Propolis Stands out as a Multifaceted Natural Product: Meta-Analysis on Its Sources, Bioactivities, Applications, and Future Perspectives
Abstract
1. Introduction
2. Methodology
3. Composition of Propolis and Extractive Procedures
- Poplar (China, New Zealand, Europe, and America) originates mainly from the bud exudates of Populus species [31]. It is rich in flavonoids such as chrysin, galangin, and pinocembrin.
- Birch (Russia) originates from Betula verrucosa Ehrh, and is characterized by high levels of phenolic acids and their esters [5].
- Mediterranean (Malta, Sicily, Crete, and Greece) is mainly collected from the resin of Cupressus sempervirens, and is noted for its abundance of diterpenes [6].
- Green (Southeastern Brazil) is derived from Baccharis dracunculifolia [32], distinguished by its high content of prenylated phenolic compounds such as artepillin C.
- Red (Southeast Mexico, Northeastern Brazil, and Cuba) is collected from resins of Dalbergia ecastaphyllum [33], and is rich in isoflavonoids and polyphenolic compounds.
- Brown (some regions of Brazil, Venezuela, and Cuba) is collected from the resins of Clusia species and Copaifera species [34], characterized by the presence of benzophenones and polyisoprenylated benzophenones.
4. Biological Activities of Propolis
4.1. Antioxidant Activity
4.2. Antimicrobial Activity
4.2.1. Antibacterial Activity
| Country/Region | Solvent | Major Components | Method | Bacteria Tested | Key Results | Ref. |
|---|---|---|---|---|---|---|
| Poland | Ethanol or propylene glycol | / | Agar well diffusion. | E. coli, S. aureus | Propolis extracts in ethanol or propylene glycol showed antibacterial activity against S. aureus. Both extracts exhibited similar activity against S. aureus. | [12] |
| China | Ethanol | flavonoids as galangin, pinocembrin, and pinobanksin. | Disk diffusion | Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Listeria monocytogenes | All the extracts showed high antimicrobial activity against S. aureus, L. monocytogenes and B. subtilis, but no effect on E. coli | [60] |
| Brazil | Ethanol | The flavonoids and aromatic compounds. | Microdilution | E. coli, S. aureus | The extracts demonstrated activity against S. aureus and E. coli, while the activity was higher against S. aureus | [84] |
| Algeria | Ethanol | Phenolics and flavonoids. | Disk diffusion | S. aureus, Bacillus cereus, E. coli, Pseudomonas aeruginosa | Ethanolic extract of Algerian propolis samples inhibited growth of all examined microorganisms with the highest activity against Gram-positive bacteria | [97] |
| Romania | Water | / | Well diffusion. Microdilution | P. aeruginosa, E. coli, S. aureus, B. cereus | Inhibitory zones of samples varied from 7 to 17 mm compared to ciprofloxacin with inhibitory zones between 25 and 30 mm. Synergistic interaction between propolis and honey | [98] |
| India | Ethanol | / | Microdilution | Multidrug-resistant bacteria, Acinetobacter sp., Enterobacter sp., Strenotrophomonas sp., | Twenty antibiotics showed low inhibitory zones due to resistance. Combination of propolis extract with phenethyl caffeate, chrysin and galangin enhanced the antibacterial activity | [99] |
| Morocco | Ethanol | Flavonoids enriched | Agar well diffusion. Microdilution | Gram-positive and Gram-negative | Gram-positive bacteria were more sensitive than Gram-negative. Variation in activity depends on the propolis origin. Inhibitory zones of propolis varied from 10 to 22.5 mm compared to chloramphenicol with inhibitory zones between 19 and 37 mm. MIC values ranged from 0.15 to 5 mg/mL, and MBC values varied from 1.25 to 5 mg/mL. Chloramphenicol showed MIC and MBC values between 0.0002 and 0.064 mg/mL | [100] |
| Europe | Ethanol or water | Phenolic acid esters and Flavonoids | Microdilution. Checkerboard. Dilution and time-kill curve assays | 32 reference strains (Gram-positive, Gram-negative and fungi). One strain of methicillin-resistant S. aureus (MRSA). One strain of vancomycin-resistant enterococci (VRE) | All samples showed moderate activity against Gram-positive. Ethanol-based propolis extracts generally demonstrated moderate effectiveness against Gram-negative bacteria, whereas aqueous extracts exhibited lower activity against these microorganisms. The propolis extract synergistically enhanced the efficacy of antibiotics | [101] |
| Turkey | Ethanol | Higher phenolics and flavonoids contents. | Agar well diffusion. Microdilution | S. aureus, E. coli, P. aeruginosa. | Propolis extract was more potent in inhibiting S. aureus than E. coli. Propolis extracts showed no activity against P. aeruginosa | [102] |
| Iran | Ethanol | / | Agar dilution | S. aureus, Streptococcus mutans, Lactobacillus acidophilus, Enterococcus faecalis | The lowest MIC values were scored for S. aureus, while the highest for L. acidophilus | [103] |
| Egypt, China, Bulgaria, Span, Australia, Greece, Italy, Canada | Ethanol | Various phenolics and flavonoids | Microdilution | S. aureus, E. coli | All propolis samples showed an inhibition in the growth of all examined microorganisms, but the inhibition varied depending on propolis origin. Propolis from Canada and Egypt showed the highest activity against S. aureus, while the propolis from Spain, Greece and Egypt was strongest against E. coli | [104] |
4.2.2. Antiparasitic Activity
4.2.3. Antifungal Activity
4.2.4. Antiviral Activity
4.3. Anti-Inflammatory Activity
4.4. Immunomodulatory Activity
4.5. Antidiabetic Activity
4.6. Wound Healing Activity
4.7. Anticancer Activity
5. Examples of Paramedical Products Based on Propolis
- Propolis is available in multiple forms, such as tinctures, capsules, sprays, and topical creams. For example, Beelife offers a Green Propolis Extract derived from Brazilian field rosemary, known for its high levels of artepillin-C, a phenolic acid associated with health benefits.
- Beekeeper’s Naturals offers a Propolis Throat Spray aimed at supporting immune health and soothing sore throats. Propolis contains compounds like flavonoids and phenolic acids, which exhibit anti-inflammatory and antimicrobial properties, providing relief from throat irritation and boosting the body’s defense mechanisms against infections [216,369].
- In skincare, propolis is valued for its potential to promote wound healing, reduce acne, and provide antioxidant benefits, making it a popular ingredient in serums and creams. Its antimicrobial and anti-inflammatory properties make it effective for both minor wounds and acne outbreaks. In wound care, propolis accelerates tissue regeneration and reduces infection rates, making it a common component in ointments and creams for cuts, burns, and skin abrasions. Similarly, its antimicrobial effects help prevent the growth of acne-causing bacteria, while its anti-inflammatory properties reduce redness and swelling, promoting faster healing of acne lesions. Propolis extracts have been shown to be particularly effective in treating inflammatory acne and are frequently included in acne treatment serums, gels, and creams [283].
- In dentistry, the antimicrobial effects on oral pathogens, as well as the anti-inflammatory activity of propolis, lead to its use for treating aphthous stomatitis, oral mucositis, acute necrotizing ulcerative gingivitis, pulpitis, gingivitis, and periodontitis. Propolis can be found in toothpaste, mouthwashes, and lozenges, aimed at improving oral hygiene and reducing inflammation and discomfort in the mouth [368].
- In ulcer diseases, propolis may serve as a successful anti-ulcerogenic agent, contributing to the development of novel phytotherapeutic approaches for treating gastric ulcers. Research has indicated that propolis exerts an anti-ulcerogenic effect by reducing gastric acid secretion and enhancing mucosal defense [370,371,372].
- Propolis has been used in veterinary care for treating wounds and infections in animals, as well as enhancing immune responses, although more clinical validation is needed.
6. Conclusions and Future Perspectives
- Antimicrobial: Effective against various bacteria, fungi, and viruses, owing to its rich content of flavonoids and phenolics.
- Antioxidant: The presence of polyphenols enables propolis to neutralize free radicals, protecting cells from oxidative stress.
- Anti-inflammatory: Propolis can modulate inflammatory responses, making it beneficial for managing conditions associated with inflammation.
- Immuno-modulatory: Propolis can enhance immune system activity, potentially aiding in the prevention of certain infections.
- Healthcare: Utilized throat sprays, lozenges, and supplements for its antimicrobial and soothing properties. For example, Beekeeper’s Naturals offers a Propolis Throat Spray aimed at supporting immune health.
- Skincare: Valued for its potential to promote wound healing, reduce acne, and provide antioxidant benefits, propolis is a popular ingredient in serums and creams.
- Veterinary medicine: Employed in products designed to enhance animal health due to its antimicrobial and healing properties.
- Standardization: The variability in propolis composition necessitates standardized extraction and formulation methods to ensure consistent efficacy and safety.
- Clinical research: Robust clinical trials are required to substantiate the therapeutic claims of propolis and fully understand its mechanisms of action.
- Regulatory frameworks: Establishing clear guidelines will facilitate the safe integration of propolis into mainstream healthcare and consumer products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, M.; Fan, T.; Watson, D.; Alenezi, S.; Saleh, K.; Sahlan, M. Preliminary Studies: The Potential Anti-Angiogenic Activities of Two Sulawesi Island (Indonesia) Propolis and Their Chemical Characterization. Heliyon 2019, 5, e01978. [Google Scholar] [CrossRef] [PubMed]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Buñay-Noboa, J.; Marceau, G.; Sapin, V.; Zellagui, A.; Baron, S.; Lahouel, M.; Lobaccaro, J.-M.A. Ethanolic Extract of Algerian Propolis Decreases Androgen Receptor Transcriptional Activity in Cultured LNCaP Cells. J. Steroid Biochem. Mol. Biol. 2019, 189, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Propolis: A Review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.-M.A. Biological Properties of Propolis Extracts: Something New from an Ancient Product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.; Umsza-Guez, M.A.; Barbosa, J.D.; Portela, R.D.; Machado, B.A. Propolis: Types, Composition, Biological Activities, and Veterinary Product Patent Prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef]
- Stojanović, S.; Najman, S.J.; Bogdanova-Popov, B.; Najman, S.S. Propolis: Chemical composition, biological and pharmacological activity-A review. Acta Med. Median. 2020, 59, 108–113. [Google Scholar] [CrossRef]
- Khalil, M.L. Biological Activity of Bee Propolis in Health and Disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Sung, S.-H.; Choi, G.-H.; Lee, N.-W.; Shin, B.-C. External Use of Propolis for Oral, Skin, and Genital Diseases: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2017, 2017, 8025752. [Google Scholar] [CrossRef]
- Havsteen, B. Flavonoids, a Class of Natural Products of High Pharmacological Potency. Biochem. Pharmacol. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the Biological Properties and Toxicity of Bee Propolis (Propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical Composition, Biological Properties and Therapeutic Activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Propolis: A New Frontier for Wound Healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Doğan, H.; Silici, S.; Ozcimen, A.A. Biological Effects of Propolis on Cancer. Turk. J. Agric. Food Sci. Technol. 2020, 8, 573–579. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Tani, H.; Hikami, S.; Takahashi, S.; Kimura, Y.; Matsuura, N.; Nakamura, T.; Yamaga, M.; Koshino, H. Isolation, Identification, and Synthesis of a New Prenylated Cinnamic Acid Derivative from Brazilian Green Propolis and Simultaneous Quantification of Bioactive Components by LC-MS/MS. J. Agric. Food Chem. 2019, 67, 12303–12312. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Hotta, S.; Uchiyama, S.; Ichihara, K. Brazilian Red Propolis Extract Enhances Expression of Antioxidant Enzyme Genes in Vitro and in Vivo. Biosci. Biotechnol. Biochem. 2020, 84, 1820–1830. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Md Lazin, M.R.M.L.; Mohamed, M. Antioxidant, Anti-Inflammatory and Synergistic Anti-Hyperglycemic Effects of Malaysian Propolis and Metformin in Streptozotocin–Induced Diabetic Rats. Food Chem. Toxicol. 2018, 120, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Ripari, N.; Sartori, A.A.; Da Silva Honorio, M.; Conte, F.L.; Tasca, K.I.; Santiago, K.B.; Sforcin, J.M. Propolis Antiviral and Immunomodulatory Activity: A Review and Perspectives for COVID-19 Treatment. J. Pharm. Pharmacol. 2021, 73, 281–299. [Google Scholar] [CrossRef]
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; Da Cunha, M.G.; Colón, D.F.; De Alencar, S.M.; Rosalen, P.L. The Use of Brazilian Propolis for Discovery and Development of Novel Anti-Inflammatory Drugs. Eur. J. Med. Chem. 2018, 153, 49–55. [Google Scholar] [CrossRef]
- De Mendonça, I.C.G.; Porto, I.C.C.D.M.; Do Nascimento, T.G.; De Souza, N.S.; Oliveira, J.M.D.S.; Arruda, R.E.D.S.; Mousinho, K.C.; Dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian Red Propolis: Phytochemical Screening, Antioxidant Activity and Effect against Cancer Cells. BMC Complement. Med. Ther. 2015, 15, 357. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and Functional Properties of Propolis (Bee Glue): A Review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Mateo, S. Procédure pour conduire avec succès une revue de littérature selon la méthode PRISMA. Kinésithérapie Rev. 2020, 20, 29–37. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, Q.; Qin, Y.; Huang, D.; Liao, Y. Agricultural Drought Research Knowledge Graph Reasoning by Using VOSviewer. Heliyon 2024, 10, e27696. [Google Scholar] [CrossRef]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef]
- Bhargava, P.; Mahanta, D.; Kaul, A.; Ishida, Y.; Terao, K.; Wadhwa, R.; Kaul, S.C. Experimental Evidence for Therapeutic Potentials of Propolis. Nutrients 2021, 13, 2528. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated Methods for the Quantification of Biologically Active Constituents of Poplar-type Propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian Red Propolis: Unreported Substances, Antioxidant and Antimicrobial Activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rastrelli, L. Cuban and Brazilian Red Propolis: Botanical Origin and Comparative Analysis by High-Performance Liquid Chromatography–Photodiode Array Detection/Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef]
