Restoring Biomechanical Gait Function with Ultrasound-Guided Acupotomy for Post-Stroke Equinovarus Foot: Two Case Reports and a Protocol (A CARE- and SPIRIT-Compliant Study)
Abstract
1. Introduction
2. Case Presentation
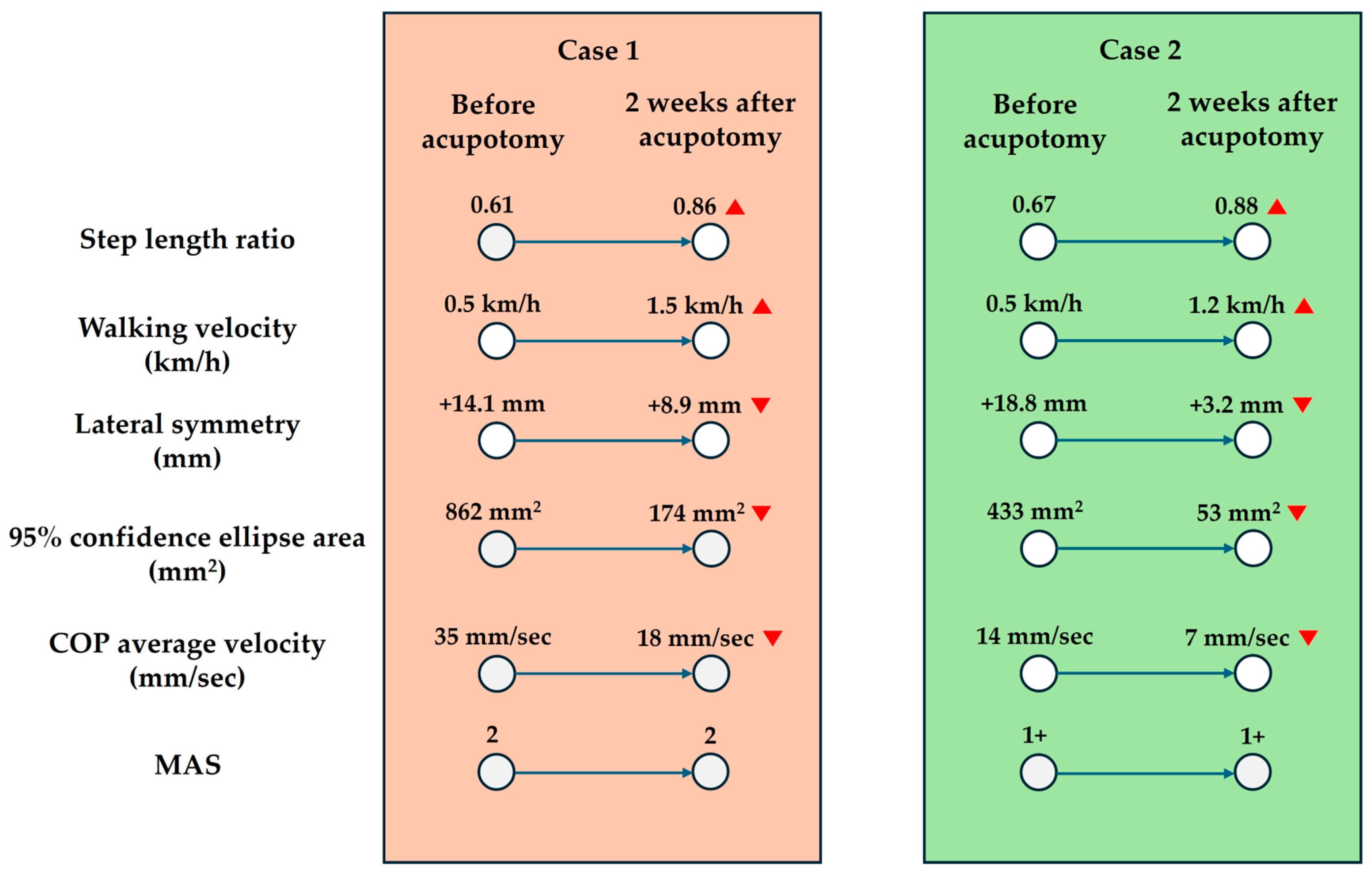
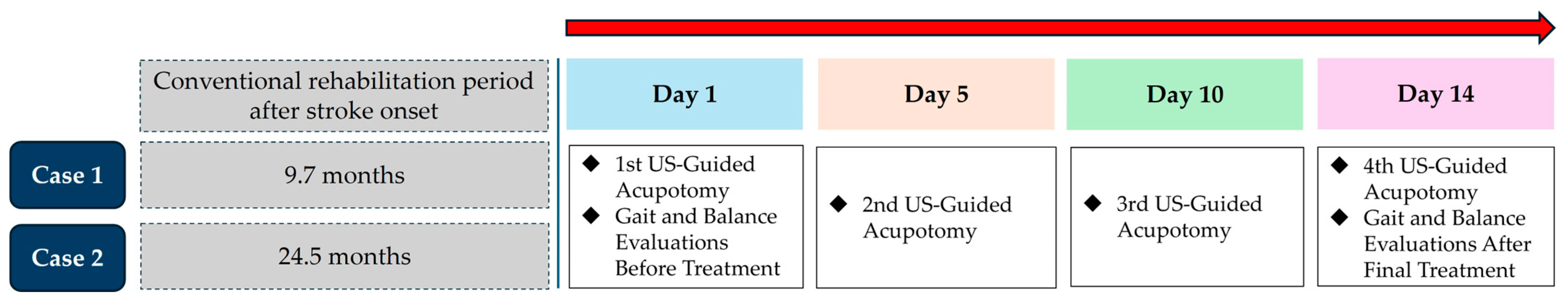
2.1. Case 1
2.2. Case 2
3. Patient Perspectives
3.1. Case 1
3.2. Case 2
4. Study Protocol
