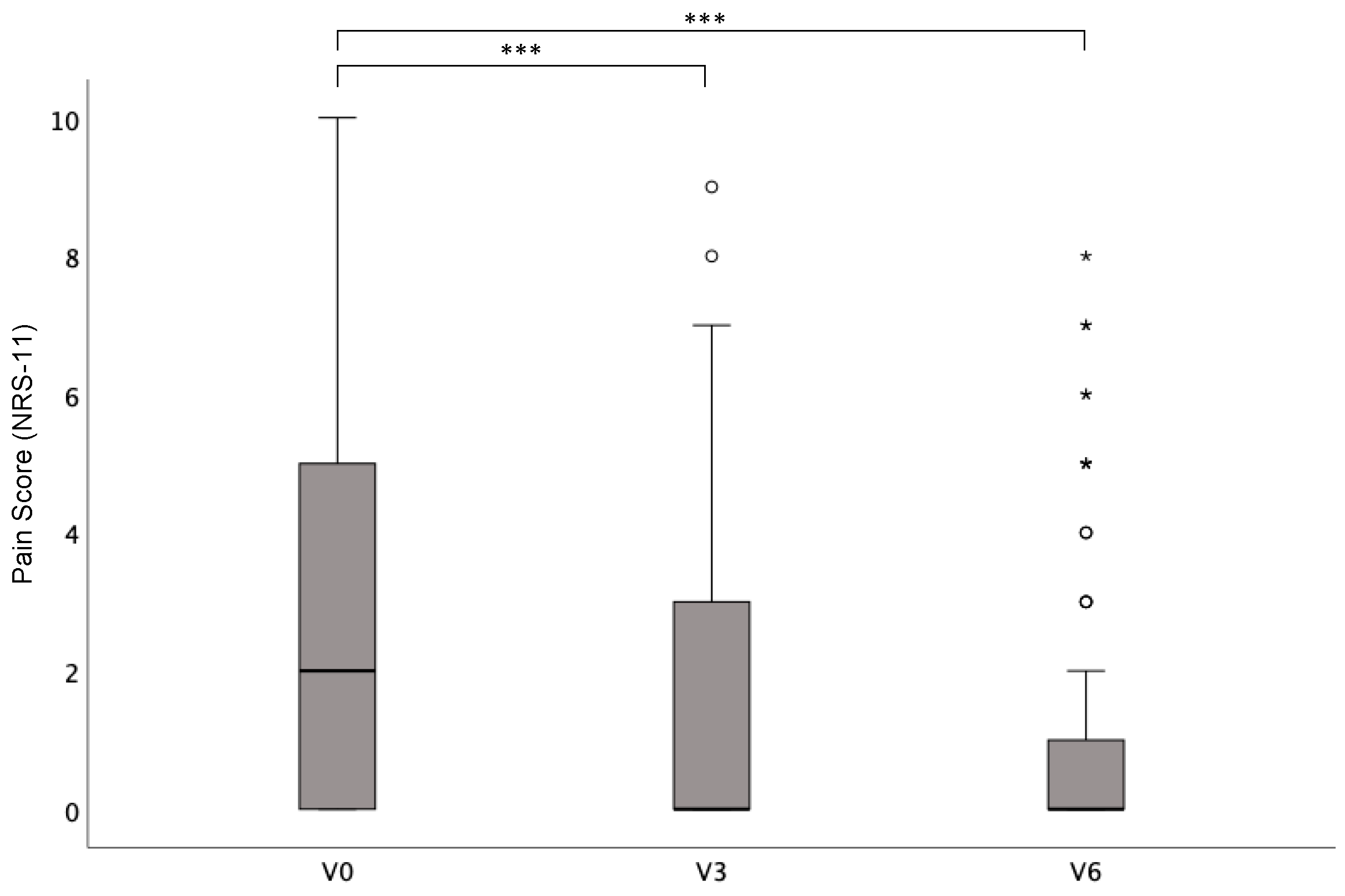
3.3. Impact of Surgery on Pain
3.4. Secondary Intention Healing vs. Skin Grafting
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, Ł.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Primer 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Kirby, J.S.; Lavian, J.; Lin, G.; Strunk, A. Sex- and Age-Adjusted Population Analysis of Prevalence Estimates for Hidradenitis Suppurativa in the United States. JAMA Dermatol. 2017, 153, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Theut Riis, P.; Pedersen, O.B.; Sigsgaard, V.; Erikstrup, C.; Paarup, H.M.; Nielsen, K.R.; Burgdorf, K.S.; Hjalgrim, H.; Rostgaard, K.; Banasik, K.; et al. Prevalence of Patients with Self-Reported Hidradenitis Suppurativa in a Cohort of Danish Blood Donors: A Cross-Sectional Study. Br. J. Dermatol. 2019, 180, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R.; Jenkins-Jones, S.; Knipe, D.W.; Morgan, C.L.I.; Cannings-John, R.; Piguet, V. Population-Based Clinical Practice Research Datalink Study Using Algorithm Modelling to Identify the True Burden of Hidradenitis Suppurativa. Br. J. Dermatol. 2018, 178, 917–924. [Google Scholar] [CrossRef]
- Savage, K.T.; Singh, V.; Patel, Z.S.; Yannuzzi, C.A.; McKenzie-Brown, A.M.; Lowes, M.A.; Orenstein, L.A.V. Pain Management in Hidradenitis Suppurativa and a Proposed Treatment Algorithm. J. Am. Acad. Dermatol. 2021, 85, 187–199. [Google Scholar] [CrossRef]
- Smith, H.S.; Chao, J.D.; Teitelbaum, J. Painful Hidradenitis Suppurativa. Clin. J. Pain 2010, 26, 435–444. [Google Scholar] [CrossRef]
- Sampogna, F.; Abeni, D.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Titeca, G.; Jemec, G.B.E.; Misery, L.; Szabó, C.; Linder, M.D.; et al. Impairment of Sexual Life in 3,485 Dermatological Outpatients from a Multicentre Study in 13 European Countries. Acta Derm. Venereol. 2017, 97, 478–482. [Google Scholar] [CrossRef]
- Ocker, L.; Abu Rached, N.; Seifert, C.; Scheel, C.; Bechara, F.G. Current Medical and Surgical Treatment of Hidradenitis Suppurativa-A Comprehensive Review. J. Clin. Med. 2022, 11, 7240. [Google Scholar] [CrossRef]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef]
- Kimball, A.B.; Jemec, G.B.E.; Alavi, A.; Reguiai, Z.; Gottlieb, A.B.; Bechara, F.G.; Paul, C.; Giamarellos Bourboulis, E.J.; Villani, A.P.; Schwinn, A.; et al. Secukinumab in Moderate-to-Severe Hidradenitis Suppurativa (SUNSHINE and SUNRISE): Week 16 and Week 52 Results of Two Identical, Multicentre, Randomised, Placebo-Controlled, Double-Blind Phase 3 Trials. Lancet Lond. Engl. 2023, 401, 747–761. [Google Scholar] [CrossRef]
- Kimball, A.B.; Jemec, G.B.E.; Sayed, C.J.; Kirby, J.S.; Prens, E.; Ingram, J.R.; Garg, A.; Gottlieb, A.B.; Szepietowski, J.C.; Bechara, F.G.; et al. Efficacy and Safety of Bimekizumab in Patients with Moderate-to-Severe Hidradenitis Suppurativa (BE HEARD I and BE HEARD II): Two 48-Week, Randomised, Double-Blind, Placebo-Controlled, Multicentre Phase 3 Trials. Lancet 2024, 403, 2504–2519. [Google Scholar] [CrossRef]