- Sawaya, A.C.H.F.; Cunha, I.B.S.; Marcucci, M.C.; De Oliveira Rodrigues, R.F.; Eberlin, M.N. Brazilian Propolis of Tetragonisca angustula and Apis mellifera. Apidologie 2006, 37, 398–407. [Google Scholar] [CrossRef]
- Alday, E.; Valencia, D.; Garibay-Escobar, A.; Domínguez-Esquivel, Z.; Piccinelli, A.L.; Rastrelli, L.; Monribot-Villanueva, J.; Guerrero-Analco, J.A.; Robles-Zepeda, R.E.; Hernandez, J.; et al. Plant Origin Authentication of Sonoran Desert Propolis: An Antiproliferative Propolis from a Semi-Arid Region. Sci. Nat. 2019, 106, 25. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Teixeira, É.W.; Message, D.; Negri, G.; Salatino, A.; Stringheta, P.C. Seasonal Variation, Chemical Composition and Antioxidant Activity of Brazilian Propolis Samples. Evid.-Based Complement. Altern. Med. 2010, 7, 307–315. [Google Scholar] [CrossRef]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent Advances in Chemistry and Plant Origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Bankova, V. Recent Trends and Important Developments in Propolis Research. Evid.-Based Complement. Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef]
- Cantarelli, M.Á.; Camiña, J.M.; Pettenati, E.M.; Marchevsky, E.J.; Pellerano, R.G. Trace Mineral Content of Argentinean Raw Propolis by Neutron Activation Analysis (NAA): Assessment of Geographical Provenance by Chemometrics. LWT Food Sci. Technol. 2011, 44, 256–260. [Google Scholar] [CrossRef]
- Silici, S.; Koç, N.A.; Ayangil, D.; Cankaya, S. Antifungal Activities of Propolis Collected by Different Races of Honeybees against Yeasts Isolated from Patients with Superficial Mycoses. J. Pharmacol. Sci. 2005, 99, 39–44. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Garcίa-Viguera, C.; Bankova, V.S.; De Castro, S.L.; Dantas, A.P.; Valente, P.H.M.; Paulino, N. Phenolic Compounds from Brazilian Propolis with Pharmacological Activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Ristivojević, P.; Trifković, J.; Dastan, T.; Yilmaz, O.; Cengiz, O.; Yesilada, E. Authentication of Turkish Propolis through HPTLC Fingerprints Combined with Multivariate Analysis and Palynological Data and Their Comparative Antioxidant Activity. LWT 2018, 87, 23–32. [Google Scholar] [CrossRef]
- Bozkuş, T.N.; Değer, O.; Yaşar, A. Chemical Characterization of Water and Ethanolic Extracts of Turkish Propolis by HPLC-DAD and GC-MS. J. Liq. Chromatogr. Relat. Technol. 2021, 44, 77–86. [Google Scholar] [CrossRef]
- Belmehdi, O.; El Menyiy, N.; Bouyahya, A.; El Baaboua, A.; El Omari, N.; Gallo, M.; Montesano, D.; Naviglio, D.; Zengin, G.; Skali Senhaji, N.; et al. Recent Advances in the Chemical Composition and Biological Activities of Propolis. Food Rev. Int. 2023, 39, 6078–6128. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Cottica, S.M.; Sabik, H.; Antoine, C.; Fortin, J.; Graveline, N.; Visentainer, J.V.; Britten, M. Characterization of Canadian Propolis Fractions Obtained from Two-Step Sequential Extraction. LWT Food Sci. Technol. 2015, 60, 609–614. [Google Scholar] [CrossRef]
- Jug, M.; Končić, M.Z.; Kosalec, I. Modulation of Antioxidant, Chelating and Antimicrobial Activity of Poplar Chemo-Type Propolis by Extraction Procures. LWT Food Sci. Technol. 2014, 57, 530–537. [Google Scholar] [CrossRef]
- Taddeo, V.A.; Epifano, F.; Fiorito, S.; Genovese, S. Comparison of Different Extraction Methods and HPLC Quantification of Prenylated and Unprenylated Phenylpropanoids in Raw Italian Propolis. J. Pharm. Biomed. Anal. 2016, 129, 219–223. [Google Scholar] [CrossRef]
- Yen, C.-H.; Chiu, H.-F.; Wu, C.-H.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Beneficial Efficacy of Various Propolis Extracts and Their Digestive Products by in Vitro Simulated Gastrointestinal Digestion. LWT 2017, 84, 281–289. [Google Scholar] [CrossRef]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis Extract and Its Bioactive Compounds—From Traditional to Modern Extraction Technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Trusheva, B.; Popova, M. Propolis Extraction Methods: A Review. J. Apic. Res. 2021, 60, 734–743. [Google Scholar] [CrossRef]
- Pellati, F.; Prencipe, F.P.; Bertelli, D.; Benvenuti, S. An Efficient Chemical Analysis of Phenolic Acids and Flavonoids in Raw Propolis by Microwave-Assisted Extraction Combined with High-Performance Liquid Chromatography Using the Fused-Core Technology. J. Pharm. Biomed. Anal. 2013, 81, 126–132. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High Hydrostatic Pressure and Ultrasound Extractions of Antioxidant Compounds, Sulforaphane and Fatty Acids from Chilean Papaya (Vasconcellea pubescens) Seeds: Effects of Extraction Conditions and Methods. LWT Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Cavalaro, R.I.; Cruz, R.G.D.; Dupont, S.; De Moura Bell, J.M.L.N.; Vieira, T.M.F.D.S. In Vitro and in Vivo Antioxidant Properties of Bioactive Compounds from Green Propolis Obtained by Ultrasound-Assisted Extraction. Food Chem. X 2019, 4, 100054. [Google Scholar] [CrossRef]
- Sambou, M.; Jean-François, J.; Ndongou Moutombi, F.J.; Doiron, J.A.; Hébert, M.P.A.; Joy, A.P.; Mai-Thi, N.-N.; Barnett, D.A.; Surette, M.E.; Boudreau, L.H.; et al. Extraction, Antioxidant Capacity, 5-Lipoxygenase Inhibition, and Phytochemical Composition of Propolis from Eastern Canada. Molecules 2020, 25, 2397. [Google Scholar] [CrossRef]
- Reis, J.H.D.O.; Barreto, G.D.A.; Cerqueira, J.C.; Anjos, J.P.D.; Andrade, L.N.; Padilha, F.F.; Druzian, J.I.; Machado, B.A.S. Evaluation of the Antioxidant Profile and Cytotoxic Activity of Red Propolis Extracts from Different Regions of Northeastern Brazil Obtained by Conventional and Ultrasound-Assisted Extraction. PLoS ONE 2019, 14, e0219063. [Google Scholar] [CrossRef]
- Khacha-ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Antioxidant and Anti-Cancer Cell Proliferation Activity of Propolis Extracts from Two Extraction Methods. Asian Pac. J. Cancer Prev. 2013, 14, 6991–6995. [Google Scholar] [CrossRef]
- Ding, Q.; Sheikh, A.R.; Gu, X.; Li, J.; Xia, K.; Sun, N.; Wu, R.A.; Luo, L.; Zhang, Y.; Ma, H. Chinese Propolis: Ultrasound-assisted Enhanced Ethanolic Extraction, Volatile Components Analysis, Antioxidant and Antibacterial Activity Comparison. Food Sci. Nutr. 2021, 9, 313–330. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Morelli, L.L.L.; Prado, M.A. Extraction Optimization for Antioxidant Phenolic Compounds in Red Grape Jam Using Ultrasound with a Response Surface Methodology. Ultrason. Sonochem. 2012, 19, 1144–1149. [Google Scholar] [CrossRef]
- Oliver, C.M.; Mawson, R.; Melton, L.D.; Dumsday, G.; Welch, J.; Sanguansri, P.; Singh, T.K.; Augustin, M.A. Sequential Low and Medium Frequency Ultrasound Assists Biodegradation of Wheat Chaff by White Rot Fungal Enzymes. Carbohydr. Polym. 2014, 111, 183–190. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen Toxicity, Oxygen Radicals, Transition Metals and Disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Botelho, C.; Rocha, C.; António Teixeira, J. Valorization of Natural Antioxidants for Nutritional and Health Applications. In Antioxidants-Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-864-5. [Google Scholar]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.P. In Vitro Methods of Assay of Antioxidants: An Overview. Food Rev. Int. 2008, 24, 392–415. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid.-Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef]
- Bittencourt, M.L.F.; Ribeiro, P.R.; Franco, R.L.P.; Hilhorst, H.W.M.; De Castro, R.D.; Fernandez, L.G. Metabolite Profiling, Antioxidant and Antibacterial Activities of Brazilian Propolis: Use of Correlation and Multivariate Analyses to Identify Potential Bioactive Compounds. Food Res. Int. 2015, 76, 449–457. [Google Scholar] [CrossRef]
- Narimane, S.; Demircan, E.; Salah, A.; Ozcelik, B.Ö.; Salah, R. Correlation between Antioxidant Activity and Phenolic Acids Profile and Content of Algerian Propolis: Influence of Solvent. Pak. J. Pharm. Sci. 2017, 30, 1417–1423. [Google Scholar]
- Andrade, J.K.S.; Denadai, M.; De Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of Bioactive Compounds Potential and Antioxidant Activity of Brown, Green and Red Propolis from Brazilian Northeast Region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Chen, J.; Jiang, X.; Hu, F. Identification of Free Radical Scavengers from Brazilian Green Propolis Using Off-Line HPLC-DPPH Assay and LC-MS. J. Food Sci. 2017, 82, 1602–1607. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abd El Hady, F.K.; Abd Allah, F.A.M. Chemical Composition and Antimicrobial Activity of European Propolis. Z. Naturforsch. C 2000, 55, 70–75. [Google Scholar] [CrossRef]
- Naik, R.R.; Shakya, A.K.; Oriquat, G.A.; Katekhaye, S.; Paradkar, A.; Fearnley, H.; Fearnley, J. Fatty Acid Analysis, Chemical Constituents, Biological Activity and Pesticide Residues Screening in Jordanian Propolis. Molecules 2021, 26, 5076. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; Da Silva, P.F.; Azevedo, L.F.; Da Rocha, L.G.; De Moraes Porto, I.C.C.; Lima E Moura, T.F.A.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; Fonseca, E.J.D.S.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res. Lett. 2016, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Y.; Niu, F.; Li, Z.; Ba, C.; Jin, B.; Chen, G.; Li, X. Enhanced Antioxidant Activity and in Vitro Release of Propolis by Acid-Induced Aggregation Using Heat-Denatured Zein and Carboxymethyl Chitosan. Food Hydrocoll. 2018, 81, 104–112. [Google Scholar] [CrossRef]
- Benković, V.; Orsolić, N.; Knežević, A.H.; Ramić, S.; Ðikić, D.; Bašić, I.; Kopjar, N. Evaluation of the Radioprotective Effects of Propolis and Flavonoids in Gamma-Irradiated Mice: The Alkaline Comet Assay Study. Biol. Pharm. Bull. 2008, 31, 167–172. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Zullkiflee, N.; Zaini, S.N.Z.; Taha, H.; Hashim, F.; Usman, A. Phytochemicals, Mineral Contents, Antioxidants, and Antimicrobial Activities of Propolis Produced by Brunei Stingless Bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J. Biol. Sci. 2020, 27, 2902–2911. [Google Scholar] [CrossRef]
- Daleprane, J.B.; Abdalla, D.S. Emerging Roles of Propolis: Antioxidant, Cardioprotective, and Antiangiogenic Actions. Evid.-Based Complement. Altern. Med. 2013, 2013, 175135. [Google Scholar] [CrossRef]
- El-ghazaly, M.A.; Abd El-Naby, D.H.; Khayyal, M.T. The Influence of Irradiation on the Potential Chondroprotective Effect of Aqueous Extract of Propolis in Rats. Int. J. Radiat. Biol. 2011, 87, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Unraveling the Chemical Composition, Antioxidant, α-Amylase and α-Glucosidase Inhibition of Moroccan Propolis. Food Biosci. 2021, 42, 101160. [Google Scholar] [CrossRef]
- Barrientos, L.; Herrera, C.L.; Montenegro, G.; Ortega, X.; Veloz, J.; Alvear, M.; Cuevas, A.; Saavedra, N.; Salazar, L.A. Chemical and Botanical Characterization of Chilean Propolis and Biological Activity on Cariogenic Bacteria Streptococcus Mutans and Streptococcus Sobrinus. Braz. J. Microbiol. 2013, 44, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Devequi-Nunes, D.; Machado, B.A.S.; Barreto, G.D.A.; Rebouças Silva, J.; Da Silva, D.F.; Da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical Characterization and Biological Activity of Six Different Extracts of Propolis through Conventional Methods and Supercritical Extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- Johnston, J.E.; Sepe, H.A.; Miano, C.L.; Brannan, R.G.; Alderton, A.L. Honey Inhibits Lipid Oxidation in Ready-to-Eat Ground Beef Patties. Meat Sci. 2005, 70, 627–631. [Google Scholar] [CrossRef]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological Activities and Chemical Composition of Three Honeys of Different Types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; Da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; I Falcão, S.; Isla, M.I.; Moreno, M.I.N.; et al. Standard Methods for Apis Mellifera Propolis Research. J. Apic. Res. 2019, 58, 1–49. [Google Scholar] [CrossRef]
- Bonamigo, T.; Campos, J.F.; Oliveira, A.S.; Torquato, H.F.V.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; De Picoli Souza, K.; Dos Santos, E.L. Antioxidant and Cytotoxic Activity of Propolis of Plebeia droryana and Apis mellifera (Hymenoptera, Apidae) from the Brazilian Cerrado Biome. PLoS ONE 2017, 12, e0183983. [Google Scholar] [CrossRef]
- Calegari, M.A.; Prasniewski, A.; Silva, C.D.; Sado, R.Y.; Maia, F.M.C.; Tonial, L.M.S.; Oldoni, T.L.C. Propolis from Southwest of Parana Produced by Selected Bees: Influence of Seasonality and Food Supplementation on Antioxidant Activity and Phenolic Profile. An. Acad. Bras. Ciências 2017, 89, 45–55. [Google Scholar] [CrossRef]
- De Lima, G.G.; De Souza, R.O.; Bozzi, A.D.; Poplawska, M.A.; Devine, D.M.; Nugent, M.J.D. Extraction Method Plays Critical Role in Antibacterial Activity of Propolis-Loaded Hydrogels. J. Pharm. Sci. 2016, 105, 1248–1257. [Google Scholar] [CrossRef]
- De Zordi, N.; Cortesi, A.; Kikic, I.; Moneghini, M.; Solinas, D.; Innocenti, G.; Portolan, A.; Baratto, G.; Dall’Acqua, S. The Supercritical Carbon Dioxide Extraction of Polyphenols from Propolis: A Central Composite Design Approach. J. Supercrit. Fluids 2014, 95, 491–498. [Google Scholar] [CrossRef]
- Lopes, A.A.; Ferreira, T.S.; Nesi, R.T.; Lanzetti, M.; Pires, K.M.P.; Silva, A.M.; Borges, R.M.; Silva, A.J.R.; Valença, S.S.; Porto, L.C. Antioxidant Action of Propolis on Mouse Lungs Exposed to Short-Term Cigarette Smoke. Bioorganic Med. Chem. 2013, 21, 7570–7577. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, C.C.S.D.M.; Fernandes, L.M.P.; Silva, M.L.; Luz, D.A.; Gomes, A.R.Q.; Monteiro, M.C.; Machado, C.S.; Torres, Y.R.; Lira, T.O.D.; Ferreira, A.G.; et al. Neurobehavioral and Antioxidant Effects of Ethanolic Extract of Yellow Propolis. Oxid. Med. Cell. Longev. 2016, 2016, 2906953. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Hayami, S.; Haruna, S.; Ogiri, Y.; Tanaka, K.; Yamada, Y.; Ikeda, K.; Yamada, H.; Sugimoto, H.; Kawai, N.; et al. In Vivo Antioxidative Activity of Propolis Evaluated by the Interaction with Vitamins C and E and the Level of Lipid Hydroperoxides in Rats. J. Agric. Food Chem. 2000, 48, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Remirez, D.; González, R.; Rodriguez, S.; Ancheta, O.; Bracho, J.C.; Rosado, A.; Rojas, E.; Ramos, M.E. Protective Effects of Propolis Extract on Allyl Alcohol-Induced Liver Injury in Mice. Phytomedicine 1997, 4, 309–314. [Google Scholar] [CrossRef]
