A Prospective Crossover Clinical Trial of Esaxerenone and Eplerenone in Patients with Chronic Heart Failure Complicated by Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Endpoints
2.3. Adverse Events
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatment Compliance
3.2. Adverse Events
3.3. Primary Endpoint
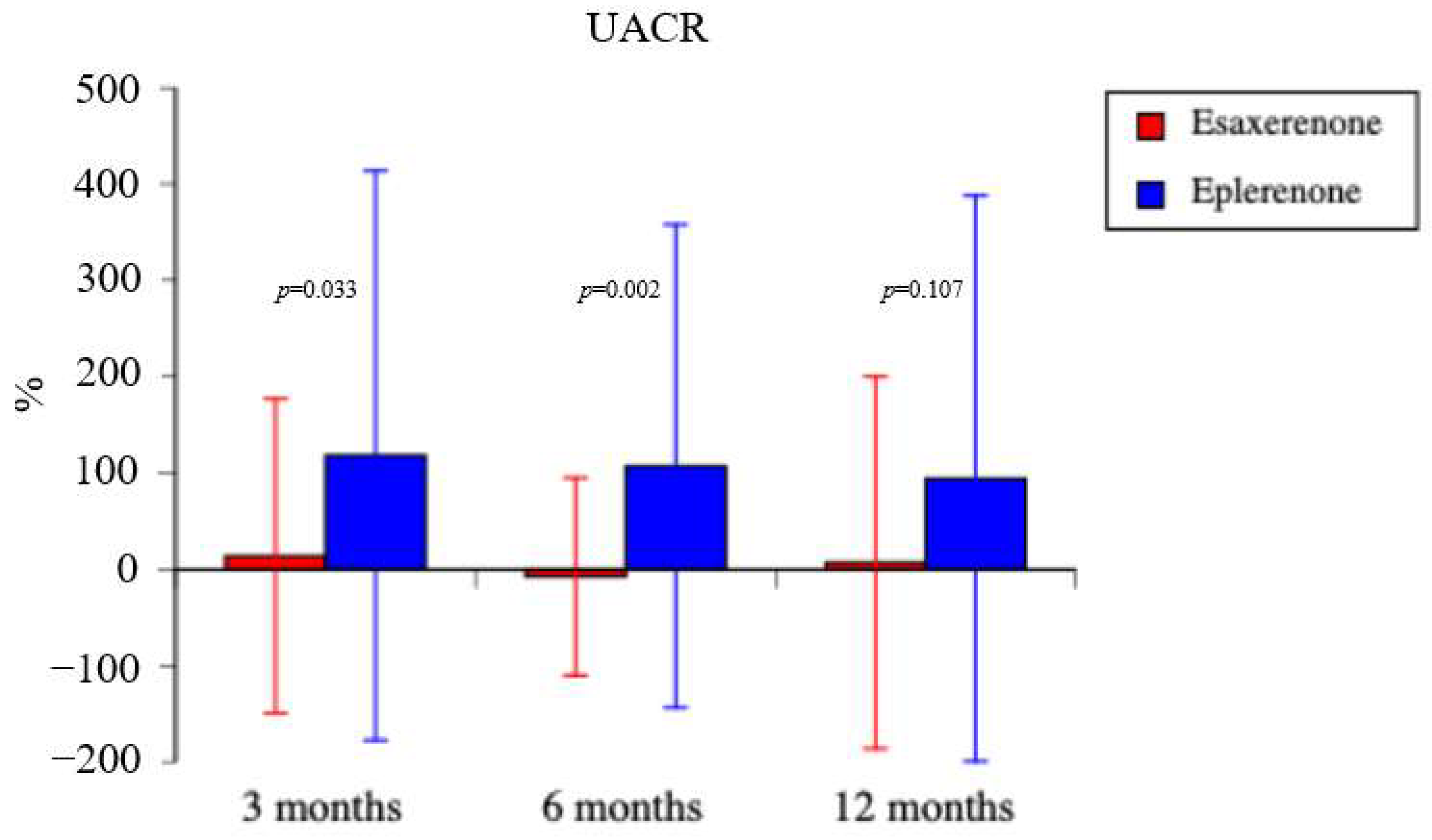
3.4. Secondary Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.V.; Le, T.N.; Truong, B.Q.; Nguyen, H.T.T. Efficacy and safety of angiotensin receptor–neprilysin inhibition in heart failure patients with end-stage kidney disease on maintenance dialysis: A systematic review and meta-analysis. Eur. J. Heart Fail. 2025, 27, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Cinar, T.; Saylik, F.; Cicek, V.; Pay, L.; Khachatryan, A.; Alejandro, J.; Erdem, A.; Hayiroglu, M.I. Effects of SGLT2 inhibitors on right ventricular function in heart failure patients: Updated meta-analysis of the current literature. Kardiol. Pol. 2024, 82, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Struthers, A.D.; MacDonald, T. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc. Res. 2004, 61, 663–670. [Google Scholar] [CrossRef]
- Nishiyama, A.; Kobori, H. Independent regulation of renin–angiotensin–aldosterone system in the kidney. Clin. Exp. Nephrol. 2018, 22, 1231–1239. [Google Scholar] [CrossRef]
- Nishiyama, A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens. Res. 2019, 42, 293–300. [Google Scholar] [CrossRef]
- Bauersachs, J. Heart failure drug treatment: The fantastic four. Eur. Heart J. 2021, 42, 681–683. [Google Scholar] [CrossRef]
- Güder, G.; Bauersachs, J.; Frantz, S.; Weismann, D.; Allolio, B.; Ertl, G.; Angermann, C.E.; Störk, S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 2007, 115, 1754–1761. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M.; et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B.; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Tsuruoka, H.; Homma, T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur. J. Pharmacol. 2015, 769, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yoshimura, M.; Yamakawa, S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN Study). Hypertension 2020, 75, 51–58. [Google Scholar] [CrossRef]
- Kario, H.; Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yoshimura, M.; Yamakawa, S. Effect of the nonsteroidal mineralocorticoid receptor blocker, esaxerenone, on nocturnal hypertension: A post hoc analysis of the ESAX-HTN study. Am. J. Hypertens. 2021, 34, 540–551. [Google Scholar] [CrossRef]