4.1. Study Registration
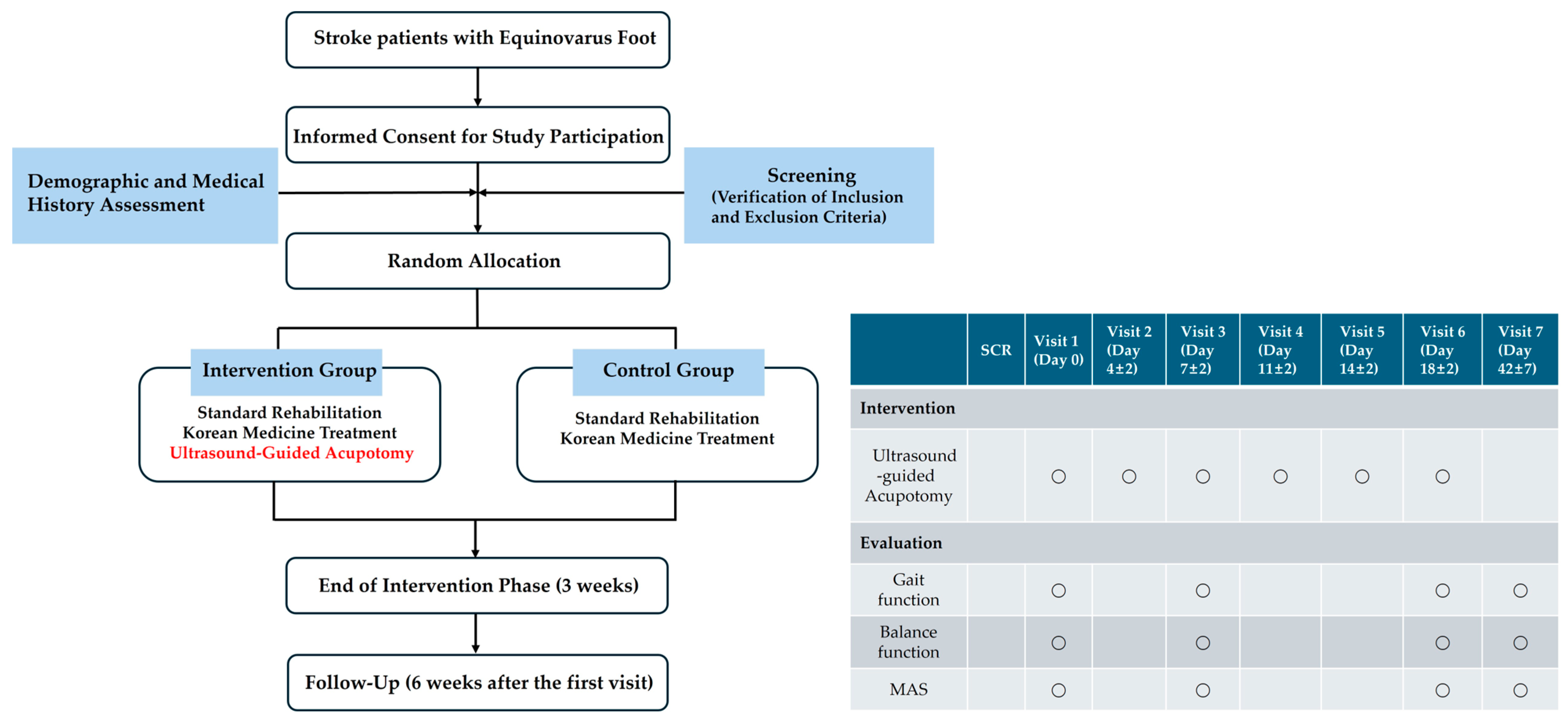
4.2. Study Design
4.2.1. Objectives
4.2.2. Study Period
4.2.3. Name and Address of the Study Institute
4.2.4. Name and Title of the Principal Investigator
4.2.5. Sample Size
- Target number of participants:A total of 48 participants (24 in the experimental group and 24 in the control group) are planned to be enrolled.