- De Lima, R.O.A.; Bazo, A.P.; Said, R.A.; Sforcin, J.M.; Bankova, V.; Darros, B.R.; Salvadori, D.M.F. Modifying Effect of Propolis on Dimethylhydrazine-Induced DNA Damage but Not Colonic Aberrant Crypt Foci in Rats. Environ. Mol. Mutagen. 2005, 45, 8–16. [Google Scholar] [CrossRef]
- Nedji, N.; Loucif-Ayad, W. Antimicrobial Activity of Algerian Propolis in Foodborne Pathogens and Its Quantitative Chemical Composition. Asian Pac. J. Trop. Dis. 2014, 4, 433–437. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedűş-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungău, S. The Antimicrobial Activity of Honey and Propolis Extracts from the Central Region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Nandre, V.S.; Bagade, A.V.; Kasote, D.M.; Lee, J.H.J.; Kodam, K.M.; Kulkarni, M.V.; Ahmad, A. Antibacterial Activity of Indian Propolis and Its Lead Compounds against Multi-Drug Resistant Clinical Isolates. J. Herb. Med. 2021, 29, 100479. [Google Scholar] [CrossRef]
- Belmehdi, O.; Bouyahya, A.; Jekő, J.; Cziáky, Z.; Zengin, G.; Sotkó, G.; El Baaboua, A.; Skali Senhaji, N.; Abrini, J. Chemical Analysis, Antibacterial, and Antioxidant Activities of Flavonoid-rich Extracts from Four Moroccan Propolis. J. Food Process. Preserv. 2021, 45, e15816. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef]
- Kahraman-Ilıkkan, Ö. Bacterial Profile and Fatty Acid Composition of Anatolian Bee Bread Samples by Metataxonomic and Metabolomic Approach. Curr. Microbiol. 2023, 80, 90. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, R.; Ghaiour, M.; Abbasi, S. Evaluation of Antibacterial Effect of Propolis and Its Application in Mouthwash Production. Front. Dent. 2019, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, A.G.; El-Fadaly, H.A.; Barakat, A.M.; Abou-El-Doubal, S.K.A. In Vitro Effects of Some Bee Products on T. gondii Tachyzoites. Glob. Vet. 2015, 13, 1043–1050. [Google Scholar]
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; ISBN 978-1-68440-066-9. [Google Scholar]
- Boufadi, Y.M.; Soubhye, J.; Riazi, A.; Rousseau, A.; Vanhaeverbeek, M.; Nève, J.; Boudjeltia, K.Z.; Van Antwerpen, P. Characterization and Antioxidant Properties of Six Algerian Propolis Extracts: Ethyl Acetate Extracts Inhibit Myeloperoxidase Activity. Int. J. Mol. Sci. 2014, 15, 2327–2345. [Google Scholar] [CrossRef]
- Seidel, V.; Peyfoon, E.; Watson, D.G.; Fearnley, J. Comparative Study of the Antibacterial Activity of Propolis from Different Geographical and Climatic Zones. Phytother. Res. 2008, 22, 1256–1263. [Google Scholar] [CrossRef]
- Muli, E.M.; Maingi, J.M. Antibacterial Activity of Apis Mellifera L. Propolis Collected in Three Regions of Kenya. J. Venom. Anim. Toxins Incl. Trop. Dis. 2007, 13, 655–663. [Google Scholar] [CrossRef]
- Mohan, P.V.M.U.; Uloopi, K.; Vinay, C.; Rao, R. In Vivo Comparison of Cavity Disinfection Efficacy with APF Gel, Propolis, Diode Laser, and 2% Chlorhexidine in Primary Teeth. Contemp. Clin. Dent. 2016, 7, 45. [Google Scholar] [CrossRef]
- Falcão, S.I.; Vale, N.; Cos, P.; Gomes, P.; Freire, C.; Maes, L.; Vilas-Boas, M. In Vitro Evaluation of Portuguese Propolis and Floral Sources for Antiprotozoal, Antibacterial and Antifungal Activity. Phytother. Res. 2014, 28, 437–443. [Google Scholar] [CrossRef]
- Xie, X.-L.; Gi, M.; Fujioka, M.; Doi, K.; Yamano, S.; Tachibana, H.; Fang, H.; Kakehashi, A.; Wanibuchi, H. Ethanol-Extracted Propolis Enhances BBN-Initiated Urinary Bladder Carcinogenesis via Non-Mutagenic Mechanisms in Rats. Food Chem. Toxicol. 2015, 83, 193–200. [Google Scholar] [CrossRef]
- Regueira-Neto, M.D.S.; Tintino, S.R.; Rolón, M.; Coronal, C.; Vega, M.C.; De Queiroz Balbino, V.; De Melo Coutinho, H.D. Antitrypanosomal, Antileishmanial and Cytotoxic Activities of Brazilian Red Propolis and Plant Resin of Dalbergia ecastaphyllum (L) Taub. Food Chem. Toxicol. 2018, 119, 215–221. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Sorkun, K.; Houcine, S.; Tsvetkova, I.; Kujumgiev, A. Propolis from the Mediterranean Region: Chemical Composition and Antimicrobial Activity. Z. Naturforsch. C 2000, 55, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antibacterial Activity of Turkish Propolis and Its Qualitative and Quantitative Chemical Composition. Phytomedicine 2005, 12, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Afrouzan, H.; Zakeri, S.; Abouie Mehrizi, A.; Molasalehi, S.; Tahghighi, A.; Shokrgozar, M.A.; Es-Haghi, A.; Dinparast Djadid, N. Anti-Plasmodial Assessment of Four Different Iranian Propolis Extracts. Arch. Iran. Med. 2017, 20, 270–281. [Google Scholar]
- Boufadi, Y.M.; Soubhye, J.; Nève, J.; Van Antwerpen, P.; Riazi, A. Antimicrobial Effects of Six Algerian Propolis Extracts. Int. J. Food Sci. Technol. 2016, 51, 2613–2620. [Google Scholar] [CrossRef]
- Kartal, M.; Yildiz, S.; Kaya, S.; Kurucu, S.; Topçu, G. Antimicrobial Activity of Propolis Samples from Two Different Regions of Anatolia. J. Ethnopharmacol. 2003, 86, 69–73. [Google Scholar] [CrossRef]
- Stepanović, S.; Antić, N.; Dakić, I.; Svabić-Vlahović, M. In Vitro Antimicrobial Activity of Propolis and Synergism between Propolis and Antimicrobial Drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef]
- Astani, A.; Zimmermann, S.; Hassan, E.; Reichling, J.; Sensch, K.H.; Schnitzler, P. Antimicrobial Activity of Propolis Special Extract GH 2002 against Multidrug-Resistant Clinical Isolates. Pharmazie 2013, 68, 695–701. [Google Scholar]
- Boyanova, L.; Kolarov, R.; Gergova, G.; Mitov, I. In Vitro Activity of Bulgarian Propolis against 94 Clinical Isolates of Anaerobic Bacteria. Anaerobe 2006, 12, 173–177. [Google Scholar] [CrossRef]
- De Souza Silva, T.; Silva, J.M.B.; Braun, G.H.; Mejia, J.A.A.; Ccapatinta, G.V.C.; Santos, M.F.C.; Tanimoto, M.H.; Bastos, J.K.; Parreira, R.L.T.; Orenha, R.P.; et al. Green and Red Brazilian Propolis: Antimicrobial Potential and Anti-Virulence against ATCC and Clinically Isolated Multidrug-Resistant Bacteria. Chem. Biodivers. 2021, 18, e2100307. [Google Scholar] [CrossRef]
- Ratajczak, M.; Kaminska, D.; Matuszewska, E.; Hołderna-Kedzia, E.; Rogacki, J.; Matysiak, J. Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products. Molecules 2021, 26, 4007. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic Effects of Honey and Propolis toward Drug Multi-Resistant Staphylococcus aureus, Escherichia coli and Candida albicans Isolates in Single and Polymicrobial Cultures. Int. J. Med. Sci. 2012, 9, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Smoleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wąsik, T.J. Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The Effects of Brazilian and Bulgarian Propolis in Vitro against Salmonella Typhi and Their Synergism with Antibiotics Acting on the Ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Kalia, P.; Kumar, N.R.; Harjai, K. Studies on the Therapeutic Effect of Propolis along with Standard Antibacterial Drug in Salmonella enterica serovar Typhimurium Infected BALB/c Mice. BMC Complement. Altern. Med. 2016, 16, 485. [Google Scholar] [CrossRef]
- Santiago, K.B.; Piana, G.M.; Conti, B.J.; Cardoso, E.D.O.; Murbach Teles Andrade, B.F.; Zanutto, M.R.; Mores Rall, V.L.; Fernandes, A.; Sforcin, J.M. Microbiological Control and Antibacterial Action of a Propolis-Containing Mouthwash and Control of Dental Plaque in Humans. Nat. Prod. Res. 2018, 32, 1441–1445. [Google Scholar] [CrossRef]
- Bazvand, L.; Aminozarbian, M.G.; Farhad, A.; Noormohammadi, H.; Hasheminia, S.M.; Mobasherizadeh, S. Antibacterial Effect of Triantibiotic Mixture, Chlorhexidine Gel, and Two Natural Materials Propolis and Aloe Vera against Enterococcus faecalis: An Ex Vivo Study. Dent. Res. J. 2014, 11, 469–474. [Google Scholar]
- Carbajal Mejía, J.B. Antimicrobial Effects of Calcium Hydroxide, Chlorhexidine, and Propolis on Enterococcus faecalis and Candida albicans. J. Investig. Clin. Dent. 2014, 5, 194–200. [Google Scholar] [CrossRef]
- Anauate-Netto, C.; Anido-Anido, A.; Lewgoy, H.R.; Matsumoto, R.; Alonso, R.C.B.; Marcucci, M.C.; Paulino, N.; Bretz, W.A. Randomized, Double-Masked, Placebo-Controlled Clinical Trial on the Effects of Propolis and Chlorhexidine Mouthrinses on Gingivitis. Braz. Dent. Sci. 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Vasconcelos, W.A.; Braga, N.M.A.; Chitarra, V.R.; Santos, V.R.; Andrade, Â.L.; Domingues, R.Z. Bioactive Glass-Green and Red Propolis Association: Antimicrobial Activity Against Oral Pathogen Bacteria. Nat. Prod. Chem. Res. 2014, 2, 154. [Google Scholar]
- Ramos, J.M.; Milla, A.; Rodríguez, J.C.; Padilla, S.; Masiá, M.; Gutiérrez, F. Seroprevalence of Toxoplasma gondii Infection among Immigrant and Native Pregnant Women in Eastern Spain. Parasitol. Res. 2011, 109, 1447–1452. [Google Scholar] [CrossRef]
- Dwivedi, K.; Mandal, A.K.; Afzal, O.; Altamimi, A.S.A.; Sahoo, A.; Alossaimi, M.A.; Almalki, W.H.; Alzahrani, A.; Barkat, M.A.; Almeleebia, T.M.; et al. Emergence of Nano-Based Formulations for Effective Delivery of Flavonoids against Topical Infectious Disorders. Gels 2023, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, E.K.; El Fadaly, H.A.M.; Soror, A.H.; Ali, F.A.Z.; Abd El-Razik, K.A.; Soliman, Y.A.; Alkhaldi, A.A.M.; Albezrah, N.K.A.; Barakat, A.M. Novel Insights on the Potential Activity of Propolis and Wheat Germ Oil against Chronic Toxoplasmosis in Experimentally Infected Mice. Biomed. Pharmacother. 2022, 156, 113811. [Google Scholar] [CrossRef]
- Hegazi, A.; Toaleb, N.; El Fadaly, H.A.; Abdel-Rahman, E.H.; Barakat, A.M. In Vivo -Cellular and Humoral Immune Response for Evaluation of Propolis Effect on Chronic Toxoplasmosis in Rats. Adv. Anim. Vet. Sci. 2021, 9, 1045–1052. [Google Scholar] [CrossRef]
- Hagras, N.A.; Mogahed, N.M.F.H.; Sheta, E.; Darwish, A.A.; El-hawary, M.A.; Hamed, M.T.; Elwakil, B.H. The Powerful Synergistic Effect of Spiramycin/Propolis Loaded Chitosan/Alginate Nanoparticles on Acute Murine Toxoplasmosis. PLOS Neglected Trop. Dis. 2022, 16, e0010268. [Google Scholar] [CrossRef] [PubMed]
- Al Nasr, I.; Ahmed, F.; Pullishery, F.; El-Ashram, S.; Ramaiah, V.V. Toxoplasmosis and Anti-Toxoplasma Effects of Medicinal Plant Extracts-A Mini-Review. Asian Pac. J. Trop. Med. 2016, 9, 730–734. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of Toxoplasmosis: Current Options and Future Perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef]
- Sousa, L.; Azevedo, M.L.; Rocha, D.; Andrade, Â.; Amparo, T.; Dos Santos, O.; Seibert, J.; Pereira, L.; Vieira, P.; Carneiro, C.; et al. Trypanocidal Activity and Increased Solubility of Benznidazole Incorporated in PEG 4000 and Its Derivatives. J. Braz. Chem. Soc. 2021, 32, 1162–1172. [Google Scholar] [CrossRef]
- Morel, C.M. Chagas Disease, from Discovery to Control-and beyond: History, Myths and Lessons to Take Home. Mem. Inst. Oswaldo Cruz 1999, 94, 03–16. [Google Scholar] [CrossRef]
- Sousa, L.R.D.; Amparo, T.R.; de Souza, G.H.B.; Ferraz, A.T.; Fonseca, K.S.; de Azevedo, A.S.; Nascimento, A.M.D.; Andrade, Â.L.; Seibert, J.B.; Valverde, T.M.; et al. Anti-Trypanosoma Cruzi Potential of Vestitol Isolated from Lyophilized Red Propolis. Molecules 2023, 28, 7812. [Google Scholar] [CrossRef]
- Salomão, K.; De Souza, E.M.; Henriques-Pons, A.; Barbosa, H.S.; De Castro, S.L. Brazilian Green Propolis: Effects In Vitro and In Vivo on Trypanosoma cruzi. Evid.-Based Complement. Altern. Med. 2011, 2011, 185918. [Google Scholar] [CrossRef]
- Dantas, A.P.; Salomão, K.; Barbosa, H.S.; De Castro, S.L. The Effect of Bulgarian Propolis against Trypanosoma Cruzi and during Its Interaction with Host Cells. Mem. Inst. Oswaldo Cruz 2006, 101, 207–211. [Google Scholar] [CrossRef]
- Nweze, N.E.; Okoro, H.O.; Al Robaian, M.; Omar, R.M.K.; Tor-Anyiin, T.A.; Watson, D.G.; Igoli, J.O. Effects of Nigerian Red Propolis in Rats Infected with Trypanosoma brucei brucei. Comp. Clin. Pathol. 2017, 26, 1129–1133. [Google Scholar] [CrossRef]
- Silva, M.P.; Silva, T.M.; Mengarda, A.C.; Salvadori, M.C.; Teixeira, F.S.; Alencar, S.M.; Luz Filho, G.C.; Bueno-Silva, B.; De Moraes, J. Brazilian Red Propolis Exhibits Antiparasitic Properties in Vitro and Reduces Worm Burden and Egg Production in an Mouse Model Harboring Either Early or Chronic Schistosoma Mansoni Infection. J. Ethnopharmacol. 2021, 264, 113387. [Google Scholar] [CrossRef] [PubMed]
- Sena-Lopes, Â.; Bezerra, F.S.B.; Das Neves, R.N.; De Pinho, R.B.; Silva, M.T.D.O.; Savegnago, L.; Collares, T.; Seixas, F.; Begnini, K.; Henriques, J.A.P.; et al. Chemical Composition, Immunostimulatory, Cytotoxic and Antiparasitic Activities of the Essential Oil from Brazilian Red Propolis. PLoS ONE 2018, 13, e0191797. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.F.; Shinohara, L.; Sforcin, J.M.; Guimarães, S. In Vitro Effects of Propolis on Giardia duodenalis trophozoites. Phytomedicine 2006, 13, 170–175. [Google Scholar] [CrossRef]
- Alday-Provencio, S.; Diaz, G.; Rascon, L.; Quintero, J.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Astiazaran, H.; Hernandez, J.; Velazquez, C. Sonoran Propolis and Some of Its Chemical Constituents Inhibit In Vitro Growth of Giardia lamblia trophozoites. Planta Medica 2015, 81, 742–747. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor Feeds Primarily on HoneyBee Fat Body Tissue and Not Hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Orantes-Bermejo, F.J.; Pajuelo, A.G.; Megías, M.M.; Fernández-Píñar, C.T. Pesticide Residues in Beeswax and Beebread Samples Collected from HoneyBee Colonies (Apis mellifera L.) in Spain. Possible Implications for Bee Losses. J. Apic. Res. 2010, 49, 243–250. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Garedew, A.; Lamprecht, I.; Schmolz, E.; Schricker, B. The Varroacidal Action of Propolis: A Laboratory Assay. Apidologie 2002, 33, 41–50. [Google Scholar] [CrossRef]
- Damiani, N.; Fernández, N.J.; Maldonado, L.M.; Alvarez, A.R.; Eguaras, M.J.; Marcangeli, J.A. Bioactivity of Propolis from Different Geographical Origins on Varroa destructor (Acari: Varroidae). Parasitol. Res. 2010, 107, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ayad, A.S.; Benchaabane, S.; Daas, T.; Smagghe, G.; Loucif-Ayad, W. Assessment of Efficacy of Algerian Propolis against the Parasitic Mite Varroa destructor and Safety for HoneyBees by Spray Treatment. Insects 2024, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- El Menyiy, N.; Bakour, M.; El Ghouizi, A.; El Guendouz, S.; Lyoussi, B. Influence of Geographic Origin and Plant Source on Physicochemical Properties, Mineral Content, and Antioxidant and Antibacterial Activities of Moroccan Propolis. Int. J. Food Sci. 2021, 2021, 5570224. [Google Scholar] [CrossRef] [PubMed]
- Drescher, N.; Klein, A.-M.; Neumann, P.; Yañez, O.; Leonhardt, S. Inside Honeybee Hives: Impact of Natural Propolis on the Ectoparasitic Mite Varroa destructor and Viruses. Insects 2017, 8, 15. [Google Scholar] [CrossRef]