- Sezai, A.; Soma, M.; Hata, M.; Yoshitake, I.; Unosawa, S.; Wakui, S.; Shiono, M. Effects of olmesartan on the renin–angiotensin–aldosterone system for patients with essential hypertension after cardiac surgery—Investigation using a candesartan change-over study. Ann. Thorac. Cardiovasc. Surg. 2011, 17, 487–493. [Google Scholar] [CrossRef]
- Sezai, A.; Osaka, S.; Yaoita, H.; Arimoto, M.; Hata, H.; Shiono, M.; Sakino, H. Changeover trial of azilsartan and olmesartan comparing effects on the renin–angiotensin–aldosterone system in patients with essential hypertension after cardiac surgery (CHAOS Study). Ann. Thorac. Cardiovasc. Surg. 2016, 22, 161–167. [Google Scholar] [CrossRef]
- Qiang, P.; Hao, J.; Yang, F.; Han, Y.; Chang, Y.; Xian, Y.; Xiong, Y.; Gao, X.; Liang, L.; Shimosawa, T.; et al. Esaxerenone inhibits the macrophage-to-myofibroblast transition through mineralocorticoid receptor/TGF-β1 pathway in mice induced with aldosterone. Front. Immunol. 2022, 13, 948658. [Google Scholar] [CrossRef]
- Okuda, Y.; Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Sawanobori, T.; Taguri, M. The renoprotective effect of esaxerenone independent of blood pressure lowering: A post hoc mediation analysis of the ESAX-DN trial. Hypertens. Res. 2023, 46, 437–444. [Google Scholar] [CrossRef]
- Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Okuda, Y.; Sawanobori, T. Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: A multicenter, single-arm, open-label phase III study. Clin. Exp. Nephrol. 2021, 25, 1070–1078. [Google Scholar] [CrossRef]
- Edwards, N.C.; Steeds, R.P.; Chue, C.D.; Stewart, P.M.; Ferro, C.J.; Townend, J.N. The safety and tolerability of spironolactone in patients with mild to moderate chronic kidney disease. Br. J. Clin. Pharmacol. 2012, 73, 447–454. [Google Scholar] [CrossRef]
- Sato, A. Does the temporary decrease in the estimated glomerular filtration rate (eGFR) after initiation of mineralocorticoid receptor (MR) antagonist treatment lead to a long-term renal protective effect? Hypertens. Res. 2019, 42, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Iijima, S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens. Res. 2021, 44, 489–497. [Google Scholar] [CrossRef]
- Arai, K.; Homma, T.; Morikawa, Y.; Ubukata, N.; Tsuruoka, H.; Aoki, K.; Ishikawa, H.; Mizuno, M.; Sada, T. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur. J. Pharmacol. 2015, 761, 226–234. [Google Scholar] [CrossRef]