- Calculation basis:This study will be an exploratory clinical trial aiming to evaluate the effects of ultrasound-guided acupotomy on improving gait function in patients with post-stroke equinovarus foot. Exploratory studies of this nature often recommend a minimum number of participants for feasibility. To calculate the target number of participants, the primary efficacy endpoint was set to a significance level (α) of 5% and a power (1 − β) of 80%. The efficacy variable selected was the step length ratio, as referenced in a previous study [9]. Due to the scarcity of studies using ultrasound-guided acupotomy for post-stroke equinovarus foot, we referred to studies that measured the step length ratio as an efficacy indicator. A study by Patterson et al. [9] compared healthy individuals and patients with stroke, demonstrating a significant difference in the step length ratio before and after treatment.The step length ratio approaches 1 as gait symmetry improves, and it decreases as asymmetry increases. In Patterson et al.’s study [9], the step length ratio was 0.87 in the stroke group and 0.97 in the healthy group, with a statistically significant difference at a significance level of 0.01. Based on these data, we assumed a clinically meaningful change in the step length ratio (ε) of 0.10 and a common standard deviation (δ) of 0.11. Using these variables, the calculated sample size per group was 19. Based on an expected dropout rate of 20%, the sample size per group was adjusted to 24, resulting in a total of 48 participants for the study.
4.2.6. Study Flow Diagram
4.3. Participants
4.3.1. Target Disease and Symptoms
4.3.2. Inclusion Criteria
- Adults aged 19 years or older;
- Patients previously diagnosed with stroke (ischemic stroke, hemorrhagic stroke, or subarachnoid hemorrhage; KCD codes I60-I63) based on clinical presentation and radiological findings;
- Patients who have passed at least 3 months (90 days) since stroke onset;
- Patients presenting with spastic equinovarus foot on the affected side with an MAS score of 2 or higher;
- Patients with a functional ambulation category score of 3 or higher;
- Patients who fully understand the study aim and voluntarily consent to participate by providing written consent either personally or through a guardian/proxy.
4.3.3. Exclusion Criteria
- Patients with traumatic hemorrhagic or subarachnoid hemorrhage;
- Patients with unstable vital signs requiring bed rest following stroke onset;
- Patients who have initiated treatments for spastic equinovarus foot (e.g., botulinum toxin injections, chemical nerve block, surgical interventions, and intrathecal baclofen therapy) within the past 3 months;
- Patients with spasticity caused by congenital deformities or skeletal abnormalities unrelated to stroke;
- Patients on anticoagulant therapy with an INR ≥ 4.0;
- Patients with severe coagulation disorders, such as hemophilia;
- Patients with signs of infection or other skin conditions at the site of acupotomy application;
- Patients deemed physically or mentally unfit for study participation based on clinical judgment.
4.3.4. Allocation Method and Blinding Limitations
- Participants enrolled in this clinical study will be assigned to either the experimental or control groups randomly to prevent biases during group allocation.
- A blocked randomization method with a block size of 4 will be used to ensure equal allocation across both groups. Randomization codes will be pregenerated, with the block size set to 4.
- Each participant who consents to join the clinical study will be assigned a screening number in the order in which they provide written consent. If multiple participants are screened on the same day, numbers will be assigned based on the order of consent.
- Participants who meet all inclusion/exclusion criteria will be randomized at visit 1 (Day 0) according to a pregenerated randomization table created by a statistical expert. A randomization number will be assigned to each participant.