- Damiani, N.; Maggi, M.D.; Gende, L.B.; Faverin, C.; Eguaras, M.J.; Marcangeli, J.A. Evaluation of the Toxicity of a Propolis Extract on Varroa destructor (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae). J. Apic. Res. 2010, 49, 257–264. [Google Scholar] [CrossRef]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.-L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune Pathways and Defence Mechanisms in Honey Bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef]
- Rex, J.H.; Rinaldi, M.G.; Pfaller, M.A. Resistance of Candida Species to Fluconazole. Antimicrob. Agents Chemother. 1995, 39, 1–8. [Google Scholar] [CrossRef]
- Ota, C.; Unterkircher, C.; Fantinato, V.; Shimizu, M.T. Antifungal Activity of Propolis on Different Species of Candida. Mycoses 2001, 44, 375–378. [Google Scholar] [CrossRef]
- Koç, A.N.; Silici, S.; Kasap, F.; Hörmet-Öz, H.T.; Mavus-Buldu, H.; Ercal, B.D. Antifungal Activity of the Honeybee Products Against Candida spp. and Trichosporon spp. J. Med. Food 2011, 14, 128–134. [Google Scholar] [CrossRef]
- Chua, E.G.; Parolia, A.; Ahlawat, P.; Pau, A.; Amalraj, F.D. Antifungal Effectiveness of Various Intracanal Medicaments against Candida albicans: An Ex-Vivo Study. BMC Oral Health 2014, 14, 53. [Google Scholar] [CrossRef]
- Joy Sinha, D.; Garg, P.; Verma, A.; Malik, V.; Maccune, E.R.; Vasudeva, A. Dentinal Tubule Disinfection with Propolis & Two Extracts of Azadirachta Indica Against Candida albicans Biofilm Formed on Tooth Substrate. Open Dent. J. 2015, 9, 369–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mutlu Sariguzel, F.; Berk, E.; Koc, A.N.; Sav, H.; Demir, G. Antifungal Activity of Propolis Against Yeasts Isolated from Blood Culture: In Vitro Evaluation. Clin. Lab. Anal. 2016, 30, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.; de Carvalho Meirelles, G.; Balestrin, L.; de Souza, P.O.; Moreira, J.C.F.; Schuh, R.S.; Bidone, J.; von Poser, G.L.; Teixeira, H.F. In Vitro Protective Effect of Topical Nanoemulgels Containing Brazilian Red Propolis Benzophenones against UV-Induced Skin Damage. Photochem. Photobiol. Sci. 2020, 19, 1460–1469. [Google Scholar] [CrossRef]
- Dudoit, A.; Mertz, C.; Chillet, M.; Cardinault, N.; Brat, P. Antifungal Activity of Brazilian Red Propolis Extract and Isolation of Bioactive Fractions by Thin-Layer Chromatography-Bioautography. Food Chem. 2020, 327, 127060. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Louten, J. Virus Replication. In Essential Human Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–70. ISBN 978-0-12-800947-5. [Google Scholar]
- Kwon, M.J.; Shin, H.M.; Perumalsamy, H.; Wang, X.; Ahn, Y.-J. Antiviral Effects and Possible Mechanisms of Action of Constituents from Brazilian Propolis and Related Compounds. J. Apic. Res. 2020, 59, 413–425. [Google Scholar] [CrossRef]
- González-Búrquez, M.D.J.; González-Díaz, F.R.; García-Tovar, C.G.; Carrillo-Miranda, L.; Soto-Zárate, C.I.; Canales-Martínez, M.M.; Penieres-Carrillo, J.G.; Crúz-Sánchez, T.A.; Fonseca-Coronado, S. Comparison between In Vitro Antiviral Effect of Mexican Propolis and Three Commercial Flavonoids against Canine Distemper Virus. Evid.-Based Complement. Altern. Med. 2018, 2018, 7092416. [Google Scholar] [CrossRef]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral Activity and Mode of Action of Propolis Extracts and Selected Compounds. Phytother. Res. 2010, 24, S20–S28. [Google Scholar] [CrossRef]
- Labska, K.; Plodkova, H.; Pumannova, M.; Sensch, K.H. Antiviral Activity of Propolis Special Extract GH 2002 against Varicella zoster Virus in Vitro. Pharmazie 2018, 73(12), 733–736. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid.-Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- Yildirim, A.; Duran, G.G.; Duran, N.; Jenedi, K.; Bolgul, B.S.; Miraloglu, M.; Muz, M. Antiviral Activity of Hatay Propolis Against Replication of Herpes Simplex Virus Type 1 and Type 2. Med. Sci. Monit. 2016, 22, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Propolis, Bee Honey, and Their Components Protect against Coronavirus Disease 2019 (COVID-19): A Review of In Silico, In Vitro, and Clinical Studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef] [PubMed]
- Refaat, H.; Naguib, Y.W.; Elsayed, M.M.A.; Sarhan, H.A.A.; Alaaeldin, E. Modified Spraying Technique and Response Surface Methodology for the Preparation and Optimization of Propolis Liposomes of Enhanced Anti-Proliferative Activity against Human Melanoma Cell Line A375. Pharmaceutics 2019, 11, 558. [Google Scholar] [CrossRef]
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and Evaluation of Propolis Liposomes as a Promising Therapeutic Approach for COVID-19. Int. J. Pharm. 2021, 592, 120028. [Google Scholar] [CrossRef]
- Toutou, Z.; Fatmi, S.; Chibani, N.; Pokajewicz, K.; Skiba, M.; Wieczorek, P.P.; Iguerouada, M. Exploring the Therapeutic Potential of Algerian Propolis: GC/MS Profiling, Protective Inclusion Complex, and In Silico Evaluation Against SARS-COV-2 Main Proteases. Pept. Sci. 2025, 117, e24381. [Google Scholar] [CrossRef]
- Maruta, H.; He, H. PAK1-Blockers: Potential Therapeutics against COVID-19. Med. Drug Discov. 2020, 6, 100039. [Google Scholar] [CrossRef]
- Amoros, M.; Simõs, C.M.O.; Girre, L.; Sauvager, F.; Cormier, M. Synergistic Effect of Flavones and Flavonols Against Herpes Simplex Virus Type 1 in Cell Culture. Comparison with the Antiviral Activity of Propolis. J. Nat. Prod. 1992, 55, 1732–1740. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Zinger, A.; Cho, W.C.; Ben-Yehuda, A. Cancer and Aging—The Inflammatory Connection. Aging Dis. 2017, 8, 611. [Google Scholar] [CrossRef]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase: Regulation of Expression and Enzyme Activity. Trends Biochem. Sci. 2007, 32, 332–341. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial Activity, Phenolic Profile and Role in the Inflammation of Propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Alvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Yanagisawa, M.; Takahashi, N.; Kawada, T.; Kumazawa, S.; Yamaotsu, N.; Nakagome, I.; Hirono, S.; Tsuda, T. Brazilian Propolis-Derived Components Inhibit TNF-α-Mediated Downregulation of Adiponectin Expression via Different Mechanisms in 3T3-L1 Adipocytes. Biochim. Biophys. Acta BBA Gen. Subj. 2011, 1810, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.; Wu, W.; Long, Y.; Wang, R. Potential Cytoprotection: Antioxidant Defence by Caffeic Acid Phenethyl Ester against Free Radical-Induced Damage of Lipids, DNA, and Proteins. Can. J. Physiol. Pharmacol. 2008, 86, 279–287. [Google Scholar] [CrossRef]
- Kumazawa, S.; Ahn, M.-R.; Fujimoto, T.; Kato, M. Radical-Scavenging Activity and Phenolic Constituents of Propolis from Different Regions of Argentina. Nat. Prod. Res. 2010, 24, 804–812. [Google Scholar] [CrossRef]
- Song, M.-Y.; Lee, D.-Y.; Kim, E.-H. Anti-Inflammatory and Anti-Oxidative Effect of Korean Propolis on Helicobacter Pylori-Induced Gastric Damage in Vitro. J. Microbiol. 2020, 58, 878–885. [Google Scholar] [CrossRef]
- Moura, S.A.; Negri, G.; Salatino, A.; Lima, L.D.; Dourado, L.P.; Mendes, J.B.; Andrade, S.P.; Ferreira, M.A.; Cara, D.C. Aqueous Extract of Brazilian Green Propolis: Primary Components, Evaluation of Inflammation and Wound Healing by Using Subcutaneous Implanted Sponges. Evid.-Based Complement. Altern. Med. 2011, 2011, 748283. [Google Scholar] [CrossRef]
- Du Toit, K.; Buthelezi, S.; Bodenstein, J. Anti-Inflammatory and Antibacterial Profiles of Selected Compounds Found in South African Propolis. South Afr. J. Sci. 2010, 105, 470–472. [Google Scholar] [CrossRef][Green Version]
- Jung, Y.C.; Kim, M.E.; Yoon, J.H.; Park, P.R.; Youn, H.-Y.; Lee, H.-W.; Lee, J.S. Anti-Inflammatory Effects of Galangin on Lipopolysaccharide-Activated Macrophages via ERK and NF-κB Pathway Regulation. Immunopharmacol. Immunotoxicol. 2014, 36, 426–432. [Google Scholar] [CrossRef]
- Cardenas, H.; Arango, D.; Nicholas, C.; Duarte, S.; Nuovo, G.; He, W.; Voss, O.; Gonzalez-Mejia, M.; Guttridge, D.; Grotewold, E.; et al. Dietary Apigenin Exerts Immune-Regulatory Activity in Vivo by Reducing NF-κB Activity, Halting Leukocyte Infiltration and Restoring Normal Metabolic Function. Int. J. Mol. Sci. 2016, 17, 323. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response through Multiple Mechanisms in Macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.O.L.; Kabeya, L.M.; Figueiredo-Rinhel, A.S.G.; Marchi, L.F.; Andrade, M.F.; Piatesi, F.; Paoliello-Paschoalato, A.B.; Azzolini, A.E.C.S.; Lucisano-Valim, Y.M. Flavonols Modulate the Effector Functions of Healthy Individuals’ Immune Complex-Stimulated Neutrophils: A Therapeutic Perspective for Rheumatoid Arthritis. Int. Immunopharmacol. 2014, 21, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.M.; Alves, A.V.F.; Queiroz, L.A.; Lima, B.S.; Filho, R.N.P.; Araújo, A.A.S.; de Albuquerque Júnior, R.L.C.; Cardoso, J.C. The Photoprotective and Anti-Inflammatory Activity of Red Propolis Extract in Rats. J. Photochem. Photobiol. B Biol. 2018, 180, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, Y.; Gao, L.; Yin, H.; Xie, Z.; Wang, D.; Zhu, Z.; Han, X. Formononetin Attenuates IL-1β-Induced Apoptosis and NF-κB Activation in INS-1 Cells. Molecules 2012, 17, 10052–10064. [Google Scholar] [CrossRef]
- Szliszka, E.; Mertas, A.; Czuba, Z.P.; Król, W. Inhibition of Inflammatory Response by Artepillin C in Activated RAW264.7 Macrophages. Evid.-Based Complement. Altern. Med. 2013, 2013, 735176. [Google Scholar] [CrossRef]
- Orsatti, C.L.; Missima, F.; Pagliarone, A.C.; Bachiega, T.F.; Búfalo, M.C.; Araújo, J.P.; Sforcin, J.M. Propolis Immunomodulatory Action in Vivo on Toll-like Receptors 2 and 4 Expression and on Pro-inflammatory Cytokines Production in Mice. Phytother. Res. 2010, 24, 1141–1146. [Google Scholar] [CrossRef]
- Balderas-Cordero, D.; Canales-Alvarez, O.; Sánchez-Sánchez, R.; Cabrera-Wrooman, A.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. Anti-Inflammatory and Histological Analysis of Skin Wound Healing through Topical Application of Mexican Propolis. Int. J. Mol. Sci. 2023, 24, 11831. [Google Scholar] [CrossRef]
- Park, E.-H.; Kahng, J.-H. Suppressive Effects of Propolis in Rat Adjuvant Arthritis. Arch. Pharmacol. Res. 1999, 22, 554–558. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Jokar, M.; Tabesh, H.; Nematy, M.; Safarian, M.; Pahlavani, N.; Maddahi, M.; Khosravi, M. The Effect of Propolis Supplementation on Inflammatory Factors and Oxidative Status in Women with Rheumatoid Arthritis: Design and Research Protocol of a Double-Blind, Randomized Controlled. Contemp. Clin. Trials Commun. 2021, 23, 100807. [Google Scholar] [CrossRef]
- Sawaya, A.C.H.F.; Barbosa Da Silva Cunha, I.; Marcucci, M.C. Analytical Methods Applied to Diverse Types of Brazilian Propolis. Chem. Cent. J. 2011, 5, 27. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An Update on Its Chemistry and Pharmacological Applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, D.; Hu, Y.; Huang, Y.; Yu, Y.; Wang, D. The Immunological Enhancement Activity of Propolis Flavonoids Liposome In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2014, 2014, 483513. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ma, L.; Zhang, W.; Xu, Y.; Zhanxi, S.L.; Zhi, X.; Cui, E.; Song, X. Microemulsion Can Improve the Immune-Enhancing Activity of Propolis Flavonoid on Immunosuppression and Immune Response. Int. J. Biol. Macromol. 2014, 63, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.O.; Funari, S.R.C.; Soares, A.M.V.C.; Calvi, S.A.; Oliveira, S.L.; Sforcin, J.M.; Bankova, V. Immunomodulatory Action of Propolis on Macrophage Activation. J. Venom. Anim. Toxins 2000, 6, 205–219. [Google Scholar] [CrossRef]
- Murad, J.M.; Calvi, S.A.; Soares, A.M.V.C.; Bankova, V.; Sforcin, J.M. Effects of Propolis from Brazil and Bulgaria on Fungicidal Activity of Macrophages against Paracoccidioides brasiliensis. J. Ethnopharmacol. 2002, 79, 331–334. [Google Scholar] [CrossRef]
- Rebouças-Silva, J.; Amorim, N.A.; Jesus-Santos, F.H.; De Lima, J.A.; Lima, J.B.; Berretta, A.A.; Borges, V.M. Leishmanicidal and Immunomodulatory Properties of Brazilian Green Propolis Extract (EPP-AF®) and a Gel Formulation in a Pre-Clinical Model. Front. Pharmacol. 2023, 14, 1013376. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Orsi, R.O.; Bankova, V. Effect of Propolis, Some Isolated Compounds and Its Source Plant on Antibody Production. J. Ethnopharmacol. 2005, 98, 301–305. [Google Scholar] [CrossRef]
- Sforcin, J.M. Propolis and the Immune System: A Review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef]
- Doi, K.; Fujioka, M.; Sokuza, Y.; Ohnishi, M.; Gi, M.; Takeshita, M.; Kumada, K.; Kakehashi, A.; Wanibuchi, H. Chemopreventive Action by Ethanol-Extracted Brazilian Green Propolis on Post-Initiation Phase of Inflammation-Associated Rat Colon Tumorigenesis. In Vivo 2017, 31, 187–198. [Google Scholar] [CrossRef]
- Mojarab, S.; Shahbazzadeh, D.; Moghbeli, M.; Eshraghi, Y.; Bagheri, K.P.; Rahimi, R.; Savoji, M.A.; Mahdavi, M. Immune Responses to HIV-1 Polytope Vaccine Candidate Formulated in Aqueous and Alcoholic Extracts of Propolis: Comparable Immune Responses to Alum and Freund Adjuvants. Microb. Pathog. 2020, 140, 103932. [Google Scholar] [CrossRef]
- Fischer, G.; Cleff, M.B.; Dummer, L.A.; Paulino, N.; Paulino, A.S.; Vilela, C.D.O.; Campos, F.S.; Storch, T.; Vargas, G.D.; Hübner, S.D.O.; et al. Adjuvant Effect of Green Propolis on Humoral Immune Response of Bovines Immunized with Bovine Herpesvirus Type 5. Vet. Immunol. Immunopathol. 2007, 116, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Conceição, F.R.; Leite, F.P.L.; Dummer, L.A.; Vargas, G.D.; Hübner, S.D.O.; Dellagostin, O.A.; Paulino, N.; Paulino, A.S.; Vidor, T. Immunomodulation Produced by a Green Propolis Extract on Humoral and Cellular Responses of Mice Immunized with SuHV-1. Vaccine 2007, 25, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Magnavacca, A.; Sangiovanni, E.; Racagni, G.; Dell’Agli, M. The Antiviral and Immunomodulatory Activities of Propolis: An Update and Future Perspectives for Respiratory Diseases. Med. Res. Rev. 2022, 42, 897–945. [Google Scholar] [CrossRef] [PubMed]
- Chinthammit, C.; Axon, D.R.; Mollon, L.; Taylor, A.M.; Pickering, M.; Black, H.; Warholak, T.; Campbell, P.J. Evaluating the Relationship between Quality Measure Adherence Definitions and Economic Outcomes in Commercial Health Plans: A Retrospective Diabetes Cohort Study. J. Manag. Care Spec. Pharm. 2021, 27, 64–72. [Google Scholar] [CrossRef]
- International Diabetes Federation. 2021 IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org (accessed on 15 February 2025).