- Itoh, H.; Ito, S.; Rakugi, H.; Okuda, Y.; Nishioka, S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: A single-arm, open-label study. Hypertens. Res. 2019, 42, 1572–1581. [Google Scholar] [CrossRef]
- Kario, H.; Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yamakawa, S. Effect of esaxerenone on nocturnal blood pressure and natriuretic peptide in different dipping phenotypes. Hypertens. Res. 2022, 45, 97–105. [Google Scholar] [CrossRef]
- Tsutsui, H.; Ito, H.; Kitakaze, M.; Komuro, I.; Murohara, T.; Izumi, T.; Sunagawa, K.; Yasumura, Y.; Yano, M.; Yamamoto, K.; et al. Double-blind, randomized, placebo-controlled trial evaluating the efficacy and safety of eplerenone in Japanese patients with chronic heart failure (J-EMPHASIS-HF). Circ. J. 2018, 82, 148–158. [Google Scholar] [CrossRef]
- Sezai, A.; Shiono, M.; Orime, Y.; Hata, H.; Hata, M.; Negishi, N.; Sezai, Y. Low-dose continuous infusion of human atrial natriuretic peptide during and after cardiac surgery. Ann. Thorac. Surg. 2000, 69, 732–738. [Google Scholar] [CrossRef]
- Sezai, A.; Hata, M.; Niino, T.; Yoshitake, I.; Unosawa, S.; Wakui, S.; Fujita, K.; Takayama, T.; Kasamaki, Y.; Hirayama, A.; et al. Continuous low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting: The NU-HIT (Nihon University Working Group Study of Low-Dose Human ANP Infusion Therapy during Cardiac Surgery) for left ventricular dysfunction. J. Am. Coll. Cardiol. 2010, 55, 1844–1851. [Google Scholar]
- Sezai, A.; Hata, M.; Niino, T.; Yoshitake, I.; Unosawa, S.; Wakui, S.; Kimura, H.; Shiono, M.; Takayama, T.; Hirayama, A. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: The NU-HIT (Nihon University Working Group Study of Low-Dose HANP Infusion Therapy during Cardiac Surgery) trial for CKD. J. Am. Coll. Cardiol. 2011, 58, 897–903. [Google Scholar]
- The RALES Investigators. Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am. J. Cardiol. 1996, 78, 902–907. [Google Scholar] [CrossRef]
- Ichikawa, S.; Tsutsumi, J.; Sugimoto, K.; Yamakawa, S. Antihypertensive effect of long-term monotherapy with esaxerenone in patients with essential hypertension: Relationship between baseline urinary sodium excretion and its antihypertensive effect. Adv. Ther. 2022, 39, 4779–4791. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Hoshide, S.; Tamaki, N.; Nagata, M.; Sasaki, K.; Kanemaru, Y.; Shimada, K.; Kario, K. Efficacy of eplerenone added to renin–angiotensin blockade in elderly hypertensive patients: The Jichi Eplerenone Treatment (JET) study. J. Renin Angiotensin Aldosterone Syst. 2011, 12, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Oshima, A.; Narang, N.; Kinugawa, K. Implication