- If a participant withdraws from the study, their assigned randomization number cannot be reused, and the withdrawn participant cannot rejoin the study.
4.3.5. Criteria for Discontinuation and Dropout
- Violation of the inclusion or exclusion criteria.
- The occurrence of a serious adverse event or participant’s request to discontinue due to adverse events.
- Withdrawal of consent by the participant or their guardian/proxy.
- The determination that continuing the study is inappropriate by the principal investigator or the study co-ordinator.
4.4. Intervention
4.4.1. Standard Rehabilitation and Korean Medicine Treatments
- Both the experimental and control groups will receive standard rehabilitation therapy and Korean medicine treatments for 6 weeks.
- Rehabilitation and Korean medicine treatments refer to commonly practiced therapies for patients with stroke and must not include any additional interventions specifically targeting lower limb spasticity relief.
- Each standard rehabilitation therapy session will comprise both physical therapy (functional electrical stimulation therapy, neurorehabilitation therapy, and gait training) and occupational therapy (complex occupational therapy and daily living activities). Each session will last for 1.5 h, with 5–10 sessions per week.
- Korean medicine treatments will consist of manual acupuncture therapy, electroacupuncture therapy, and herbal medicine therapy. Manual acupuncture and electroacupuncture treatments will be administered once daily, with seven sessions per week, while herbal medicine will be taken daily at doses of 2–3 packs per day (110 cc per pack).
4.4.2. Ultrasound-Guided Acupotomy
- The experimental group will receive ultrasound-guided acupotomy in addition to standard rehabilitation and Korean medicine treatments, administered six times over three weeks.
- The acupotomy needles will have the following dimensions: 0.5 mm × 80 mm.
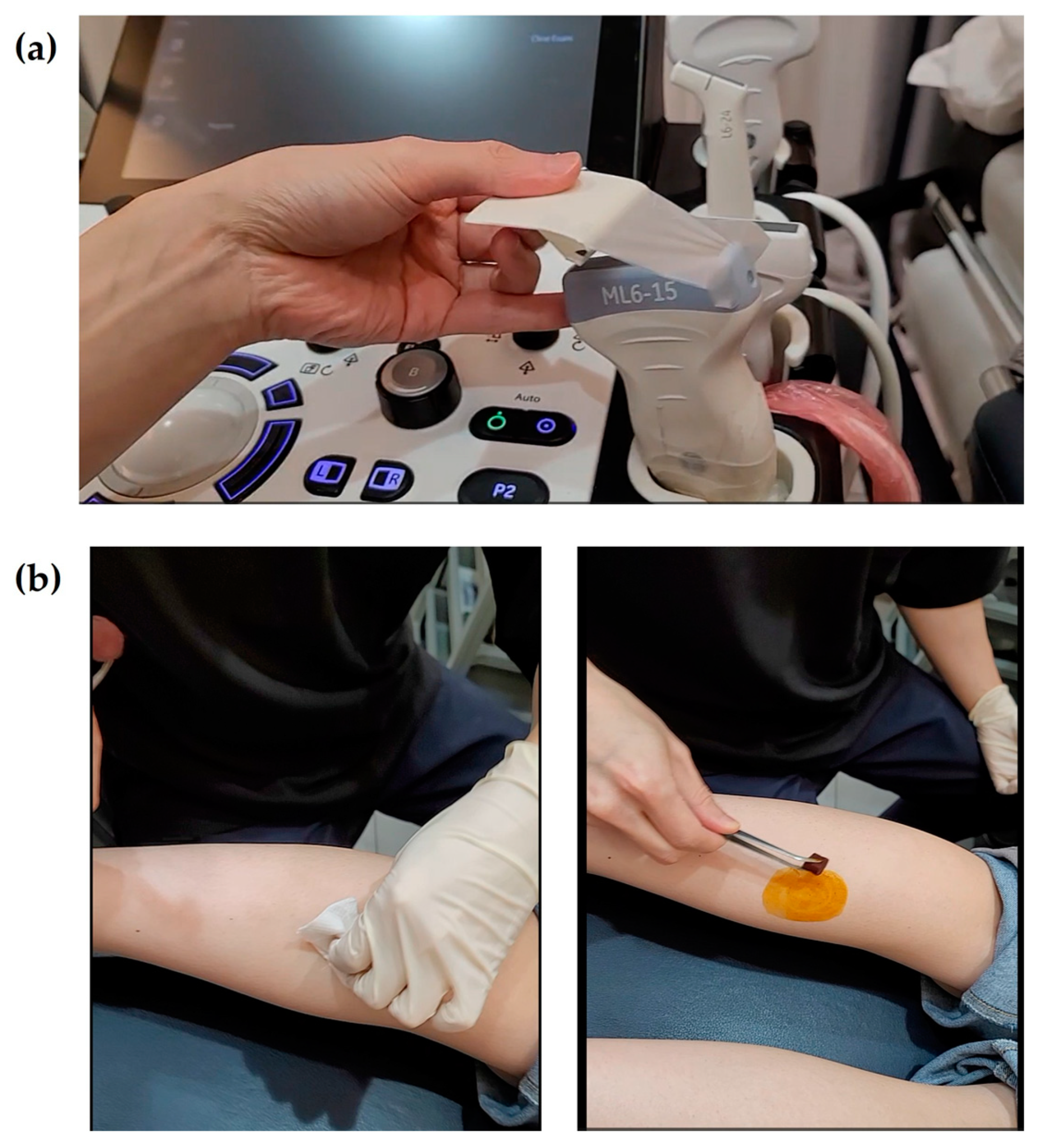
- The treatment will target three regions and four specific muscles: the medial and lateral heads of the gastrocnemius, the muscle belly of the soleus, and the muscle belly of the tibialis posterior. Using an in-plane ultrasound-guided technique, the gastrocnemius and soleus muscles will be treated with the patient in a prone position (Figure 1a). Two insertion pathways will be used to sequentially target both gastrocnemius heads and the underlying soleus muscle, enabling effective stimulation through shared access points. The tibialis posterior muscle will be treated in the supine position, with the affected limb externally rotated approximately 30°, using a medial approach along the tibia (Figure 1b). The precise scanning points will be determined based on the tenderness points in each muscle. If tenderness points are difficult to identify due to sensory loss, the treatment site will be selected based on anatomical landmarks and the region of maximal muscle thickness. For the tibialis posterior muscle, scanning will focus on avoiding major blood vessels and nerves. At each insertion point, the acupotomy needle will be manipulated up to three times to elicit a deqi sensation, which will be verbally confirmed by the patient whenever possible.