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative Stress and Inflammatory Markers in Prediabetes and Diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for Prevention of Type 2 Diabetes Mellitus: The STOP-NIDDM Randomised Trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Chiba, S. Molecular Mechanism in α-Glucosidase and Glucoamylase. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239. [Google Scholar] [CrossRef]
- El Adaouia Taleb, R.; Djebli, N.; Chenini, H.; Sahin, H.; Kolayli, S. In Vivo and in Vitro Anti-diabetic Activity of Ethanolic Propolis Extract. J. Food Biochem. 2020, 44, e13267. [Google Scholar] [CrossRef]
- Popova, M.; Lyoussi, B.; Aazza, S.; Antunes, D.; Bankova, V.; Miguel, G. Antioxidant and α-Glucosidase Inhibitory Properties and Chemical Profiles of Moroccan Propolis. Nat. Prod. Commun. 2015, 10, 1961–1964. [Google Scholar] [CrossRef]
- Hernández-Martínez, J.A.; Zepeda-Bastida, A.; Morales-Rodríguez, I.; Fernández-Luqueño, F.; Campos-Montiel, R.; Hereira-Pacheco, S.E.; Medina-Pérez, G. Potential Antidiabetic Activity of Apis Mellifera Propolis Extraction Obtained with Ultrasound. Foods 2024, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Alaribe, C.S.; Esposito, T.; Sansone, F.; Sunday, A.; Pagano, I.; Piccinelli, A.L.; Celano, R.; Cuesta Rubio, O.; Coker, H.A.; Nabavi, S.M.; et al. Nigerian Propolis: Chemical Composition, Antioxidant Activity and α-Amylase and α-Glucosidase Inhibition. Nat. Prod. Res. 2021, 35, 3095–3099. [Google Scholar] [CrossRef] [PubMed]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Antunes, M.D.; Faleiro, M.L.; Miguel, M.G. Anti-acetylcholinesterase, Antidiabetic, Anti-inflammatory, Antityrosinase and Antixanthine Oxidase Activities of Moroccan Propolis. Int. J. Food Sci. Technol. 2016, 51, 1762–1773. [Google Scholar] [CrossRef]
- Vongsak, B.; Kongkiatpaiboon, S.; Jaisamut, S.; Machana, S.; Pattarapanich, C. In Vitro Alpha Glucosidase Inhibition and Free-Radical Scavenging Activity of Propolis from Thai Stingless Bees in Mangosteen Orchard. Rev. Bras. Farm. 2015, 25, 445–450. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.-H.; Chen, M.-L.; Hu, F.-L. Protective Effects of Chinese and Brazilian Propolis Treatment against Hepatorenal Lesion in Diabetic Rats. Hum. Exp. Toxicol. 2011, 30, 1246–1255. [Google Scholar] [CrossRef]
- Chen, L.-H.; Chien, Y.-W.; Chang, M.-L.; Hou, C.-C.; Chan, C.-H.; Tang, H.-W.; Huang, H.-Y. Taiwanese Green Propolis Ethanol Extract Delays the Progression of Type 2 Diabetes Mellitus in Rats Treated with Streptozotocin/High-Fat Diet. Nutrients 2018, 10, 503. [Google Scholar] [CrossRef]
- El Menyiy, N.; Al-Waili, N.; El Ghouizi, A.; El-Guendouz, S.; Salom, K.; Lyoussi, B. Potential Therapeutic Effect of Moroccan Propolis in Hyperglycemia, Dyslipidemia, and Hepatorenal Dysfunction in Diabetic Rats. Iran. J. Basic Med. Sci. 2019, 22, 1331–1339. [Google Scholar] [CrossRef]
- Al-Hariri, M.T.; Eldin, T.A.G.; Al-Harb, M.M. Protective Effect and Potential Mechanisms of Propolis on Streptozotocin-Induced Diabetic Rats. J. Taibah Univ. Med. Sci. 2016, 11, 7–12. [Google Scholar] [CrossRef][Green Version]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of Antioxidant-Rich Propolis and Bee Pollen Extracts against D-Glucose Induced Type 2 Diabetes in Rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef]
- Samadi, N.; Mozaffari-Khosravi, H.; Rahmanian, M.; Askarishahi, M. Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: A randomized, double-blind clinical trial. J. Integr. Med. 2017, 15, 124–134. [Google Scholar] [CrossRef]
- Sartori, D.; Kawakami, C.; Orsatti, C.; Sforcin, J. Propolis Effect on Streptozotocin-Induced Diabetic Rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 93–102. [Google Scholar] [CrossRef]
- Aoi, W.; Hosogi, S.; Niisato, N.; Yokoyama, N.; Hayata, H.; Miyazaki, H.; Kusuzaki, K.; Fukuda, T.; Fukui, M.; Nakamura, N.; et al. Improvement of Insulin Resistance, Blood Pressure and Interstitial pH in Early Developmental Stage of Insulin Resistance in OLETF Rats by Intake of Propolis Extracts. Biochem. Biophys. Res. Commun. 2013, 432, 650–653. [Google Scholar] [CrossRef]
- Babatunde, I.R.; Abdulbasit, A.; Oladayo, M.I.; Olasile, O.I.; Olamide, F.R.; Gbolahan, B.W. Hepatoprotective and Pancreatoprotective Properties of the Ethanolic Extract of Nigerian Propolis. J. Intercult. Ethnopharmacol. 2015, 4, 102–108. [Google Scholar] [CrossRef]
- Mustafa, I. Nigerian Propolis Improves Blood Glucose, Glycated Hemoglobin (HbA1c), VLDL and HDL Levels in Rat Models of Diabetes. J. Intercult. Ethnopharmacol. 2016, 5, 233. [Google Scholar] [CrossRef]
- Ochoa-Morales, P.D.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Patiño-Laguna, A.D.J. Anti-Hyperglycemic Effects of Propolis or Metformin in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Int. J. Vitam. Nutr. Res. 2023, 93, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Medellín-Luna, M.F.; Castañeda-Delgado, J.E.; Martínez-Balderas, V.Y.; Cervantes-Villagrana, A.R. Medicinal Plant Extracts and Their Use as Wound Closure Inducing Agents. J. Med. Food 2019, 22, 435–443. [Google Scholar] [CrossRef]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research Progress on Therapeutic Effect and Mechanism of Propolis on Wound Healing. Evid.-Based Complement. Altern. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Rojczyk, E.; Klama-Baryła, A.; Łabuś, W.; Wilemska-Kucharzewska, K.; Kucharzewski, M. Historical and Modern Research on Propolis and Its Application in Wound Healing and Other Fields of Medicine and Contributions by Polish Studies. J. Ethnopharmacol. 2020, 262, 113159. [Google Scholar] [CrossRef]
- Baum, C.L.; Arpey, C.J. Normal Cutaneous Wound Healing: Clinical Correlation with Cellular and Molecular Events. Dermatol. Surg. 2006, 31, 674–686. [Google Scholar] [CrossRef]
- Hiromatsu, Y.; Toda, S. Mast Cells and Angiogenesis. Microsc. Res. Tech. 2003, 60, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Mast cells and oral inflammation. Crit. Rev. Oral Biol. Med. 2003, 14, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Noli, C.; Miolo, A. The Mast Cell in Wound Healing. Vet. Dermatol. 2001, 12, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Hasegawa, A.; Yamasaki, S.; Uchida, R.; Ohashi, W.; Kurashima, Y.; Kunisawa, J.; Kimura, S.; Iwanaga, T.; Watarai, H.; et al. Mast Cells Play Role in Wound Healing through the ZnT2/GPR39/IL-6 Axis. Sci. Rep. 2019, 9, 10842. [Google Scholar] [CrossRef] [PubMed]
- Weller, K.; Foitzik, K.; Paus, R.; Syska, W.; Maurer, M.; Weller, K.; Foitzik, K.; Paus, R.; Syska, W.; Maurer, M. Mast Cells Are Required for Normal Healing of Skin Wounds in Mice. FASEB J. 2006, 20, 2366–2368. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Salama, A.; Tohamy, H.-A.S. Applications of Propolis-Based Materials in Wound Healing. Arch. Dermatol. Res. 2023, 316, 61. [Google Scholar] [CrossRef]
- Barroso, P.R.; Lopes-Rocha, R.; Pereira, E.M.F.; Marinho, S.A.; De Miranda, J.L.; Lima, N.L.; Verli, F.D. Effect of Propolis on Mast Cells in Wound Healing. Inflammopharmacology 2012, 20, 289–294. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential Role of Propolis in Wound Healing: Biological Properties and Therapeutic Activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Stojko, M.; Wolny, D.; Włodarczyk, J. Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Molecules 2021, 26, 5701. [Google Scholar] [CrossRef]
- Ramadan, A.; Soliman, G.; Mahmoud, S.S.; Nofal, S.M.; Abdel-Rahman, R.F. Evaluation of the Safety and Antioxidant Activities of Crocus Sativus and Propolis Ethanolic Extracts. J. Saudi Chem. Soc. 2012, 16, 13–21. [Google Scholar] [CrossRef]
- Ramos, I.F.D.A.S.; Biz, M.T.; Paulino, N.; Scremin, A.; Della Bona, Á.; Barletta, F.B.; Figueiredo, J.A.P.D. Histopathological Analysis of Corticosteroid-Antibiotic Preparation and Propolis Paste Formulation as Intracanal Medication after Pulpectomy: An in Vivo Study. J. Appl. Oral Sci. 2012, 20, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Pellavio, G.; Laforenza, U.; Ranzato, E. Propolis Induces AQP3 Expression: A Possible Way of Action in Wound Healing. Molecules 2019, 24, 1544. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Hosoya, T.; Shimamura, Y.; Masuda, S.; Ogawa, T.; Kobayashi, H.; Shirafuji, K.; Moli, R.T.; Kozone, I.; Shin-ya, K.; et al. Solophenols B–D and Solomonin: New Prenylated Polyphenols Isolated from Propolis Collected from The Solomon Islands and Their Antibacterial Activity. J. Agric. Food Chem. 2012, 60, 11765–11770. [Google Scholar] [CrossRef] [PubMed]
- Ayad, A.S.; Hébert, M.P.A.; Doiron, J.A.; Loucif-Ayad, W.; Daas, T.; Smagghe, G.; Alburaki, M.; Barnett, D.A.; Touaibia, M.; Surette, M.E. Algerian Propolis from Distinct Geographical Locations: Chemical Profiles, Antioxidant Capacity, Cytotoxicity and Inhibition of 5-Lipoxygenase Product Biosynthesis. Chem. Biodivers. 2024, 21, e202301758. [Google Scholar] [CrossRef]
- Búfalo, M.C.; Ferreira, I.; Costa, G.; Francisco, V.; Liberal, J.; Cruz, M.T.; Lopes, M.C.; Batista, M.T.; Sforcin, J.M. Propolis and Its Constituent Caffeic Acid Suppress LPS-Stimulated pro-Inflammatory Response by Blocking NF-κB and MAPK Activation in Macrophages. J. Ethnopharmacol. 2013, 149, 84–92. [Google Scholar] [CrossRef]
- Franchin, M.; Colón, D.F.; Da Cunha, M.G.; Castanheira, F.V.S.; Saraiva, A.L.L.; Bueno-Silva, B.; Alencar, S.M.; Cunha, T.M.; Rosalen, P.L. Neovestitol, an Isoflavonoid Isolated from Brazilian Red Propolis, Reduces Acute and Chronic Inflammation: Involvement of Nitric Oxide and IL-6. Sci. Rep. 2016, 6, 36401. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Badr, G.; Al Ghamdi, A.A.; Sayed, A.; Al-Waili, N.S.; Garraud, O. Topical Application of Propolis Enhances Cutaneous Wound Healing by Promoting TGF-Beta/Smad-Mediated Collagen Production in a Streptozotocin-Induced Type I Diabetic Mouse Model. Cell. Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef]
- Cao, X.-P.; Chen, Y.-F.; Zhang, J.-L.; You, M.-M.; Wang, K.; Hu, F.-L. Mechanisms Underlying the Wound Healing Potential of Propolis Based on Its in Vitro Antioxidant Activity. Phytomedicine 2017, 34, 76–84. [Google Scholar] [CrossRef]