of mineralocorticoid receptor antagonist esaxerenone in patients with heart failure with preserved ejection fraction. Circ. Rep. 2021, 3, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Naruke, T.; Maemura, K.; Oki, T.; Yazaki, M.; Fujita, T.; Ikeda, Y.; Nabeta, T.; Ishii, S.; Minami, Y.; Fukaya, H.; et al. Efficacy and safety of esaxerenone in patients with hypertension and concomitant heart failure. Hypertens. Res. 2021, 44, 601–603. [Google Scholar] [CrossRef]
- Berry, C.; Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta-analysis. Eur. Heart J. 2012, 33, 1750–1757. [Google Scholar]
- Tsuchihashi-Makaya, M.; Hamaguchi, S.; Kinugawa, S.; Yokota, T.; Goto, D.; Yokoshiki, H.; Kato, N.; Takeshita, A.; Tsutsui, H.; JCARE-CARD Investigators. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ. J. 2009, 73, 1893–1900. [Google Scholar] [CrossRef]
- Tsuji, K.; Sakata, Y.; Nochioka, K.; Miura, M.; Yamauchi, T.; Onose, T.; Abe, R.; Oikawa, T.; Kasahara, S.; Sato, M.; et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction—A report from the CHART-2 Study. Eur. J. Heart Fail. 2017, 19, 1258–1269. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- Jhund, P.S.; Talebi, A.; Henderson, A.D.; Claggett, B.L.; Vaduganathan, M.; Desai, A.S.; Lam, C.S.P.; Pitt, B.; Senni, M.; Shah, S.J.; et al. Mineralocorticoid receptor antagonists in heart failure: An individual patient level meta-analysis. Lancet 2024, 404, 1119–1131. [Google Scholar] [CrossRef]

| Characteristic | Data Value |
|---|---|
| Cases, No. | 66 |
| Age, years, mean ± SD (range) | 72.3 ± 9.7 (38–89) |
| Gender, male/female, No. | 51:15 |
| Classification of heart failure, No. (%) | |
| HFrEF | 2 (3) |
| HFmrEF | 1 (2) |
| HFpEF | 63 (95) |
| Cause of heart failure, No. (%) | |
| Ischemic heart disease | 17 (26) |
| Valve disease | 31 (47) |
| Hypertension | 15 (23) |
| Cardiomyopathy | 1 (2) |
| Arrhythmia | 2 (3) |
| Complication, No. (%) | |
| Diabetes mellitus | 30 (45) |
| Dyslipidemia | 61 (92) |
| Hyperuricemia | 29 (44) |
| CKD (stage G3a) | 30 (45) |
| CKD (stage G3b) | 18 (27) |
| Obesity | 16 (24) |
| Cerebral infarct | 6 (9) |
| Oral medicine, No. (%) | |
| ACE-I | 2 (3) |
| ARB | 44 (67) |
| Beta-blocker | 55 (83) |
| SGLT2 inhibitor | 8 (12) |
| Diuretic | 15 (23) |
| Digoxin | 2 (3) |
| Pimobendan | 1 (1) |
| Calcium channel blocker | 31 (47) |
| Alpha-blocker | 4 (6) |
| Systolic BP (mmHg) | 144.5 ± 7.2 |
| Diastolic BP (mmHg) | 78.2 ± 12.0 |
| BNP (pg/mL) | 166.3 ± 283.0 |
| ANP (pg/mL) | 87.7 ± 58.9 |