- Thomsen, S.F. A Note on Improvement in Dermatology Life Quality Index (DLQI) among Patients with Hidradenitis Suppurativa Treated with Adalimumab. J. Dermatol. Treat. 2018, 29, 823–824. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, H.H.; Longcore, M.; Geng, Z.; Garg, A. Weekly Adalimumab Treatment Decreased Disease Flare in Hidradenitis Suppurativa over 36 Weeks: Integrated Results from the Phase 3 PIONEER Trials. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1050–1056. [Google Scholar] [CrossRef]
- Cramer, P.; Schneider-Burrus, S.; Kovács, M.; Scholl, L.; Podda, M.; Bechara, F.G. Hidradenitis suppurativa/acne inversa-surgical options, reconstruction and combinations with drug therapies-an update. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2021, 72, 692–699. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Del Marmol, V.; Mrowietz, U.; Prens, E.P.; Tzellos, T.; Jemec, G.B.E. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—a Simple Practical Measure for Routine Clinical Use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Harry, J. Hurley Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: Surgical approach. In Roenigk & Roenigk’s Dermatologic Surgery: Principles and Practice, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 623–646. ISBN 978-0-8247-9503-0. [Google Scholar]
- Sartorius, K.; Emtestam, L.; Jemec, G.b.e.; Lapins, J. Objective Scoring of Hidradenitis Suppurativa Reflecting the Role of Tobacco Smoking and Obesity. Br. J. Dermatol. 2009, 161, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.; Hoggart, B. Pain: A Review of Three Commonly Used Pain Rating Scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef]
- Chernyshov, P.V.; Finlay, A.Y.; Tomas-Aragones, L.; Poot, F.; Sampogna, F.; Marron, S.E.; Zemskov, S.V.; Abeni, D.; Tzellos, T.; Szepietowski, J.C.; et al. Quality of Life in Hidradenitis Suppurativa: An Update. Int. J. Environ. Res. Public Health 2021, 18, 6131. [Google Scholar] [CrossRef]
- Vellaichamy, G.; Braunberger, T.L.; Jones, J.L.; Peacock, A.; Nahhas, A.F.; Hamzavi, I.H. Patient-Reported Outcomes in Hidradenitis Suppurativa. G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2019, 154, 137–147. [Google Scholar] [CrossRef]
- Hongbo, Y.; Thomas, C.L.; Harrison, M.A.; Sam Salek, M.; Finlay, A.Y. Translating the Science of Quality of Life into Practice: What Do Dermatology Life Quality Index Scores Mean? J. Investig. Dermatol. 2005, 125, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.S.; Thorlacius, L.; Villumsen, B.; Ingram, J.R.; Garg, A.; Christensen, K.B.; Butt, M.; Esmann, S.; Tan, J.; Jemec, G.B.E. The Hidradenitis Suppurativa Quality of Life (HiSQOL) Score: Development and Validation of a Measure for Clinical Trials. Br. J. Dermatol. 