- After the acupotomy procedure, the treated area will be disinfected with an alcohol swab, followed by cupping therapy for five minutes. The intensity of cupping will be minimized to prevent excessive muscle relaxation, and the cups will be immediately removed once bleeding has ceased at the treatment site. The area will then be disinfected again with an alcohol swab, and a sterile circular adhesive bandage will be applied.
- Post-procedure reactions, such as bruising, pain, or minor bleeding, will be carefully monitored. To prevent dizziness-related falls, patients will be advised not to stand immediately after the procedure. Instead, they will be encouraged to remain seated until their body has fully adjusted. After standing, patients will be closely observed for any signs of dizziness or instability, and movement will be restricted until sufficient stabilization is ensured.
4.4.3. Prohibited Concomitant Medications and Pharmacological Treatments
4.5. Outcomes
4.5.1. Primary Efficacy Outcome
- Step length ratioStep length is defined as the distance from the heel of one foot to the heel of the opposite foot. According to Patterson et al. [9], the step length ratio is one of the most useful gait variables for evaluating walking in patients with stroke. The step length ratio is calculated by dividing the step length of the affected side by that of the unaffected side to facilitate interpretation. For example, a step length ratio of 0.5 indicates that the step length on the unaffected side is twice as long as that on the affected side. A step length ratio closer to 1 indicates a gait pattern more similar to that of a healthy individual [9].
4.5.2. Secondary Efficacy Outcomes
- Gait function
- ○
- Walking velocity (km/h):
- ○
- Lateral symmetry (mm):Lateral symmetry refers to the side-to-side movement of the center of pressure (COP) during walking [11]. A larger positive value indicates a shift of the COP toward the right side, while a larger negative value indicates a shift toward the left side [11]. In this study, to ensure ease of interpretation, the unaffected and affected sides will be consistently denoted with positive (+) and negative (–) values.
- Balance function
- ○
- 95% confidence ellipse area (mm2):
- ○
- COP average velocity (mm/s):
- Modified Ashworth Scale (MAS)The MAS is a clinical scale used to assess muscle tone by evaluating the resistance encountered during passive joint movement [12]. This assessment does not require specialized equipment and can be performed quickly.