| Blood urea nitrogen (mg/dL) | 19.4 ± 5.7 |
| Serum creatinine (mg/dL) | 1.11 ± 0.33 |
| eGFR (mL/min/1.73 m2) | 51.6 ± 14.4 |
| Serum sodium (mEq/L) | 140.8 ± 2.6 |
| Serum potassium (mEq/L) | 4.22 ± 0.43 |
| PRA (ng/mL/h) | 2.58 ± 4.76 |
| Angiotensin-II (U/L) | 30.3 ± 117.8 |
| PAC (pg/mL) | 109.5 ± 62.0 |
| Cortisol (µg/dL) | 10.9 ± 3.4 |
| UACR (g/gCr) | 94.7 ± 141.7 |
| Urinary sodium (mEq/L) | 91.1 ± 38.4 |
| Urinary potassium (mEq/L) | 35.5 ± 21.5 |
| Urinary sodium/potassium ratio | 3.39 ± 1.86 |
| β2-MG (µg/L) | 704.8 ± 1150.9 |
| L-FABP (µg/g Cr) | 8.85 ± 14.3 |
| Test | Drug and p Value | 1 Month | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|---|
| Systolic BP | Esaxerenone | −12.5 ± 12.8 | −12.3 ± 12.9 | −12.1 ± 12.7 | −12.3 ± 12.4 |
| Eplerenone | −2.6 ± 9.6 | −2.2 ± 10.4 | −3.1 ± 10.1 | −1.9 ± 9.0 | |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Diastolic BP | Esaxerenone | −5.4 ± 5.4 | −5.2 ± 4.5 | −5.9 ± 8.0 | −4.4 ± 7.7 |
| Eplerenone | 5.5 ± 6.1 | 5.4 ± 8.8 | 5.7 ± 9.0 | 5.3 ± 6.8 | |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | |
| BUN | Esaxerenone | 11.5 ± 25.1 | 12.6 ± 28.3 | 12.4 ± 24.8 | 10.1 ± 28.6 |
| Eplerenone | 10.3 ± 25.6 | 10.8 ± 34.4 | 11.4 ± 33.5 | 7.1 ± 29.5 | |
| p value | 0.898 | 0.898 | >0.999 | 0.832 | |
| Serum creatinine | Esaxerenone | 0.1 ± 10.9 | 3.5 ± 16.6 | 4.1 ± 14.4 | 1.4 ± 17.1 |
| Eplerenone | 5.4 ± 9.9 | 6.0 ± 12.4 | 6.7 ± 14.2 | 4.6 ± 14.4 | |
| p value | 0.003 | 1 | 0.372 | 0.221 | |
| eGFR | Esaxerenone | 0.14 ± 10.6 | −1.9 ± 16.8 | −3.1 ± 17.1 | 0.26 ± 18.4 |
| Eplerenone | −5.2 ± 10.3 | −6.0 ± 12.8 | −5.9 ± 13.1 | −3.7 ± 15.0 | |
| p value | 0.002 | 0.134 | 0.344 | 0.116 | |
| Serum sodium | Esaxerenone | −0.41 ± 1.72 | −0.35 ± 2.16 | −0.29 ± 1.97 | −0.03 ± 1.76 |
| Eplerenone | −0.13 ± 1.32 | 0.22 ± 1.67 | 0.18 ± 1.57 | −0.26 ± 2.53 | |
| p value | 0.308 | 0.111 | 0.093 | 0.593 | |
| Serum potassium | Esaxerenone | 4.6 ± 10.2 | 5.8 ± 12.2 | 5.4 ± 12.2 | 4.0 ± 10.4 |
| Eplerenone | 3.1 ± 8.1 | 2.9 ± 10.5 | 3.0 ± 11.2 | 1.9 ± 10.3 | |
| p value | 0.521 | 0.251 | 0.322 | 0.501 | |
| PRA | Esaxerenone | - | 194.0 ± 49.6 | 285.7 ± 84.1 | 349.9 ± 66.9 |
| Eplerenone | - | 126.0 ± 48.7 | 166.1 ± 82.6 | 106.1 ± 66.6 | |
| p value | - | 0.334 | 0.316 | 0.012 | |
| Angiotensin II | Esaxerenone | - | 194.9 ± 88.1 | 139.4 ± 75.9 | 174.8 ± 69.6 |
| Eplerenone | - | 109.5 ± 50.1 | 83.7 ± 45.1 | 59.7 ± 48.7 | |
| p value | - | 0.299 | 0.392 | 0.104 | |
| PAC | Esaxerenone | - | 58.0 ± 14.7 | 66.9 ± 24.0 | 42.9 ± 25.5 |