- Kawai, M.; Hirano, T.; Higa, S.; Arimitsu, J.; Maruta, M.; Kuwahara, Y.; Ohkawara, T.; Hagihara, K.; Yamadori, T.; Shima, Y.; et al. Flavonoids and Related Compounds as Anti-Allergic Substances. Allergol. Int. 2007, 56, 113–123. [Google Scholar] [CrossRef]
- Yano, S.; Umeda, D.; Yamashita, T.; Ninomiya, Y.; Sumida, M.; Fujimura, Y.; Yamada, K.; Tachibana, H. Dietary Flavones Suppresses IgE and Th2 Cytokines in OVA-Immunized BALB/c Mice. Eur. J. Nutr. 2007, 46, 257–263. [Google Scholar] [CrossRef]
- Nakamura, R.; Nakamura, R.; Watanabe, K.; Oka, K.; Ohta, S.; Mishima, S.; Teshima, R. Effects of Propolis from Different Areas on Mast Cell Degranulation and Identification of the Effective Components in Propolis. Int. Immunopharmacol. 2010, 10, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Lee, S.; Kim, S.-H. Chrysin Suppresses Mast Cell-Mediated Allergic Inflammation: Involvement of Calcium, Caspase-1 and Nuclear Factor-κB. Toxicol. Appl. Pharmacol. 2011, 254, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Park, W.S.; Jung, W.-K.; Qian, Z.; Lee, D.-S.; Choi, J.-S.; Lee, D.-Y.; Park, S.-G.; Seo, S.-K.; Kim, H.-J.; et al. Caffeic Acid Phenethyl Ester Promotes Anti-Inflammatory Effects by Inhibiting MAPK and NF-κB Signaling in Activated HMC-1 Human Mast Cells. Pharm. Biol. 2014, 52, 926–932. [Google Scholar] [CrossRef]
- Nader, M.A. Caffeic Acid Phenethyl Ester Attenuates IgE-Induced Immediate Allergic Reaction. Inflammopharmacology 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Kapare, H.S.; Giram, P.S.; Raut, S.S.; Gaikwad, H.K.; Paiva-Santos, A.C. Formulation Development and Evaluation of Indian Propolis Hydrogel for Wound Healing. Gels 2023, 9, 375. [Google Scholar] [CrossRef]
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Bowen, W.H. Effects of Compounds Found in Propolis on Streptococcus Mutans Growth and on Glucosyltransferase Activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. [Google Scholar] [CrossRef]
- Conceição, M.; Gushiken, L.F.S.; Aldana-Mejía, J.A.; Tanimoto, M.H.; Ferreira, M.V.D.S.; Alves, A.C.M.; Miyashita, M.N.; Bastos, J.K.; Beserra, F.P.; Pellizzon, C.H. Histological, Immunohistochemical and Antioxidant Analysis of Skin Wound Healing Influenced by the Topical Application of Brazilian Red Propolis. Antioxidants 2022, 11, 2188. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Elakkad, Y.E.; Elwakeel, A.A.; Allam, R.M.; Mousa, M.R. Formulation and Characterization of Propolis and Tea Tree Oil Nanoemulsion Loaded with Clindamycin Hydrochloride for Wound Healing: In-Vitro and in-Vivo Wound Healing Assessment. Saudi Pharm. J. 2021, 29, 1238–1249. [Google Scholar] [CrossRef]
- Chirumbolo, S. Flavonoids in Propolis Acting on Mast Cell-Mediated Wound Healing. Inflammopharmacology 2012, 20, 99–101. [Google Scholar] [CrossRef]
- Canales-Alvarez, O.; Canales-Martinez, M.M.; Dominguez-Verano, P.; Balderas-Cordero, D.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Rodriguez-Monroy, M.A. Effect of Mexican Propolis on Wound Healing in a Murine Model of Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 2201. [Google Scholar] [CrossRef]
- Eyarefe, O.D.; Ozota, C.A.; Jarikre, T.A.; Emikpe, B.O. Pathological and Immunohistochemical Evaluation of Wound Healing Potential of Nigerian Bee Propolis in Albino Rats. Comp. Clin. Pathol. 2019, 28, 455–466. [Google Scholar] [CrossRef]
- Moghtaday Khorasgani, E.; Karimi, A.H.; Nazem, M.R. A Comparison of Healing Effects of Propolis and Silver Sulfadiazine on Full Thickness SkinWounds in Rats. Pak. Vet. J. 2010, 30, 72–74. [Google Scholar]
- Kucharzewski, M.; Kózka, M.; Urbanek, T. Topical Treatment of Nonhealing Venous Leg Ulcer with Propolis Ointment. Evid.-Based Complement. Altern. Med. 2013, 2013, 254017. [Google Scholar] [CrossRef][Green Version]
- Samet, N.; Laurent, C.; Susarla, S.M.; Samet-Rubinsteen, N. The Effect of Bee Propolis on Recurrent Aphthous Stomatitis: A Pilot Study. Clin. Oral Investig. 2007, 11, 143–147. [Google Scholar] [CrossRef]
- Olczyk, P.; Wisowski, G.; Komosinska-Vassev, K.; Stojko, J.; Klimek, K.; Olczyk, M.; Kozma, E.M. Propolis Modifies Collagen Types I and III Accumulation in the Matrix of Burnt Tissue. Evid.-Based Complement. Altern. Med. 2013, 2013, 423809. [Google Scholar] [CrossRef]
- Staniczek, J.; Jastrzębska-Stojko, Ż.; Stojko, R. Biological Activity of Propolis Ointment with the Addition of 1% Nanosilver in the Treatment of Experimentally-Evoked Burn Wounds. Polymers 2021, 13, 2312. [Google Scholar] [CrossRef]
- Afkhamizadeh, M.; Aboutorabi, R.; Ravari, H.; Fathi Najafi, M.; Ataei Azimi, S.; Javadian Langaroodi, A.; Yaghoubi, M.A.; Sahebkar, A. Topical Propolis Improves Wound Healing in Patients with Diabetic Foot Ulcer: A Randomized Controlled Trial. Nat. Prod. Res. 2018, 32, 2096–2099. [Google Scholar] [CrossRef]
- Mujica, V.; Orrego, R.; Fuentealba, R.; Leiva, E.; Zúñiga-Hernández, J. Propolis as an Adjuvant in the Healing of Human Diabetic Foot Wounds Receiving Care in the Diagnostic and Treatment Centre from the Regional Hospital of Talca. J. Diabetes Res. 2019, 2019, 2507578. [Google Scholar] [CrossRef]
- Da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef]
- Russo, A.; Longo, R.; Vanella, A. Antioxidant Activity of Propolis: Role of Caffeic Acid Phenethyl Ester and Galangin. Fitoterapia 2002, 73, S21–S29. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant Activity of Propolis of Various Geographic Origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Valente, M.J.; Baltazar, A.F.; Henrique, R.; Estevinho, L.; Carvalho, M. Biological Activities of Portuguese Propolis: Protection against Free Radical-Induced Erythrocyte Damage and Inhibition of Human Renal Cancer Cell Growth in Vitro. Food Chem. Toxicol. 2011, 49, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Okińczyc, P.; Widelski, J.; Szperlik, J.; Żuk, M.; Mroczek, T.; Skalicka-Woźniak, K.; Sakipova, Z.; Widelska, G.; Kuś, P.M. Impact of Plant Origin on Eurasian Propolis on Phenolic Profile and Classical Antioxidant Activity. Biomolecules 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Komosińska-Vassev, K.; Winsz-Szczotka, K.; Koźma, E.M.; Wisowski, G.; Stojko, J.; Klimek, K.; Olczyk, K. Propolis Modulates Vitronectin, Laminin, and Heparan Sulfate/Heparin Expression during Experimental Burn Healing. J. Zhejiang Univ. Sci. B 2012, 13, 932–941. [Google Scholar] [CrossRef]
- Gregory, S.R.; Piccolo, N.; Piccolo, M.T.; Piccolo, M.S.; Heggers, J.P. Comparison of Propolis Skin Cream to Silver Sulfadiazine: A Naturopathic Alternative to Antibiotics in Treatment of Minor Burns. J. Altern. Complement. Med. 2002, 8, 77–83. [Google Scholar] [CrossRef]
- Olczyk, P.; Ramos, P.; Bernas, M.; Komosinska-Vassev, K.; Stojko, J.; Pilawa, B. Application of Electron Paramagnetic Resonance Spectroscopy to Comparative Examination of Different Groups of Free Radicals in Thermal Injuries Treated with Propolis and Silver Sulphadiazine. Evid.-Based Complement. Altern. Med. 2013, 2013, 851940. [Google Scholar] [CrossRef]
- Pessolato, A.G.T.; Martins, D.D.S.; Ambrósio, C.E.; Mançanares, C.A.F.; De Carvalho, A.F. Propolis and Amnion Reepithelialise Second-Degree Burns in Rats. Burns 2011, 37, 1192–1201. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Dolgin, E. Bringing down the Cost of Cancer Treatment. Nature 2018, 555, S26–S29. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Darvishi, N.; Yousefinejad, V.; Akbari, M.E.; Abdi, M.; Moradi, N.; Darvishi, S.; Mehrabi, Y.; Ghaderi, E.; Jamshidi-Naaeini, Y.; Ghaderi, B.; et al. Antioxidant and Anti-Inflammatory Effects of Oral Propolis in Patients with Breast Cancer Treated with Chemotherapy: A Randomized Controlled Trial. J. Herb. Med. 2020, 23, 100385. [Google Scholar] [CrossRef]
- Ebeid, S.A.; Abd El Moneim, N.A.; El-Benhawy, S.A.; Hussain, N.G.; Hussain, M.I. Assessment of the Radioprotective Effect of Propolis in Breast Cancer Patients Undergoing Radiotherapy. New Perspective for an Old Honeybee Product. J. Radiat. Res. Appl. Sci. 2016, 9, 431–440. [Google Scholar] [CrossRef]
- Watanabe, M.A.E.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic Constituents of Propolis Inducing Anticancer Effects: A Review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Sun, G.; Qiu, Z.; Wang, W.; Sui, X.; Sui, D. Flavonoids Extraction from Propolis Attenuates Pathological Cardiac Hypertrophy through PI3K/AKT Signaling Pathway. Evid.-Based Complement. Altern. Med. 2016, 2016, 6281376. [Google Scholar] [CrossRef]
- Fuliang, H.; Hepburn, H.; Xuan, H.; Chen, M.; Daya, S.; Radloff, S. Effects of Propolis on Blood Glucose, Blood Lipid and Free Radicals in Rats with Diabetes Mellitus. Pharmacol. Res. 2005, 51, 147–152. [Google Scholar] [CrossRef]
- Ertürküner, S.P.; Yaprak Saraç, E.; Göçmez, S.S.; Ekmekçi, H.; Öztürk, Z.B.; Seçkin, İ.; Sever, Ö.; Keskinbora, K. Anti-Inflammatory and Ultrastructural Effects of Turkish Propolis in a Rat Model of Endotoxin-Induced Uveitis. Folia Histochem. Cytobiol. 2015, 54, 49–57. [Google Scholar] [CrossRef]
- Demir, S.; Aliyazicioglu, Y.; Turan, I.; Misir, S.; Mentese, A.; Yaman, S.O.; Akbulut, K.; Kilinc, K.; Deger, O. Antiproliferative and Proapoptotic Activity of Turkish Propolis on Human Lung Cancer Cell Line. Nutr. Cancer 2016, 68, 165–172. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic Acid Composition, Antiatherogenic and Anticancer Potential of Honeys Derived from Various Regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef]
- Valença, I.; Morais-Santos, F.; Miranda-Gonçalves, V.; Ferreira, A.M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese Propolis Disturbs Glycolytic Metabolism of Human Colorectal Cancer in Vitro. BMC Complement. Altern. Med. 2013, 13, 184. [Google Scholar] [CrossRef]
