Beta-Lactam Antibiotic Concentrations and the Acquisition of Multi-Drug Resistant Bacteria in Critically Ill Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Microbiological Data
2.3. Therapeutic Drug Monitoring
2.4. Data Collection
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Insufficient Drug Levels (Less than 100%fT > 1xMIC)
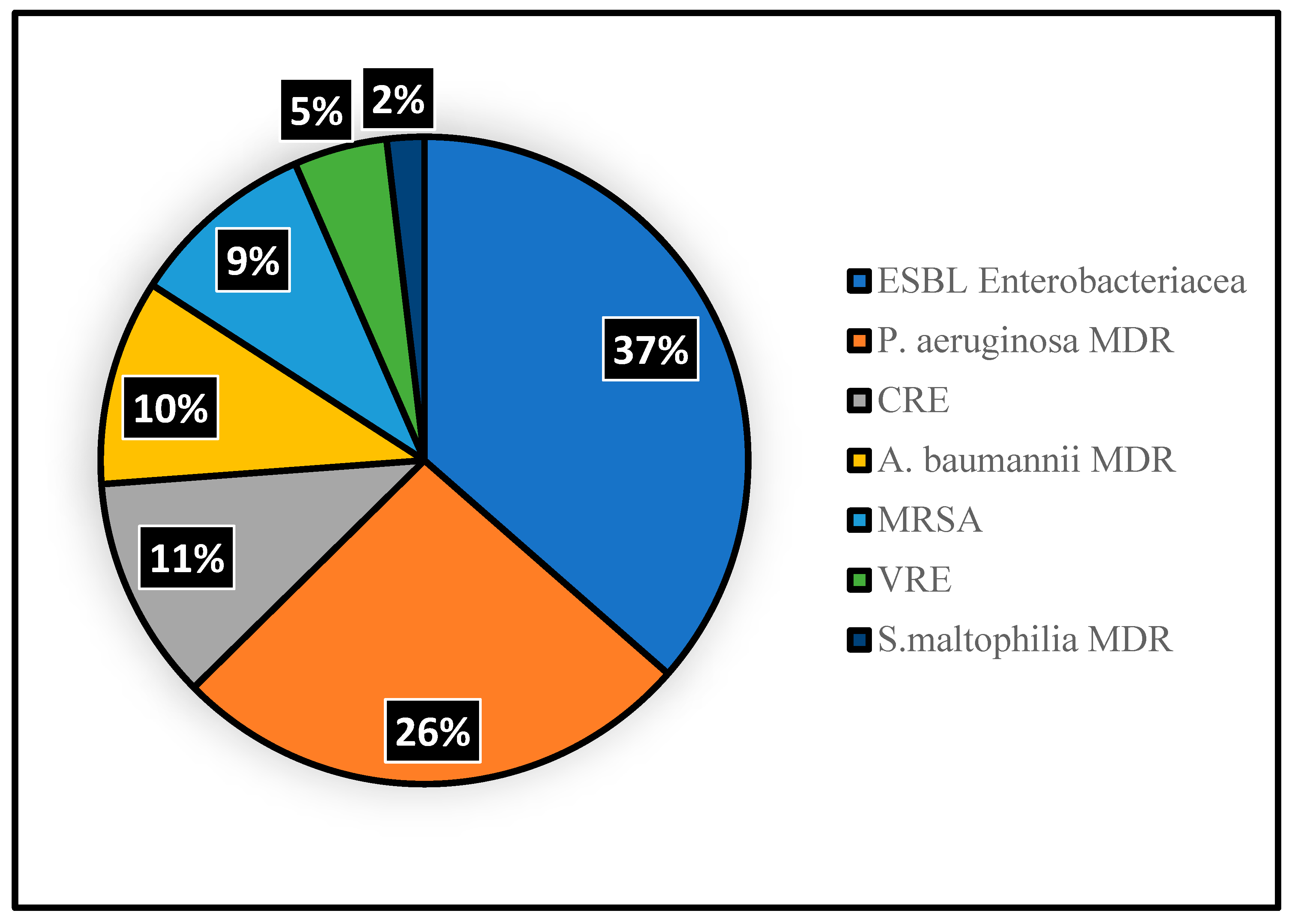
3.3. MDR Acquisition
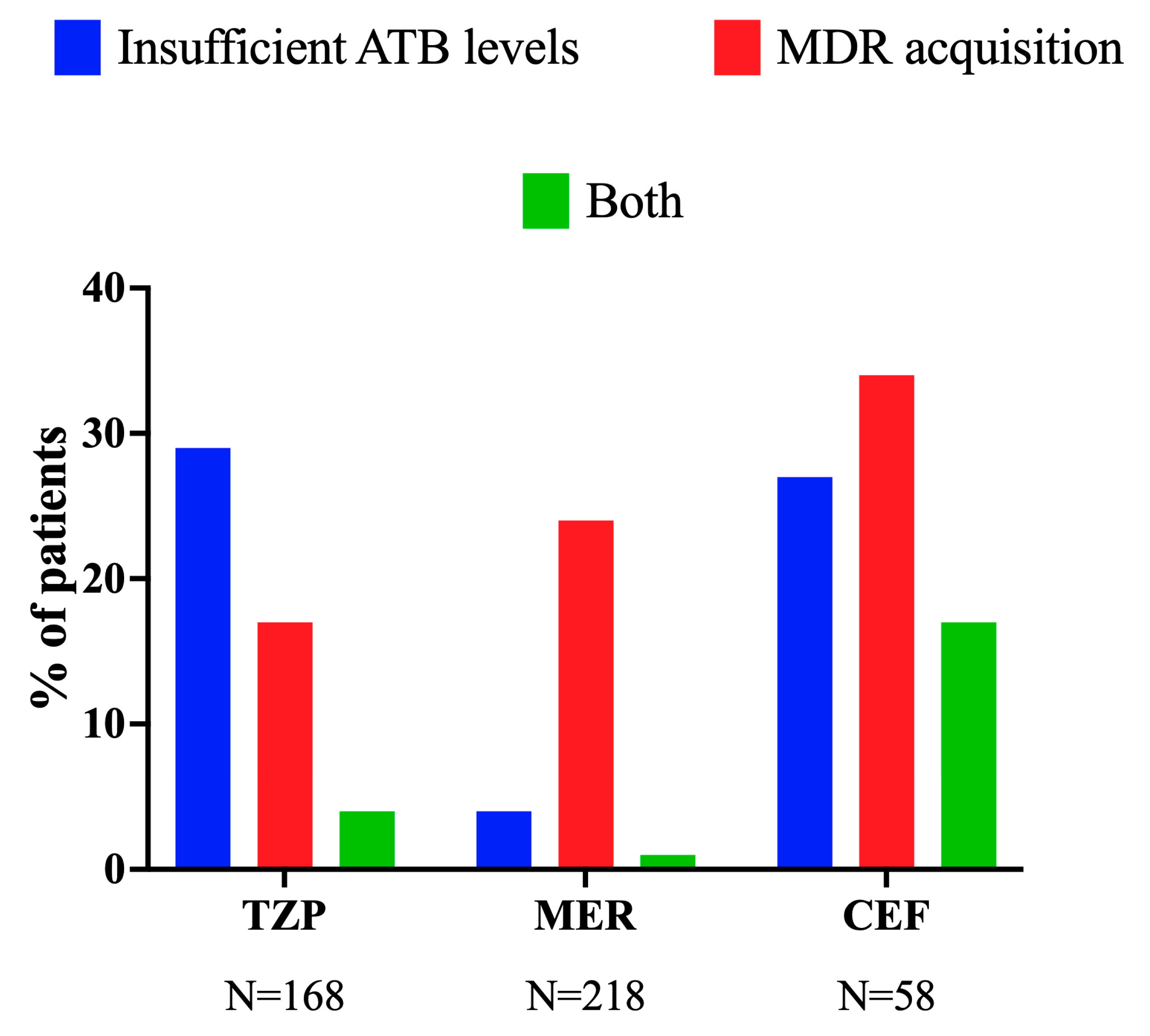
3.4. Insufficient Drug Levels (Less than 100%fT > 1xMIC) and MDR Acquisition
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDR | Multi-drug resistance |
| AMR | Antimicrobial resistance |
| TDM | Therapeutic drug monitoring |
| MPC | Mutant prevention concentration |
| MIC | Minimum inhibitory concentration |
| Pk/Pd | Pharmacokinetic/pharmacodynamic |
| CEF | Ceftazidime/Cefepime |
| TZP | Tazobactam–piperacillin |
| MEM | Meropenem |
| ICU | Intensive Care Unit |
| APACHE | acute physiological and chronic health evaluation |
| SOFA | sequential organ dysfunction assessment |
| COPD | chronic obstructive pulmonary disease |
| ECMO | extracorporeal membrane oxygenation |
| RRT | renal replacement therapy |
References
- Antimicrobial Resistence. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 October 2023).