4.5.3. Safety Assessment
- Safety outcomes will be evaluated by assessing reactions observed following ultrasound-guided acupotomy treatment. These reactions will be categorized into bruising, pain, microbleeding, extensive bleeding, hematoma, local infection, edema, fatigue, autonomic nervous system dysfunction, and others. The frequency of each reaction will then be analyzed.
4.5.4. Time Points for Assessment and Data Collection Methods
- The assessment time points are illustrated in Figure 4. The step length ratio, walking velocity, and lateral symmetry will be measured using a treadmill with an embedded force plate (FDM-T, Zebris Co., Ltd., Germany). Participants will walk on the treadmill at their most comfortable speed for 30 s, during which the above variable will be automatically recorded. The 95% confidence ellipse area and COP average velocity will be measured while the participant stands still on the same treadmill for 10 s. The MAS will be assessed by a trained Korean medicine practitioner, who will passively move the participant’s ankle joint to evaluate the degree of resistance in the muscles.
4.6. Statistical Analysis
4.6.1. General Principles
- The stroke data obtained from the participants in this clinical study will be analyzed using both the intention-to-treat (ITT) and per-protocol (PP) methods. The primary analysis will be conducted using the ITT approach, while the PP analysis will be performed separately to ensure consistency with the ITT results. All statistical analyses will be two-tailed, with a significance level of 5%. Descriptive statistics for demographic, sociological, and baseline characteristics will be presented for each group.
- For continuous data, independent t-tests or Mann–Whitney U tests will be used, depending on the normality of the data distribution. Categorical data will be analyzed using the Chi-square test or Fisher’s exact test. If significant differences are found in the baseline characteristics between the two groups, differences will be adjusted during the efficacy analysis to account for heterogeneity. Statistical analyses will be performed using SPSS Version 23.0 (IBM Corp., Armonk, NY, USA).
4.6.2. Efficacy Outcome Variables
- For the analysis of efficacy outcome variables, independent t-tests will be applied to verify the homogeneity of baseline values between the intervention and control groups, provided that normality is assumed. If homogeneity is confirmed, changes in outcome variables from baseline to the final intervention will be analyzed within each group using paired t-tests.
- If there are significant baseline differences between the two groups, an analysis of covariance (ANCOVA) will be performed, using the group as a fixed factor and the baseline values as covariates. If normality is not assumed, a Wilcoxon signed-rank test will be applied to assess within-group changes.
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANCOVA | analysis of covariance |
| COP | center of pressure |
| CRIS | Clinical Research Information Service |
| EVF | equinovarus foot |
| FAC | functional ambulation category |
| ITT | intention to treat |
| KHIDI | Korea Health Industry Development Institute |
| MAS | Modified Ashworth Scale |
| NIH | National Institute of Health |
| PP | per protocol |
| WUGMC | Wonkwang University Gwangju Medical Center |
References
- Chau, J.P.C.; Lo, S.H.S.; Butt, L.; Liang, S. Post-Stroke Experiences and Rehabilitation Needs of Community-Dwelling Chinese Stroke Survivors: A Qualitative Study. Int. J. Environ. Res. Public Health 2022, 19, 16345. [Google Scholar] [CrossRef]
- Freitas, M.; Fonseca, P.; Alves, L.; Pinho, L.; Silva, S.; Figueira, V.; Félix, J.; Pinho, F.; Vilas-Boas, J.P.; Silva, A. Neurobiomechanical Characterization of Feedforward Phase of Gait Initiation in Chronic Stroke: A Linear and Non-Linear Approach. Appl. Sci. 2025, 15, 4762. [Google Scholar] [CrossRef]
- Spina, S.; Facciorusso, S.; Botticelli, C.; Intiso, D.; Ranieri, M.; Colamaria, A.; Fiore, P.; Ciritella, C.; Genêt, F.; Santamato, A. Ultrasonographic Evaluation of Three Approaches for Botulinum Toxin Injection into Tibialis Posterior Muscle in Chronic Stroke Patients with Equinovarus Foot: An Observational Study. Toxins 2021, 13, 829. [Google Scholar] [CrossRef]