- Xuan, H.; Li, Z.; Yan, H.; Sang, Q.; Wang, K.; He, Q.; Wang, Y.; Hu, F. Antitumor Activity of Chinese Propolis in Human Breast Cancer MCF-7 and MDA-MB-231 Cells. Evid.-Based Complement. Altern. Med. 2014, 2014, 280120. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Kabała-Dzik, A.; Moździerz, A.; Kubina, R.; Wojtyczka, R.; Stojko, R.; Dziedzic, A.; Jastrzębska-Stojko, Ż.; Jurzak, M.; Buszman, E.; et al. Caffeic Acid Phenethyl Ester and Ethanol Extract of Propolis Induce the Complementary Cytotoxic Effect on Triple-Negative Breast Cancer Cell Lines. Molecules 2015, 20, 9242–9262. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Yañez, N.; Rodriguez-Canales, M.; Nieto-Yañez, O.; Jimenez-Estrada, M.; Ibarra-Barajas, M.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. Hypoglycaemic and Antioxidant Effects of Propolis of Chihuahua in a Model of Experimental Diabetes. Evid.-Based Complement. Altern. Med. 2018, 2018, 4360356. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Tezuka, Y.; Adnyana, I.K.; Midorikawa, K.; Matsushige, K.; Message, D.; Huertas, A.A.G.; Kadota, S. Cytotoxic, Hepatoprotective and Free Radical Scavenging Effects of Propolis from Brazil, Peru, the Netherlands and China. J. Ethnopharmacol. 2000, 72, 239–246. [Google Scholar] [CrossRef]
- Búfalo, M.C.; Candeias, J.M.G.; Sousa, J.P.B.; Bastos, J.K.; Sforcin, J.M. In Vitro Cytotoxic Activity of Baccharis Dracunculifolia and Propolis against HEp-2 Cells. Nat. Prod. Res. 2010, 24, 1710–1718. [Google Scholar] [CrossRef]
- Mora, D.P.P.; Santiago, K.B.; Conti, B.J.; De Oliveira Cardoso, E.; Conte, F.L.; Oliveira, L.P.G.; De Assis Golim, M.; Uribe, J.F.C.; Gutiérrez, R.M.; Buitrago, M.R.; et al. The Chemical Composition and Events Related to the Cytotoxic Effects of Propolis on Osteosarcoma Cells: A Comparative Assessment of Colombian Samples. Phytother. Res. 2019, 33, 591–601. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Ishihara, M.; Naoi, K.; Hashita, M.; Itoh, Y.; Suzui, M. Growth Inhibitory Activity of Ethanol Extracts of Chinese and Brazilian Propolis in Four Human Colon Carcinoma Cell Lines. Oncol. Rep. 2009, 22, 349–354. [Google Scholar]
- Misir, S.; Aliyazicioglu, Y.; Demir, S.; Turan, I.; Hepokur, C. Effect of Turkish Propolis on miRNA Expression, Cell Cycle, and Apoptosis in Human Breast Cancer (MCF-7) Cells. Nutr. Cancer 2020, 72, 133–145. [Google Scholar] [CrossRef]
- Al-Oudat, B.A.; Alqudah, M.A.; Audat, S.A.; Al-Balas, Q.A.; El-Elimat, T.; Hassan, M.A.; Frhat, I.N.; Azaizeh, M.M. Design, Synthesis, and Biologic Evaluation of Novel Chrysin Derivatives as Cytotoxic Agents and Caspase-3/7 Activators. Drug Des. Dev. Ther. 2019, 13, 423–433. [Google Scholar] [CrossRef]
- Zingue, S.; Maxeiner, S.; Rutz, J.; Ndinteh, D.T.; Chun, F.K.-H.; Fohouo, F.T.; Njamen, D.; Blaheta, R.A. Ethanol-extracted Cameroonian Propolis: Antiproliferative Effects and Potential Mechanism of Action in Prostate Cancer. Andrologia 2020, 52, e13698. [Google Scholar] [CrossRef] [PubMed]
- KabaÅ‚a-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, Bioactive Components of Propolis, Exhibit Cytotoxic Activity and Induce Cell Cycle Arrest and Apoptosis in Human Breast Cancer Cells MDA-MB-231 and MCF-7–a Comparative Study. Cell. Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Heideman, L.; Chung, C.S.; Pelling, J.C.; Koehler, K.J.; Birt, D.F. Cell-Cycle Arrest at G2/M and Growth Inhibition by Apigenin in Human Colon Carcinoma Cell Lines. Mol. Carcinog. 2000, 28, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A Comprehensive Review of Its Chemistry, Bioavailability, and Pharmacological Properties. Fitoterapia 2020, 147, 104775. [Google Scholar] [CrossRef]
- Coskun, Z.M.; Ersoz, M.; Gecili, M.; Ozden, A.; Acar, A. Cytotoxic and Apoptotic Effects of Ethanolic Propolis Extract on C6 Glioma Cells. Environ. Toxicol. 2020, 35, 768–773. [Google Scholar] [CrossRef]
- Scifo, C.; Cardile, V.; Russo, A.; Consoli, R.; Vancheri, C.; Capasso, F.; Vanella, A.; Renis, M. Resveratrol and Propolis as Necrosis or Apoptosis Inducers in Human Prostate Carcinoma Cells. Oncol. Res. 2004, 14, 415–426. [Google Scholar] [CrossRef]
- Oliveira, L.P.G.; Conte, F.L.; Cardoso, E.D.O.; Conti, B.J.; Santiago, K.B.; Golim, M.D.A.; Cruz, M.T.; Sforcin, J.M. Immunomodulatory/Inflammatory Effects of Geopropolis Produced by Melipona fasciculata Smith in Combination with Doxorubicin on THP-1 Cells. J. Pharm. Pharmacol. 2016, 68, 1551–1558. [Google Scholar] [CrossRef]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular Characterization and Enhancement of Anticancer Activity of Caffeic Acid Phenethyl Ester by γ Cyclodextrin. J. Cancer 2016, 7, 1755–1771. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Cuban Brown Propolis Interferes in the Crosstalk between Colorectal Cancer Cells and M2 Macrophages. Nutrients 2020, 12, 2040. [Google Scholar] [CrossRef]
- Ishida, Y.; Gao, R.; Shah, N.; Bhargava, P.; Furune, T.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer Activity in Honeybee Propolis: Functional Insights to the Role of Caffeic Acid Phenethyl Ester and Its Complex With γ-Cyclodextrin. Integr. Cancer Ther. 2018, 17, 867–873. [Google Scholar] [CrossRef]
- Banzato, T.P.; Gubiani, J.R.; Bernardi, D.I.; Nogueira, C.R.; Monteiro, A.F.; Juliano, F.F.; De Alencar, S.M.; Pilli, R.A.; Lima, C.A.D.; Longato, G.B.; et al. Antiproliferative Flavanoid Dimers Isolated from Brazilian Red Propolis. J. Nat. Prod. 2020, 83, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.M.D.A.; Schneider, J.K.; De Jesus, C.V.F.; De Andrade, L.N.; Amaral, R.G.; David, J.M.; Krause, L.C.; Severino, P.; Soares, C.M.F.; Caramão Bastos, E.; et al. Brazilian Red Propolis: Extracts Production, Physicochemical Characterization, and Cytotoxicity Profile for Antitumor Activity. Biomolecules 2020, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Nishihara, H.; Hara, H.; Adachi, T. Ethanol Extract of Brazilian Red Propolis Induces Apoptosis in Human Breast Cancer MCF-7 Cells through Endoplasmic Reticulum Stress. J. Agric. Food Chem. 2012, 60, 11065–11070. [Google Scholar] [CrossRef] [PubMed]
- Kohtz, P.D.; Halpern, A.L.; Eldeiry, M.A.; Hazel, K.; Kalatardi, S.; Ao, L.; Meng, X.; Reece, T.B.; Fullerton, D.A.; Weyant, M.J. Toll-Like Receptor-4 Is a Mediator of Proliferation in Esophageal Adenocarcinoma. Ann. Thorac. Surg. 2019, 107, 233–241. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, H.; Li, C.; You, M.; Zheng, Y.; Li, G.Q.; Chen, X.; Zhang, C.; Hu, F. Chinese Propolis Inhibits the Proliferation of Human Gastric Cancer Cells by Inducing Apoptosis and Cell Cycle Arrest. Evid.-Based Complement. Altern. Med. 2020, 2020, 2743058. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Núñez, O.; Torres, A.; Guzmán, L.; Wehinger, S. Antitumor Activity of Propolis: Recent Advances in Cellular Perspectives, Animal Models and Possible Applications. Food Rev. Int. 2020, 36, 429–455. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Cevallos-Solorzano, G.; Romero-Benavides, J.; Ramirez Orellana, M. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017, 18, 106–131. [Google Scholar] [CrossRef]
- Kashani, B.; Zandi, Z.; Pourbagheri-Sigaroodi, A.; Bashash, D.; Ghaffari, S.H. The Role of Toll-like Receptor 4 (TLR4) in Cancer Progression: A Possible Therapeutic Target? J. Cell. Physiol. 2021, 236, 4121–4137. [Google Scholar] [CrossRef]
- Kimoto, T.; Aga, M.; Hino, K.; Koya-Miyata, S.; Yamamoto, Y.; Micallef, M.J.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Apoptosis of Human Leukemia Cells Induced by Artepillin C, an Active Ingredient of Brazilian Propolis. Anticancer Res. 2001, 21, 221–228. [Google Scholar]
- Pai, J.-T.; Lee, Y.-C.; Chen, S.-Y.; Leu, Y.-L.; Weng, M.-S. Propolin C Inhibited Migration and Invasion via Suppression of EGFR-Mediated Epithelial-to-Mesenchymal Transition in Human Lung Cancer Cells. Evid.-Based Complement. Altern. Med. 2018, 2018, 7202548. [Google Scholar] [CrossRef]
- Chiang, K.-C.; Yang, S.-W.; Chang, K.-P.; Feng, T.-H.; Chang, K.-S.; Tsui, K.-H.; Shin, Y.-S.; Chen, C.-C.; Chao, M.; Juang, H.-H. Caffeic Acid Phenethyl Ester Induces N-Myc Downstream Regulated Gene 1 to Inhibit Cell Proliferation and Invasion of Human Nasopharyngeal Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1397. [Google Scholar] [CrossRef] [PubMed]
- Frión-Herrera, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Maurício Sforcin, J. Mechanisms Underlying the Cytotoxic Effect of Propolis on Human Laryngeal Epidermoid Carcinoma Cells. Nat. Prod. Res. 2018, 32, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Boik, J. Natural Compounds in Cancer Therapy: Promising Nontoxic Antitumor Agents from Plants and Other Natural Sources; Oregon Medical Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Ganesan, M.; Kanimozhi, G.; Pradhapsingh, B.; Khan, H.A.; Alhomida, A.S.; Ekhzaimy, A.; Brindha, G.; Prasad, N.R. Phytochemicals Reverse P-Glycoprotein Mediated Multidrug Resistance via Signal Transduction Pathways. Biomed. Pharmacother. 2021, 139, 111632. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Kebsa, W.; Lahouel, M.; Rouibah, H.; Zihlif, M.; Ahram, M.; Abu-Irmaileh, B.; Mustafa, E.; Al-Ameer, H.J.; Al Shhab, M. Reversing Multidrug Resistance in Chemo-Resistant Human Lung Adenocarcinoma (A549/DOX) Cells by Algerian Propolis Through Direct Inhibiting the P-Gp Efflux-Pump, G0/G1 Cell Cycle Arrest and Apoptosis Induction. Anti-Cancer Agents Med. Chem. 2018, 18, 1330–1337. [Google Scholar] [CrossRef]
- Abubakar, M.B.; Abdullah, W.Z.; Sulaiman, S.A.; Ang, B.S. Polyphenols as Key Players for the Antileukaemic Effects of Propolis. Evid.-Based Complement. Altern. Med. 2014, 2014, 371730. [Google Scholar] [CrossRef]
- Bogdanov, S. Propolis: Composition, Health, Medicine: A Review; Bee Product Science: Morges, Switzerland, 2016. [Google Scholar]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzębska-Stojko, Ż.; Stojko, R.; Wojtyczka, R.; Stojko, J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules 2017, 22, 1554. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, D.; He, Y.; Xie, J.; Zhong, Z.; Li, Z.; Xie, J. Caffeic Acid Phenethyl Ester Induces Growth Arrest and Apoptosis of Colon Cancer Cells via the β-Catenin/T-Cell Factor Signaling. Anti-Cancer Drugs 2006, 17, 753–762. [Google Scholar] [CrossRef]