| Eplerenone | - | 21.2 ± 14.7 | 6.6 ± 23.6 | 14.3 ± 25.1 | |
| p value | - | 0.042 | 0.039 | 0.115 | |
| Cortisol | Esaxerenone | - | 8.4 ± 33.2 | 8.6 ± 32.4 | 8.6 ± 35.0 |
| Eplerenone | - | 10.2 ± 39.8 | 10.4 ± 40.9 | 12.3 ± 35.6 | |
| p value | - | 0.722 | 0.639 | 0.646 |
| Test | Drug and p Value | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|
| Sodium | Esaxerenone | 25.2 ± 82.3 | 10.8 ± 56.2 | 19.7 ± 81.3 |
| Eplerenone | 47.5 ± 227.9 | 21.3 ± 101.8 | 40.5 ± 211.7 | |
| p value | 0.419 | 0.301 | 0.382 | |
| Potassium | Esaxerenone | 97.8 ± 308.3 | 76.8 ± 387.8 | 82.7 ± 336.5 |
| Eplerenone | 35.6 ± 148.5 | 33.3 ± 131.3 | 24.4 ± 131.9 | |
| p value | 1 | 0.561 | 0.278 | |
| Urinary sodium/potassium ratio | Esaxerenone | 21.3 ± 95.0 | 16.9 ± 84.8 | 26.2 ± 97.2 |
| Eplerenone | 89.6 ± 472.5 | 31.9 ± 108.1 | 40.9 ± 109.7 | |
| p value | 0.995 | 0.287 | 1 | |
| Collagen IV | Esaxerenone | 67.7 ± 505.5 | −2.6 ± 53.7 | −1.7 ± 62.8 |
| Eplerenone | 45.6 ± 90.5 | 34.5 ± 100.0 | 39.0 ± 79.3 | |
| p value | 0.771 | 0.013 | 0.042 | |
| β2-MG | Esaxerenone | 157.5 ± 124.3 | 81.7 ± 32.1 | 263.9 ± 87.0 |
| Eplerenone | 314.5 ± 122.1 | 130.1 ± 30.8 | 130.3 ± 85.0 | |
| p value | 0.374 | 0.230 | 0.280 | |
| L-FABP | Esaxerenone | 61.4 ± 73.7 | 64.0 ± 145.1 | 53.4 ± 149.2 |
| Eplerenone | 172.9 ± 77.7 | 101.9 ± 35.1 | 15.6 ± 39.3 | |
| p value | 0.326 | 0.458 | >0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sezai, A.; Abe, M.; Maruyama, T.; Taoka, M.; Sekino, H.; Tanaka, M. A Prospective Crossover Clinical Trial of Esaxerenone and Eplerenone in Patients with Chronic Heart Failure Complicated by Hypertension. Life 2025, 15, 741. https://doi.org/10.3390/life15050741
Sezai A, Abe M, Maruyama T, Taoka M, Sekino H, Tanaka M. A Prospective Crossover Clinical Trial of Esaxerenone and Eplerenone in Patients with Chronic Heart Failure Complicated by Hypertension. Life. 2025; 15(5):741. https://doi.org/10.3390/life15050741
Chicago/Turabian StyleSezai, Akira, Msasnori Abe, Takashi Maruyama, Makoto Taoka, Hisakuni Sekino, and Masashi Tanaka. 2025. "A Prospective Crossover Clinical Trial of Esaxerenone and Eplerenone in Patients with Chronic Heart Failure Complicated by Hypertension" Life 15, no. 5: 741. https://doi.org/10.3390/life15050741
APA StyleSezai, A., Abe, M., Maruyama, T., Taoka, M., Sekino, H., & Tanaka, M. (2025). A Prospective Crossover Clinical Trial of Esaxerenone and Eplerenone in Patients with Chronic Heart Failure Complicated by Hypertension. Life, 15(5), 741. https://doi.org/10.3390/life15050741