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Luepke, K.H.; Mohr, J.F., 3rd. The antibiotic pipeline: Reviving research and development and speeding drugs to market. Expert. Rev. Anti Infect. Ther. 2017, 15, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.; Diaz Caballero, J.; Kapel, N.; de Winter, F.H.R.; Jangir, P.; Quinn, A.; Del Barrio-Tofino, E.; Lopez-Causape, C.; Hedge, J.; Torrens, G.; et al. Rapid evolution and host immunity drive the rise and fall of carbapenem resistance during an acute Pseudomonas aeruginosa infection. Nat. Commun. 2021, 12, 2460. [Google Scholar] [CrossRef] [PubMed]
- Cabot, G.; Bruchmann, S.; Mulet, X.; Zamorano, L.; Moya, B.; Juan, C.; Haussler, S.; Oliver, A. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob. Agents Chemother. 2014, 58, 3091–3099. [Google Scholar] [CrossRef]
- Shields, R.K.; Chen, L.; Cheng, S.; Chavda, K.D.; Press, E.G.; Snyder, A.; Pandey, R.; Doi, Y.; Kreiswirth, B.N.; Nguyen, M.H.; et al. Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob. Agents Chemother. 2017, 61, e02097-16. [Google Scholar]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Canton, R.; Akova, M.; Carmeli, Y.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; Livermore, D.M.; Miriagou, V.; Naas, T.; Rossolini, G.M.; et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2012, 18, 413–431. [Google Scholar] [CrossRef]
- Brusselaers, N.; Vogelaers, D.; Blot, S. The rising problem of antimicrobial resistance in the intensive care unit. Ann. Intensive Care 2011, 1, 47. [Google Scholar] [CrossRef]
- Martinez, M.N.; Papich, M.G.; Drusano, G.L. Dosing regimen matters: The importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 2012, 56, 2795–2805. [Google Scholar] [CrossRef]
- Pinder, M.; Bellomo, R.; Lipman, J. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth. Intensive Care 2002, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Bloos, F.; Ruddel, H.; Thomas-Ruddel, D.; Schwarzkopf, D.; Pausch, C.; Harbarth, S.; Schreiber, T.; Grundling, M.; Marshall, J.; Simon, P.; et al. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: A cluster randomized trial. Intensive Care Med. 2017, 43, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit. Care 2019, 23, 104. [Google Scholar]
- Goncalves-Pereira, J.; Povoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of beta-lactams. Crit. Care 2011, 15, R206. [Google Scholar] [CrossRef]
- Lodise, T.P.; Lomaestro, B.M.; Drusano, G.L.; Society of Infectious Diseases Pharmacists. Application of antimicrobial pharmacodynamic concepts into clinical practice: Focus on beta-lactam antibiotics: Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2006, 26, 1320–1332. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851, quiz 859. [Google Scholar] [CrossRef]
- Sinnollareddy, M.G.; Roberts, M.S.; Lipman, J.; Roberts, J.A. Beta-lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: A structured review. Clin. Exp. Pharmacol. Physiol. 2012, 39, 489–496. [Google Scholar] [CrossRef]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 12.0. 2022. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 21 October 2023).