- Verplancke, D.; Snape, S.; Salisbury, C.; Jones, P.; Ward, A. A randomized controlled trial of botulinum toxin on lower limb spasticity following acute acquired severe brain injury. Clin. Rehabil. 2005, 19, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Han, T.R.; Bang, M.S.; Chung, S.G. Rehabilitation Medicine, 6th ed.; Koonja Publishing Inc.: Paju, Republic of Korea, 2019. [Google Scholar]
- Jun, H.; Yoon, S.-H.; Ryu, M.; Chae, H.; Chu, H.; Leem, J.; Kim, T.-H. Acupotomy in Korean Medicine Doctors: A Preliminary Survey on Experiences, Perceptions, and Clinical Usage Status. Healthcare 2023, 11, 2577. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.; Cho, E.; Kim, S.; Oh, S.; Kim, S.; Park, J.; Kim, S. Performing ultrasound-guided pharmacopuncture and acupotomy for nerve entrapment in the upper extremity: A guide for teaching procedural skills. J. Acupunct. Res. 2024, 41, 135–141. [Google Scholar] [CrossRef]
- Kim, C.-H.; Moon, Y.-J. A clinical study of a stroke patient with a worsened gait pattern after discontinuing rehabilitation. J. Intern. Korean Med. 2017, 38, 118–124. [Google Scholar] [CrossRef]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef]
- Kim, C.H.; Chu, H.; Kang, G.H.; Sung, K.K.; Kang, D.G.; Lee, H.S.; Lee, S. Difference in gait recovery rate of hemiparetic patients with stroke according to paralyzed side: A cross-sectional study based on a retrospective chart review. Medicine 2019, 98, e18023. [Google Scholar] [CrossRef]
- Kim, C.-H.; Chu, H.; Kang, G.-H.; Kim, K.-H.; Lee, Y.-U.; Lim, H.-S.; Sung, K.-K.; Lee, S. Comparison of gait recovery patterns according to the paralyzed side in patients with stroke: An observational study based on a retrospective chart review (STROBE compliant). Medicine 2021, 100, e25212. [Google Scholar] [CrossRef]
- Harb, A.; Margetis, K.; Kishner, S. Modified Ashworth Scale. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554572/ (accessed on 5 May 2025).
- Yoon, S.-H.; Jo, H.-G.; Song, M.-Y. Post-stroke spasticity treated by miniscalpel-acupuncture: Three case report. J. Korean Med. Rehabil. 2018, 28, 145–152. [Google Scholar] [CrossRef]
- Li, S. Ankle and foot spasticity patterns in chronic stroke survivors with abnormal gait. Toxins 2020, 12, 646. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kim, J.-H.; Kim, J.-H. Effects of eccentric activation training of plantar flexors for the patients with stroke. J. Korean Soc. Neurother. 2020, 24, 33–39. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Q.X.; Lu, L.M.; Guo, Z.X.; Liu, H.; Zhang, L.Z.; Xiu, Z.B. Effect of acupotomy intervention on the morphology and ultrastructure of rectus femoris muscle in rabbits with knee osteoarthritis. Zhongguo Gu Shang 2022, 35, 281–286. [Google Scholar] [PubMed]
- Liu, F.-S.; Zhou, F.-Y.; Zhang, Y.; Guo, C.-Q. Effects of acupotomy therapy on mRNA expressions of Bcl-2, Bax, Caspase-3 in posteri-or cervical extensor muscles in cervical spondylosis rabbits. Zhen Ci Yan Jiu 2017, 42, 514–517. [Google Scholar]
- Lee, J.; Kim, J.; Kim, H.; Choi, S. Protocol for ultrasound-guided acupotomy procedure. J. Korean Med. Soc. Acupotomol. 2023, 7, 154–159. [Google Scholar] [CrossRef]
- Oh, H.-M.; Park, G.-Y.; Choi, Y.-M.; Koo, H.-J.; Jang, Y.; Im, S. The effects of botulinum toxin injections on plantar flexor spasticity in different phases after stroke: A secondary analysis from a double-blind, randomized trial. PM&R 2018, 10, 789–797. [Google Scholar]
- Ada, L.; O’Dwyer, N.; O’Neill, E. Relation between spasticity, weakness and contracture of the elbow flexors and upper limb activity after stroke: An observational study. Disabil. Rehabil. 2006, 28, 891–897. [Google Scholar] [CrossRef]
- Malhotra, S.; Pandyan, A.D.; Rosewilliam, S.; Roffe, C.; Hermens, H. Spasticity and contractures at the wrist after stroke: Time course of development and their association with functional recovery of the upper limb. Clin. Rehabil. 2011, 25, 184–191. [Google Scholar] [CrossRef]