2020, 183, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Marrón, S.E.; Gómez-Barrera, M.; Tomás-Aragonés, L.; Díaz Díaz, R.M.; Vilarrasa Rull, E.; Madrid Álvarez, M.B.; Puig, L. Development and Preliminary Validation of the HSQoL-24 Tool to Assess Quality of Life in Patients with Hidradenitis Suppurativa. Actas Dermosifiliogr. 2019, 110, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Pinard, J.; Vleugels, R.A.; Joyce, C.; Merola, J.F.; Patel, M. Hidradenitis Suppurativa Burden of Disease Tool: Pilot Testing of a Disease-Specific Quality of Life Questionnaire. J. Am. Acad. Dermatol. 2018, 78, 215–217.e2. [Google Scholar] [CrossRef]
- Sisic, M.; Kirby, J.S.; Boyal, S.; Plant, L.; McLellan, C.; Tan, J. Development of a Quality-of-Life Measure for Hidradenitis Suppurativa. J. Cutan. Med. Surg. 2017, 21, 152–155. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Okun, M.M.; Prens, E.P.; Gniadecki, R.; Foley, P.A.; Lynde, C.; Weisman, J.; Gu, Y.; Williams, D.A.; Jemec, G.B.E. Long-Term Adalimumab Efficacy in Patients with Moderate-to-Severe Hidradenitis Suppurativa/Acne Inversa: 3-Year Results of a Phase 3 Open-Label Extension Study. J. Am. Acad. Dermatol. 2019, 80, 60–69.e2. [Google Scholar] [CrossRef]
- Shukla, R.; Karagaiah, P.; Patil, A.; Farnbach, K.; Ortega-Loayza, A.G.; Tzellos, T.; Szepietowski, J.C.; Giulini, M.; Schepler, H.; Grabbe, S.; et al. Surgical Treatment in Hidradenitis Suppurativa. J. Clin. Med. 2022, 11, 2311. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Bechara, F.G.; Fritz, K.; Goebeler, M.; Hetzer, F.H.; Just, E.; Kirsten, N.; Kokolakis, G.; Kurzen, H.; Nikolakis, G.; et al. S2k-Leitlinie zur Therapie der Hidradenitis suppurativa/Acne inversa (ICD-10-Code: L73.2). Aktuelle Dermatol. 2024, 50, 30–83. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Bechara, F.G.; Benhadou, F.; Bettoli, V.; Bukvić Mokos, Z.; Del Marmol, V.; Dolenc-Voljč, M.; Giamarellos-Bourboulis, E.J.; Grimstad, Ø.; Guillem, P.; et al. European S2k Guidelines for Hidradenitis Suppurativa/Acne Inversa Part 2: Treatment. J. Eur. Acad. Dermatol. Venereol. JEADV 2025, 39, 899–941. [Google Scholar] [CrossRef]
- Wong, H.-S.; Jiang, J.-Y.; Huang, S.-D.; Zhu, P.; Ji, X.; Wang, D.-G. A Review of Surgical and Reconstructive Techniques for Hidradenitis Suppurativa. Arch. Dermatol. Res. 2024, 316, 270. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Tsaousi, A.; Barbus, S.; Huss-Marp, J.; Witte, K.; Wolk, K.; Fritz, B.; Sabat, R. Features Associated With Quality of Life Impairment in Hidradenitis Suppurativa Patients. Front. Med. 2021, 8, 676241. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, Ø.; Tzellos, T.; Dufour, D.N.; Bremnes, Ø.; Skoie, I.M.; Snekvik, I.; Jarnaess, E.; Kyrgidis, A.; Ingvarsson, G. Evaluation of Medical and Surgical Treatments for Hidradenitis Suppurativa Using Real-Life Data from the Scandinavian Registry (HISREG). J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 1164–1171. [Google Scholar] [CrossRef]