- Ren, X.; Liu, J.; Hu, L.; Liu, Q.; Wang, D.; Ning, X. Caffeic Acid Phenethyl Ester Inhibits the Proliferation of HEp2 Cells by Regulating Stat3/Plk1 Pathway and Inducing S Phase Arrest. Biol. Pharm. Bull. 2019, 42, 1689–1693. [Google Scholar] [CrossRef]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol Extract of Propolis and Its Constituent Caffeic Acid Phenethyl Ester Inhibit Breast Cancer Cells Proliferation in Inflammatory Microenvironment by Inhibiting TLR4 Signal Pathway and Inducing Apoptosis and Autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef]
- Banskota, A.H.; Nagaoka, T.; Sumioka, L.Y.; Tezuka, Y.; Awale, S.; Midorikawa, K.; Matsushige, K.; Kadota, S. Antiproliferative Activity of the Netherlands Propolis and Its Active Principles in Cancer Cell Lines. J. Ethnopharmacol. 2002, 80, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, P.; Araujo, J.; Pinho, M.J.; Martel, F. In Vitro Studies on the Inhibition of Colon Cancer by Butyrate and Polyphenolic Compounds. Nutr. Cancer 2011, 63, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham, U.-H.; Iqra, Y.; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and Therapeutic Properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, H.; Hage-Sleiman, R.; Wehbi, B.; Fayyad-Kazan, H.; Hayar, S.; Traboulssi, M.; Alyamani, O.A.; Faour, W.H.; ElMakhour, Y. Chemical Characterization and Cytotoxic Activity Evaluation of Lebanese Propolis. Biomed. Pharmacother. 2017, 95, 298–307. [Google Scholar] [CrossRef]
- Benguedouar, L.; Lahouel, M.; Gangloff, S.C.; Durlach, A.; Grange, F.; Bernard, P.; Antonicelli, F. Ethanolic Extract of Algerian Propolis and Galangin Decreased Murine Melanoma Tumor Progression in Mice. Anti-Cancer Agents Med. Chem. 2016, 16, 1172–1183. [Google Scholar] [CrossRef]
- Liu, C.; Ma, M.; Zhang, J.; Gui, S.; Zhang, X.; Xue, S. Galangin Inhibits Human Osteosarcoma Cells Growth by Inducing Transforming Growth Factor-Β1-Dependent Osteogenic Differentiation. Biomed. Pharmacother. 2017, 89, 1415–1421. [Google Scholar] [CrossRef]
- Liu, D.; You, P.; Luo, Y.; Yang, M.; Liu, Y. Galangin Induces Apoptosis in MCF-7 Human Breast Cancer Cells Through Mitochondrial Pathway and Phosphatidylinositol 3-Kinase/Akt Inhibition. Pharmacology 2018, 102, 58–66. [Google Scholar] [CrossRef]
- Freires, I.A.; De Alencar, S.M.; Rosalen, P.L. A Pharmacological Perspective on the Use of Brazilian Red Propolis and Its Isolated Compounds against Human Diseases. Eur. J. Med. Chem. 2016, 110, 267–279. [Google Scholar] [CrossRef]
- Bhargava, P.; Grover, A.; Nigam, N.; Kaul, A.; Doi, M.; Ishida, Y.; Kakuta, H.; Kaul, S.; Terao, K.; Wadhwa, R. Anticancer Activity of the Supercritical Extract of Brazilian Green Propolis and Its Active Component, artepillin�C: Bioinformatics and Experimental Analyses of Its Mechanisms of Action. Int. J. Oncol. 2018, 52(3), 925–932. [Google Scholar] [CrossRef]
- Turktekin, M.; Konac, E.; Onen, H.I.; Alp, E.; Yilmaz, A.; Menevse, S. Evaluation of the Effects of the Flavonoid Apigenin on Apoptotic Pathway Gene Expression on the Colon Cancer Cell Line (HT29). J. Med. Food 2011, 14, 1107–1117. [Google Scholar] [CrossRef]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxic Constituents from Brazilian Red Propolis and Their Structure–Activity Relationship. Bioorg. Med. Chem. 2008, 16, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, N.L.; Obradovic, A.D.; Vukic, M.D.; Jovanovic, D.; Djurdjevic, P.M. Cytotoxic, Proapoptotic and Antioxidative Potential of Flavonoids Isolated from Propolis against Colon (HCT-116) and Breast (MDA-MB-231) Cancer Cell Lines. Food Res. Int. 2018, 106, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, L. Formononetin-Induced Apoptosis by Activation of Ras/P38 Mitogen-Activated Protein Kinase in Estrogen Receptor-Positive Human Breast Cancer Cells. Horm. Metab. Res. 2012, 44, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.A.; Finger, D.; Machado, C.S.; Schmidt, E.M.; Costa, P.M.D.; Alves, A.P.N.N.; Morais, T.M.F.; Queiroz, M.G.R.D.; Quináia, S.P.; Rosa, M.R.D.; et al. In Vivo Antitumoural Activity and Composition of an Oil Extract of Brazilian Propolis. Food Chem. 2011, 126, 1239–1245. [Google Scholar] [CrossRef]
- Inoue, K.; Saito, M.; Kanai, T.; Kawata, T.; Shigematsu, N.; Uno, T.; Isobe, K.; Liu, C.-H.; Ito, H. Anti-Tumor Effects of Water-Soluble Propolis on a Mouse Sarcoma Cell Line In Vivo and In Vitro. Am. J. Chin. Med. 2008, 36, 625–634. [Google Scholar] [CrossRef]
- Gal, A.F.; Stan, L.; Tăbăran, F.; Rugină, D.; Cătoi, A.F.; Andrei, S. Chemopreventive Effects of Propolis in the MNU-Induced Rat Mammary Tumor Model. Oxid. Med. Cell. Longev. 2020, 2020, 4014838. [Google Scholar] [CrossRef]
- Ghaderi, A.; Stice, E.; Andersson, G.; Enö Persson, J.; Allzén, E. A Randomized Controlled Trial of the Effectiveness of Virtually Delivered Body Project (vBP) Groups to Prevent Eating Disorders. J. Consult. Clin. Psychol. 2020, 88, 643–656. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Don, M.-J.; Hung, P.-S.; Shen, Y.-C.; Lo, Y.-S.; Chang, K.-W.; Chen, C.-F.; Ho, L.-K. Cytotoxicity of Phenolic Acid Phenethyl Esters on Oral Cancer Cells. Cancer Lett. 2005, 223, 19–25. [Google Scholar] [CrossRef]
- Lavinas, F.C.; Macedo, E.H.B.C.; Sá, G.B.L.; Amaral, A.C.F.; Silva, J.R.A.; Azevedo, M.M.B.; Vieira, B.A.; Domingos, T.F.S.; Vermelho, A.B.; Carneiro, C.S.; et al. Brazilian Stingless Bee Propolis and Geopropolis: Promising Sources of Biologically Active Compounds. Rev. Bras. Farm. 2019, 29, 389–399. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of Propolis in Antimicrobial and Antioxidative Protection of Food Quality–A Review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Ueda, T.; Inden, M.; Shirai, K.; Sekine, S.; Masaki, Y.; Kurita, H.; Ichihara, K.; Inuzuka, T.; Hozumi, I. The Effects of Brazilian Green Propolis That Contains Flavonols against Mutant Copper-Zinc Superoxide Dismutase-Mediated Toxicity. Sci. Rep. 2017, 7, 2882. [Google Scholar] [CrossRef] [PubMed]
- Alghutaimel, H.; Matoug-Elwerfelli, M.; Alhaji, M.; Albawardi, F.; Nagendrababu, V.; Dummer, P.M.H. Propolis Use in Dentistry: A Narrative Review of Its Preventive and Therapeutic Applications. Int. Dent. J. 2024, 74, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Shinmei, Y.; Yano, H.; Kagawa, Y.; Izawa, K.; Akagi, M.; Inoue, T.; Kamei, C. Effect of Brazilian Propolis on Sneezing and Nasal Rubbing in Experimental Allergic Rhinitis of Mice. Immunopharmacol. Immunotoxicol. 2009, 31, 688–693. [Google Scholar] [CrossRef] [PubMed]
- De Barros, M.P.; Sousa, J.P.B.; Bastos, J.K.; De Andrade, S.F. Effect of Brazilian Green Propolis on Experimental Gastric Ulcers in Rats. J. Ethnopharmacol. 2007, 110, 567–571. [Google Scholar] [CrossRef]
- Barros, M.P.D.; Lemos, M.; Maistro, E.L.; Leite, M.F.; Sousa, J.P.B.; Bastos, J.K.; Andrade, S.F.D. Evaluation of Antiulcer Activity of the Main Phenolic Acids Found in Brazilian Green Propolis. J. Ethnopharmacol. 2008, 120, 372–377. [Google Scholar] [CrossRef]
- Boeing, T.; Mejia, J.A.; Ccana-Ccapatinta, G.V.; Mariott, M.; de Souza, P.; Mariano, L.N.; Oliveira, G.R.; da Rocha, I.M.; da Costa, G.A.; de Andrade, S.F.; et al. The Gastroprotective Effect of Red Propolis Extract from Northeastern Brazil and the Role of Its Isolated Compounds. J. Ethnopharmacol. 2021, 267, 113623. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid.-Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Lecce, L.; Incoronato, A.L.; Del Nobile, M.A. Microencapsulated Propolis to Enhance the Antioxidant Properties of Fresh Fish Burgers. J. Food Process Eng. 2015, 38, 527–535. [Google Scholar] [CrossRef]
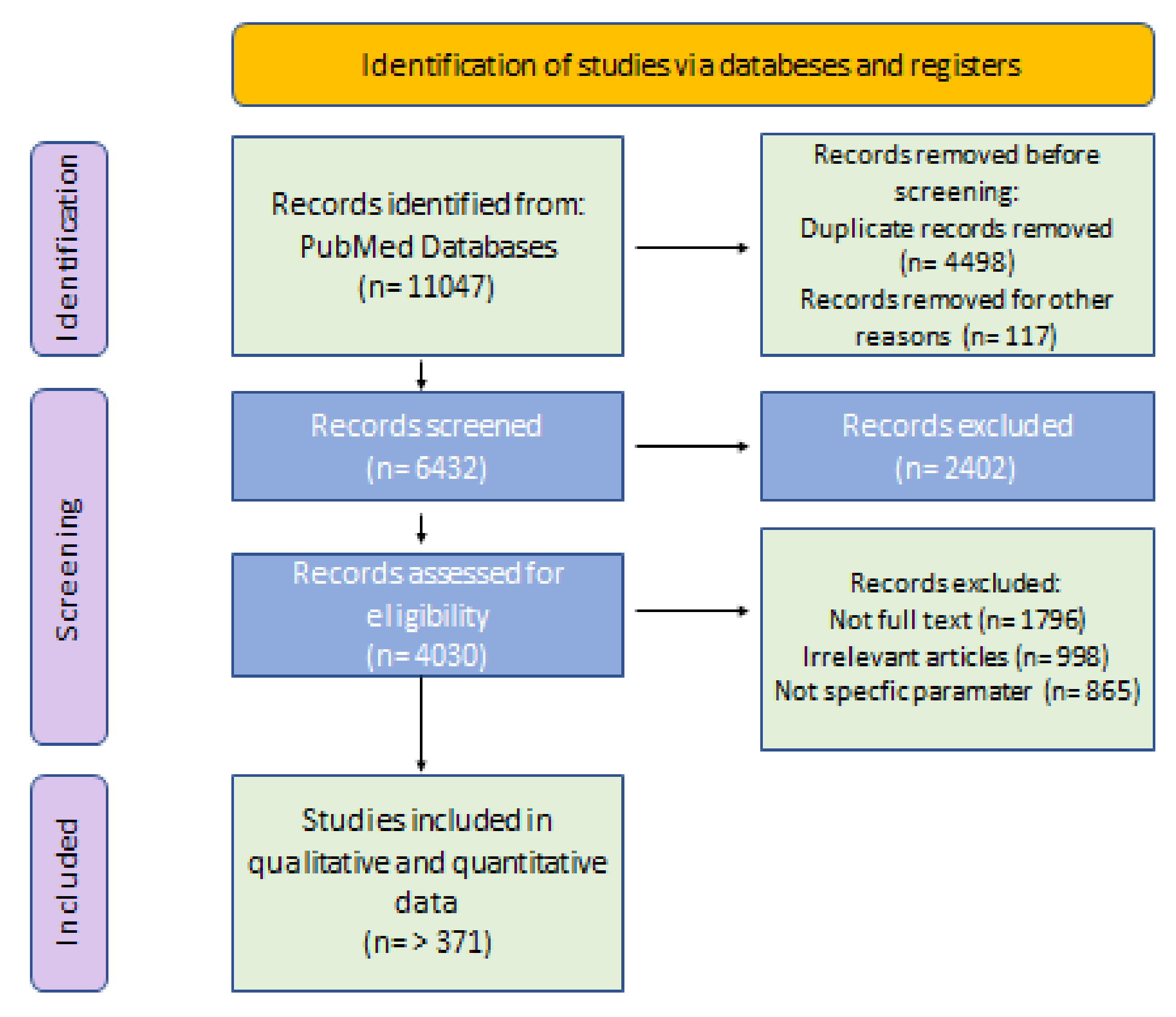
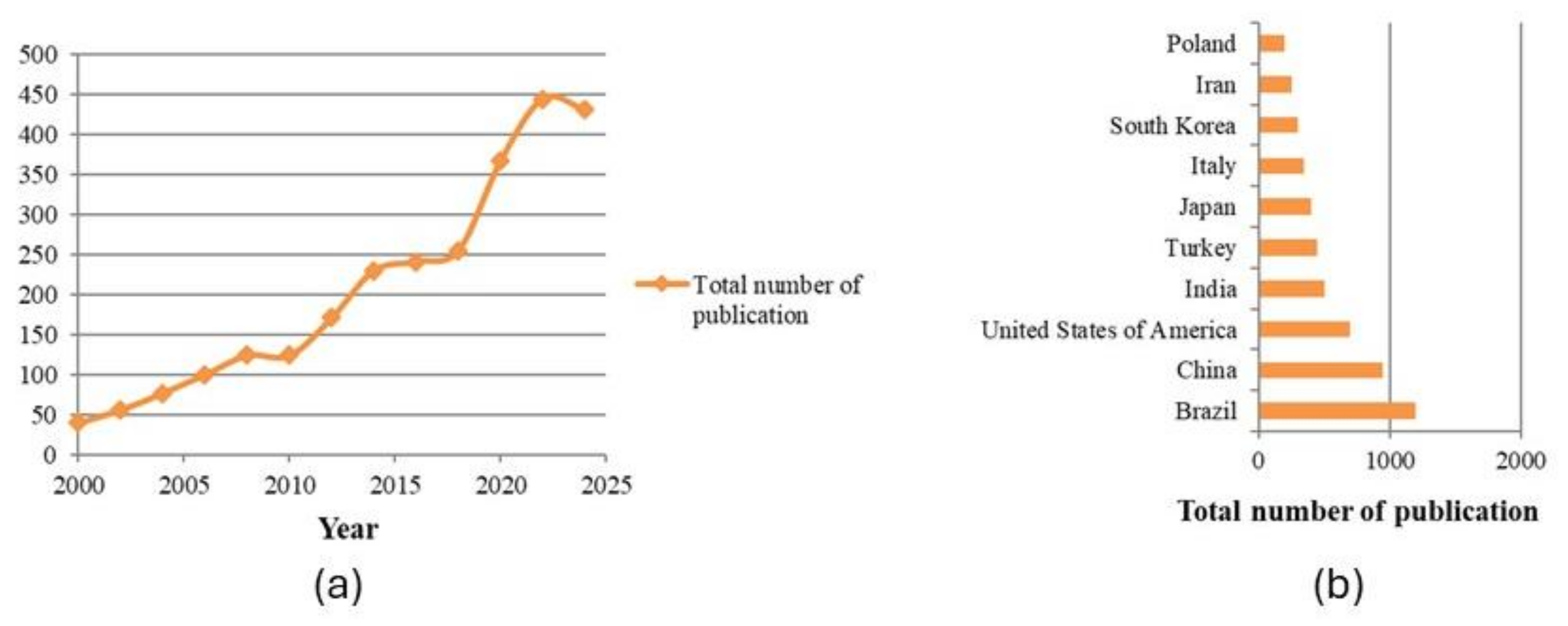
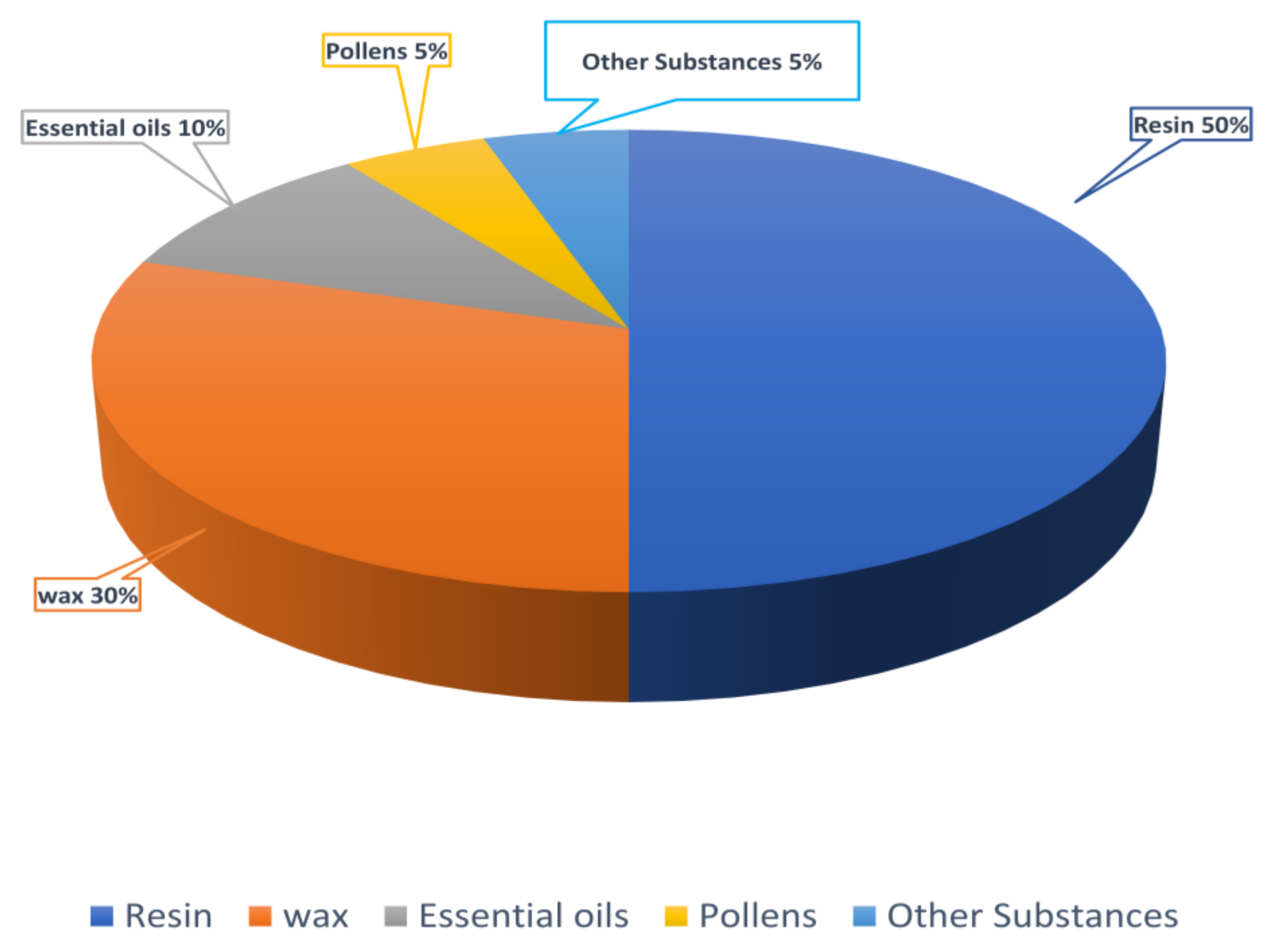
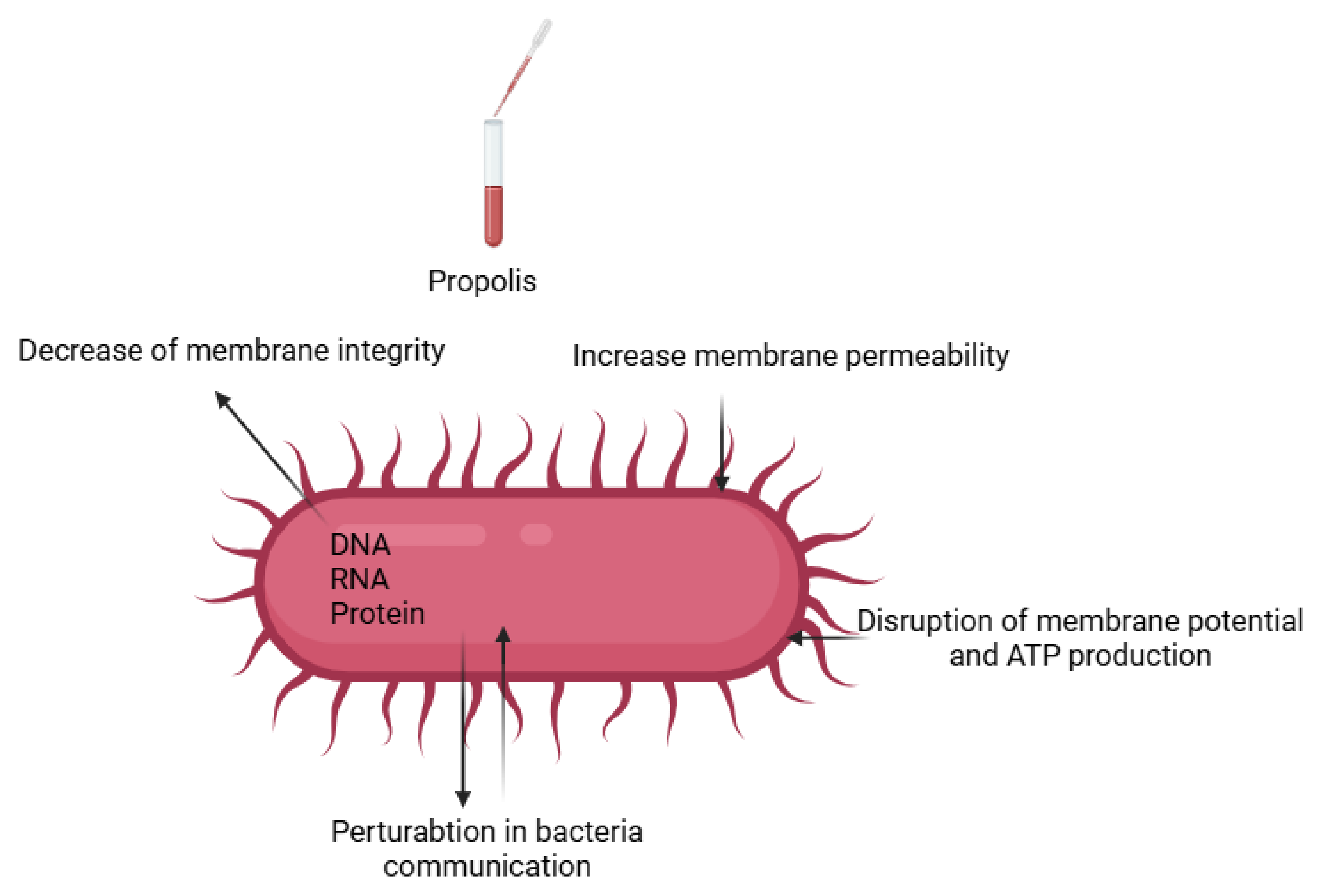


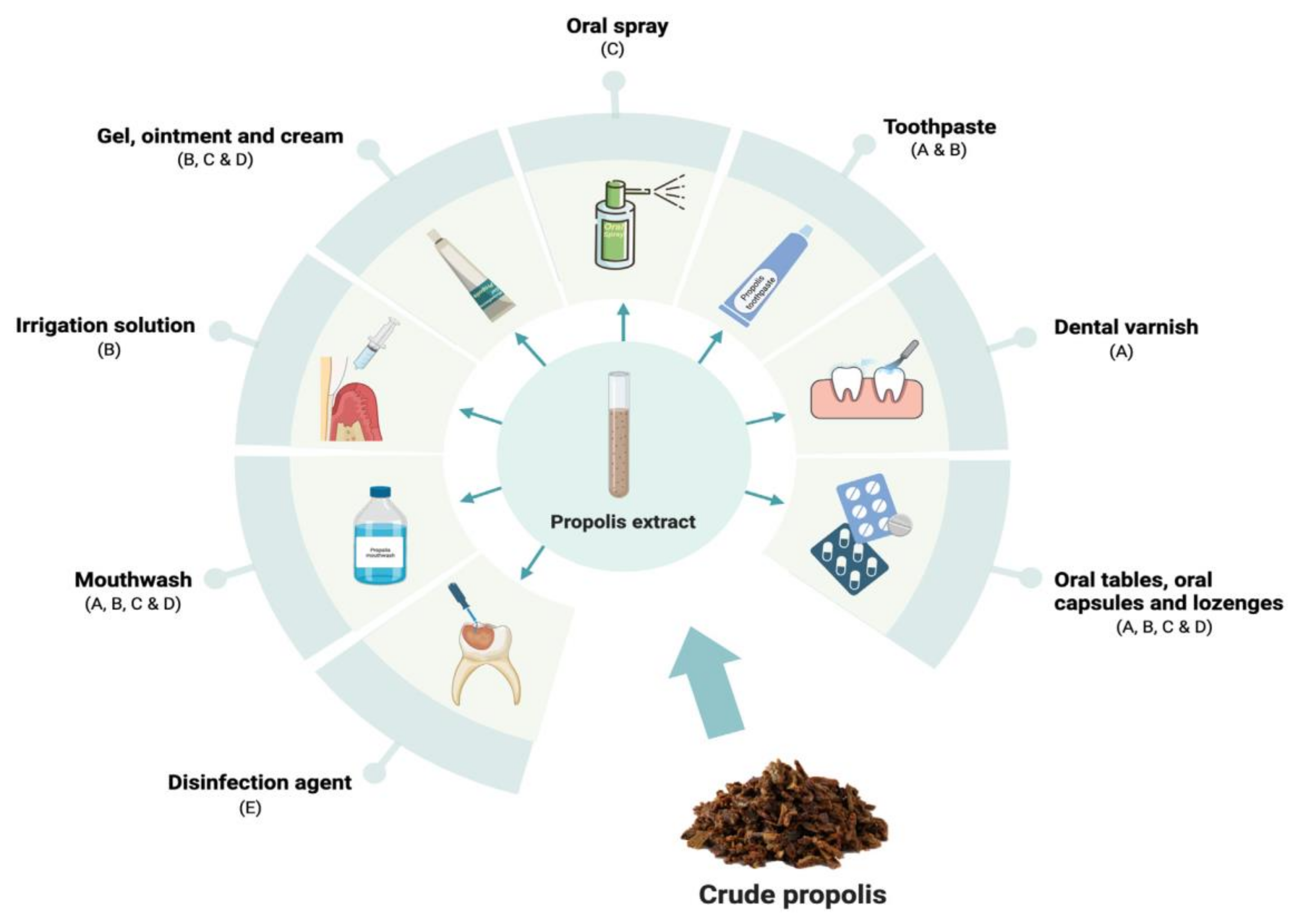
| Searched Topics and Keywords | Number of Publications |
|---|---|
| Propolis | 4720 |
| Propolis extraction | 2308 |
| Composition of propolis | 790 |
| Biological activities of propolis | 2307 |
| Application of propolis | 665 |
| Formulation of propolis | 257 |
| Name of Institution (Country) | Key Research Focus Areas |
|---|---|
| University of São Paulo (Brazil) | Brazil is a leading producer of propolis, and the University of São Paulo has been at the forefront of research on its antimicrobial, anti-inflammatory, and antioxidant properties. |
| Bulgarian Academy of Sciences | The institute has significantly contributed to understanding the chemical profile, diversity and bioactivity of propolis. |
| University of Zagreb (Croatia) | It is known for studies on the chemical composition and bioactive properties of European propolis. |
| National Institute of Agricultural Research (INRA) (France) | They have conducted research on the health benefits and applications of propolis in food and medicine. |
| Chinese Academy of Agricultural Sciences (CAAS) (China) | They have engaged in research on the bioactive components and applications of Chinese propolis. |
| United States Department of Agriculture (USDA), Arizona, and University of Minnesota (USA) | Researchers at this institution have investigated the microbiota associated with propolis application, enhancing the understanding of its role in honeybee health. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayad, A.S.; Benchaabane, S.; Daas, T.; Smagghe, G.; Loucif-Ayad, W. Propolis Stands out as a Multifaceted Natural Product: Meta-Analysis on Its Sources, Bioactivities, Applications, and Future Perspectives. Life 2025, 15, 764. https://doi.org/10.3390/life15050764
Ayad AS, Benchaabane S, Daas T, Smagghe G, Loucif-Ayad W. Propolis Stands out as a Multifaceted Natural Product: Meta-Analysis on Its Sources, Bioactivities, Applications, and Future Perspectives. Life. 2025; 15(5):764. https://doi.org/10.3390/life15050764
Chicago/Turabian StyleAyad, Ahmed Sabri, Samia Benchaabane, Tarek Daas, Guy Smagghe, and Wahida Loucif-Ayad. 2025. "Propolis Stands out as a Multifaceted Natural Product: Meta-Analysis on Its Sources, Bioactivities, Applications, and Future Perspectives" Life 15, no. 5: 764. https://doi.org/10.3390/life15050764
APA StyleAyad, A. S., Benchaabane, S., Daas, T., Smagghe, G., & Loucif-Ayad, W. (2025). Propolis Stands out as a Multifaceted Natural Product: Meta-Analysis on Its Sources, Bioactivities, Applications, and Future Perspectives. Life, 15(5), 764. https://doi.org/10.3390/life15050764