- Dietz, V.; Sinkjaer, T. Spastic movement disorder: Impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007, 6, 725–733. [Google Scholar] [CrossRef]
- Levin, M.F.; Feldman, A.G.; Mullick, A.A.; Rodrigues, M.R.M. A New Standard in Objective Measurement of Spasticity. J. Med. Devices-Trans. Asme 2013, 7, 030909. [Google Scholar] [CrossRef]

| Generic Names | Dosages and Frequencies | |
|---|---|---|
| Case 1 | Choline alfoscerate 400 mg | 3 capsules, three times daily |
| Mosapride citrate hydrate 5.29 mg | 3 tablets, three times daily | |
| Acetyl-L-camitine hydrochloride 590 mg | 3 tablets, three times daily | |
| Amlodipine besylate 6.944 mg | 1 tablet, once daily | |
| Case 2 | Clopidogrel bisulfate 97.875 mg | 1 tablet, once daily |
| Atorvastatin calcium trihydrate 10.85 mg | 1 tablet, once daily |
| Variables | Explanation |
|---|---|
| Step length ratio | Step length represents the linear distance between the heel contacts of opposite feet during successive steps [8]. In patients with gait impairments, a reduced step length is often observed, reflecting compromised gait stability and increased energy demands [8]. As noted by Patterson et al. [9], step length is a critical variable for assessing gait symmetry after stroke, with the step length ratio providing a simplified metric; values closer to 1 suggest more symmetric gait patterns. |
| Walking velocity | Walking velocity, defined as the speed of ambulation over a given distance, is a primary indicator of functional recovery in individuals post-stroke [10]. Increases in walking velocity are associated with better rehabilitation outcomes and overall improvements in quality of life [10]. |
| Lateral symmetry | Lateral symmetry quantifies the medial–lateral displacement of the center of pressure (COP) during gait. Larger positive or negative values indicate greater deviations toward the right or left side, respectively [11]. In this study, shifts toward the nonparetic side were recorded as positive, while shifts toward the paretic side were recorded as negative, relative to the midline. A value approaching zero signifies improved lateral balance and gait symmetry [11]. |
| 95% confidence ellipse area | The 95% confidence ellipse area of the COP measures the dispersion of postural sway during quiet standing [11]. It represents the smallest ellipse encompassing 95% of COP trajectories [11]. A smaller ellipse area corresponds to enhanced postural stability and reduced sway amplitude [11]. |
| COP average velocity | COP average velocity describes the mean speed of center-of-pressure movement during a standing task [11]. Lower COP average velocities reflect greater postural control, indicating reduced instability and more efficient balance maintenance [11]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ahn, T.; Moon, J.; So, Y.; Cho, H.-g.; Ji, S.; Oh, M.; Lee, S.; Kim, C.-H. Restoring Biomechanical Gait Function with Ultrasound-Guided Acupotomy for Post-Stroke Equinovarus Foot: Two Case Reports and a Protocol (A CARE- and SPIRIT-Compliant Study). Life 2025, 15, 766. https://doi.org/10.3390/life15050766
Kim J, Ahn T, Moon J, So Y, Cho H-g, Ji S, Oh M, Lee S, Kim C-H. Restoring Biomechanical Gait Function with Ultrasound-Guided Acupotomy for Post-Stroke Equinovarus Foot: Two Case Reports and a Protocol (A CARE- and SPIRIT-Compliant Study). Life. 2025; 15(5):766. https://doi.org/10.3390/life15050766
Chicago/Turabian StyleKim, Jiwoo, Taeseok Ahn, Jihyun Moon, Youngjo So, Hyeon-gyu Cho, Sangho Ji, Myungjin Oh, Sangkwan Lee, and Cheol-Hyun Kim. 2025. "Restoring Biomechanical Gait Function with Ultrasound-Guided Acupotomy for Post-Stroke Equinovarus Foot: Two Case Reports and a Protocol (A CARE- and SPIRIT-Compliant Study)" Life 15, no. 5: 766. https://doi.org/10.3390/life15050766
APA StyleKim, J., Ahn, T., Moon, J., So, Y., Cho, H.-g., Ji, S., Oh, M., Lee, S., & Kim, C.-H. (2025). Restoring Biomechanical Gait Function with Ultrasound-Guided Acupotomy for Post-Stroke Equinovarus Foot: Two Case Reports and a Protocol (A CARE- and SPIRIT-Compliant Study). Life, 15(5), 766. https://doi.org/10.3390/life15050766