- Kohorst, J.J.; Baum, C.L.; Otley, C.C.; Roenigk, R.K.; Pemberton, J.H.; Dozois, E.J.; Tran, N.V.; Davis, M.D.P. Patient Satisfaction and Quality of Life Following Surgery for Hidradenitis Suppurativa. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al 2017, 43, 125–133. [Google Scholar] [CrossRef]
- Kofler, L.; Schweinzer, K.; Heister, M.; Kohler, M.; Breuninger, H.; Häfner, H.-M. Surgical Treatment of Hidradenitis Suppurativa: An Analysis of Postoperative Outcome, Cosmetic Results and Quality of Life in 255 Patients. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Prens, L.M.; Huizinga, J.; Janse, I.C.; Horváth, B. Surgical Outcomes and the Impact of Major Surgery on Quality of Life, Activity Impairment and Sexual Health in Hidradenitis Suppurativa Patients: A Prospective Single Centre Study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1941–1946. [Google Scholar] [CrossRef]
- Posch, C.; Monshi, B.; Quint, T.; Vujic, I.; Lilgenau, N.; Rappersberger, K. The Role of Wide Local Excision for the Treatment of Severe Hidradenitis Suppurativa (Hurley Grade III): Retrospective Analysis of 74 Patients. J. Am. Acad. Dermatol. 2017, 77, 123–129.e5. [Google Scholar] [CrossRef]
- Dick, J.; Kröhl, V.; Enk, A.; Hartschuh, W.; Gholam, P. Improvement in Quality of Life and Pain in Patients With Hidradenitis Suppurativa After Wide Local Excision: A Prospective Study. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al 2021, 47, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Bechara, F.G.; Podda, M.; Prens, E.P.; Horváth, B.; Giamarellos-Bourboulis, E.J.; Alavi, A.; Szepietowski, J.C.; Kirby, J.; Geng, Z.; Jean, C.; et al. Efficacy and Safety of Adalimumab in Conjunction With Surgery in Moderate to Severe Hidradenitis Suppurativa: The SHARPS Randomized Clinical Trial. JAMA Surg. 2021, 156, 1001–1009. [Google Scholar] [CrossRef]
- Offidani, A.; Marzano, A.V.; Peris, K.; Molinelli, E.; Bettoli, V.; Magnoni, C.; Vaienti, L.; Pappagallo, G.; Amerio, P.; Atzori, L.; et al. Guidelines How to Integrate Surgery and Targeted Therapy with Biologics for the Treatment of Hidradenitis Suppurativa: Delphi Consensus Statements from an Italian Expert Panel. Dermatology 2024, 240, 885–896. [Google Scholar] [CrossRef]
- Aarts, P.; van Huijstee, J.C.; van der Zee, H.H.; van Doorn, M.B.A.; van Straalen, K.R.; Prens, E.P. Adalimumab in Conjunction with Surgery Compared with Adalimumab Monotherapy for Hidradenitis Suppurativa: A Randomized Controlled Trial in a real-world setting. J. Am. Acad. Dermatol. 2023, 89, 677–684. [Google Scholar] [CrossRef]
- Lai, P.-T.; Tseng, H.-C. Adopting Adalimumab Combined Surgery in the Management of Moderate to Severe Hidradenitis Suppurativa: Experience from a Single Medical Center in Southern Taiwan. J. Dermatol. 2024, 51, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Ocker, L.; Abu Rached, N.; Bechara, F.G. Surgical Management of Hidradenitis Suppurativa; Dermatology—The Latest Research on the Most Common Diseases; IntechOpen: Rijeka, Croatia, 2024; ISBN 978-0-85014-899-2. [Google Scholar]
- Morgan, W.P.; Harding, K.G.; Hughes, L.E. A Comparison of Skin Grafting and Healing by Granulation, Following Axillary Excision for Hidradenitis Suppurativa. Ann. R. Coll. Surg. Engl. 1983, 65, 235–236. [Google Scholar] [PubMed]
- Marvel, J.; Vlahiotis, A.; Sainski-Nguyen, A.; Willson, T.; Kimball, A. Disease Burden and Cost of Hidradenitis Suppurativa: A Retrospective Examination of US Administrative Claims Data. BMJ Open 2019, 9, e030579. [Google Scholar] [CrossRef] [PubMed]
| Sex n (%) | female | 42 (51.2) |
| male | 40 (48.8) | |
| Age at surgery, mean (±SD), y | 37.5 (±12.7) | |
| BMI, mean (±SD); kg/m2 | 30.2 (±6.8)) | |
| Positive family history of HS, n (%) | 27 (32.9) | |
| Active smokers, n (%) | 50 (61.0) | |
| Age of HS onset, median (IQR), y | 20.5 (10–60) | |
| Prior treatments, n (%) | prior surgery | 62 (75.6) |
| prior antibiotics | 51 (62.2) | |
| Affected body regions, n (%) | Axilla | 38 (46.3) |
| Groins/genital | 55 (67.1) | |
| Glutes/perianal | 38 (46.3) | |
| Other | 8 (9.8) | |
| Surgery site, n (%) | Axilla | 28 (34.1) |
| Groins/genital | 47 (57.3) | |
| Glutes/perianal | 28 (34.1) | |
| Other | 13 (15.8) | |
| Hurley stages, n (%) | I | 0 (0.0) |
| II | 66 (80.5) | |
| III | 16 (19.5) | |
| modified HSS, mean (±SD) | 50.3 (±49.3) | |
| DLQI, mean (±SD) | 11.6 (±8.3) | |
| Pain (NRS-11), mean (±SD) | 3.01 (±3.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocker, L.; Abu Rached, N.; Koller, A.; Frost, C.; Käpynen, R.; Bechara, F.G. The Impact of Surgery on Quality of Life in Hidradenitis Suppurativa: Results from a Prospective Single-Center Study. Life 2025, 15, 769. https://doi.org/10.3390/life15050769
Ocker L, Abu Rached N, Koller A, Frost C, Käpynen R, Bechara FG. The Impact of Surgery on Quality of Life in Hidradenitis Suppurativa: Results from a Prospective Single-Center Study. Life. 2025; 15(5):769. https://doi.org/10.3390/life15050769
Chicago/Turabian StyleOcker, Lennart, Nessr Abu Rached, Anna Koller, Carolin Frost, Riina Käpynen, and Falk G. Bechara. 2025. "The Impact of Surgery on Quality of Life in Hidradenitis Suppurativa: Results from a Prospective Single-Center Study" Life 15, no. 5: 769. https://doi.org/10.3390/life15050769
APA StyleOcker, L., Abu Rached, N., Koller, A., Frost, C., Käpynen, R., & Bechara, F. G. (2025). The Impact of Surgery on Quality of Life in Hidradenitis Suppurativa: Results from a Prospective Single-Center Study. Life, 15(5), 769. https://doi.org/10.3390/life15050769