- Taccone, F.S.; Laterre, P.F.; Dugernier, T.; Spapen, H.; Delattre, I.; Wittebole, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.L.; et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Vitrat, V.; Hautefeuille, S.; Janssen, C.; Bougon, D.; Sirodot, M.; Pagani, L. Optimizing antimicrobial therapy in critically ill patients. Infect. Drug Resist. 2014, 7, 261–271. [Google Scholar]
- Depuydt, P.; De Waele, J.J. Optimal and responsible use of antibiotics. Curr. Opin. Crit. Care 2019, 25, 458–464. [Google Scholar] [CrossRef]
- Carrie, C.; Legeron, R.; Petit, L.; Ollivier, J.; Cottenceau, V.; d’Houdain, N.; Boyer, P.; Lafitte, M.; Xuereb, F.; Sztark, F.; et al. Higher than standard dosing regimen are needed to achieve optimal antibiotic exposure in critically ill patients with augmented renal clearance receiving piperacillin-tazobactam administered by continuous infusion. J. Crit. Care 2018, 48, 66–71. [Google Scholar] [CrossRef]
- Lipman, J.; Wallis, S.C.; Boots, R.J. Cefepime versus cefpirome: The importance of creatinine clearance. Anesth. Analg. 2003, 97, 1149–1154. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Ellis-Grosse, E.J.; Drusano, G.L. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: Look before you leap! Clin. Infect. Dis. 2010, 51 (Suppl. 1), S103–S110. [Google Scholar] [CrossRef]
- Udy, A.; Boots, R.; Senthuran, S.; Stuart, J.; Deans, R.; Lassig-Smith, M.; Lipman, J. Augmented creatinine clearance in traumatic brain injury. Anesth. Analg. 2010, 111, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Conil, J.M.; Georges, B.; Fourcade, O.; Seguin, T.; Lavit, M.; Samii, K.; Houin, G.; Tack, I.; Saivin, S. Assessment of renal function in clinical practice at the bedside of burn patients. Br. J. Clin. Pharmacol. 2007, 63, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Minville, V.; Asehnoune, K.; Ruiz, S.; Breden, A.; Georges, B.; Seguin, T.; Tack, I.; Jaafar, A.; Saivin, S.; Fourcade, O.; et al. Increased creatinine clearance in polytrauma patients with normal serum creatinine: A retrospective observational study. Crit. Care 2011, 15, R49. [Google Scholar] [CrossRef] [PubMed]
- May, C.C.; Arora, S.; Parli, S.E.; Fraser, J.F.; Bastin, M.T.; Cook, A.M. Augmented Renal Clearance in Patients with Subarachnoid Hemorrhage. Neurocrit. Care 2015, 23, 374–379. [Google Scholar] [CrossRef]
- Brown, R.; Babcock, R.; Talbert, J.; Gruenberg, J.; Czurak, C.; Campbell, M. Renal function in critically ill postoperative patients: Sequential assessment of creatinine osmolar and free water clearance. Crit. Care Med. 1980, 8, 68–72. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef]
- Conil, J.M.; Georges, B.; Mimoz, O.; Dieye, E.; Ruiz, S.; Cougot, P.; Samii, K.; Houin, G.; Saivin, S. Influence of renal function on trough serum concentrations of piperacillin in intensive care unit patients. Intensive Care Med. 2006, 32, 2063–2066. [Google Scholar] [CrossRef]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic initial beta-lactam concentrations in select critically ill patients: Association between augmented renal clearance and low trough drug concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- Taccone, F.S.; Bogossian, E.G.; Tironi, R.M.; Antonucci, E.; Hites, M.; Knoop, C.; Etienne, I.; Jacobs, F.; Creteur, J. Early beta-lactam concentrations and infectious complications after lung transplantation. Am. J. Transplant. 2021, 21, 2489–2497. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Hites, M.; Taccone, F.S.; Wolff, F.; Cotton, F.; Beumier, M.; De Backer, D.; Roisin, S.; Lorent, S.; Surin, R.; Seyler, L.; et al. Case-control study of drug monitoring of beta-lactams in obese critically ill patients. Antimicrob. Agents Chemother. 2013, 57, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10, quiz 11–12. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Chang, K.T.; Zhou, J.; Ledesma, K.R.; Phe, K.; Gao, S.; Van Bambeke, F.; Sanchez-Diaz, A.M.; Zamorano, L.; Oliver, A.; et al. Determining beta-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J. Antimicrob. Chemother. 2017, 72, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Schilling, A.N.; Neshat, S.; Poole, K.; Melnick, D.A.; Coyle, E.A. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 4920–4927. [Google Scholar] [CrossRef]
- Felton, T.W.; Goodwin, J.; O’Connor, L.; Sharp, A.; Gregson, L.; Livermore, J.; Howard, S.J.; Neely, M.N.; Hope, W.W. Impact of Bolus dosing versus continuous infusion of Piperacillin and Tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 5811–5819. [Google Scholar] [CrossRef]
- Tam, V.H.; Schilling, A.N.; Poole, K.; Nikolaou, M. Mathematical modelling response of Pseudomonas aeruginosa to meropenem. J. Antimicrob. Chemother. 2007, 60, 1302–1309. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Lipsitch, M.; Samore, M.H. Antimicrobial use and antimicrobial resistance: A population perspective. Emerg. Infect. Dis. 2002, 8, 347–354. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Hoste, E.A.; De Waele, J.J. Why We May Need Higher Doses of Beta-Lactam Antibiotics: Introducing the ‘Maximum Tolerable Dose’. Antibiotics 2022, 11, 889. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhang, X.J.; Yang, Y.; Gong, W.T.; Xu, B.; Zhu, Y.Q.; Liu, W. Evaluation of meropenem regimens suppressing emergence of resistance in Acinetobacter baumannii with human simulated exposure in an in vitro intravenous-infusion hollow-fiber infection model. Antimicrob. Agents Chemother. 2014, 58, 6773–6781. [Google Scholar] [CrossRef]
- Drusano, G.L.; Hope, W.; MacGowan, A.; Louie, A. Suppression of Emergence of Resistance in Pathogenic Bacteria: Keeping Our Powder Dry, Part 2. Antimicrob. Agents Chemother. 2015, 60, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Firsov, A.A.; Vostrov, S.N.; Lubenko, I.Y.; Drlica, K.; Portnoy, Y.A.; Zinner, S.H. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Gugel, J.; Dos Santos Pereira, A.; Pignatari, A.C.; Gales, A.C. Beta-Lactam MICs correlate poorly with mutant prevention concentrations for clinical isolates of Acinetobacter spp. and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2276–2277. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Femenia, M.À.; Gomis-Font, M.A.; Oliver, A. Mutant prevention concentrations and phenotypic and genomic profiling of first-step resistance mechanisms to classical and novel β-lactams in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2025, 69, e0194224. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; De Kezel, M.; Callant, M.; Boelens, J.; De Bus, L.; Depuydt, P.; De Waele, J.J. Emergence of antimicrobial resistance to piperacillin/tazobactam or meropenem in the ICU: Intermittent versus continuous infusion. A retrospective cohort study. J. Crit. Care 2018, 47, 164–168. [Google Scholar] [CrossRef]
- Beumier, M.; Casu, G.S.; Hites, M.; Wolff, F.; Cotton, F.; Vincent, J.L.; Jacobs, F.; Taccone, F.S. Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015, 81, 497–506. [Google Scholar]
- Roberts, J.A.; Joynt, G.M.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.L.; Stephens, D.; Taccone, F.S.; et al. The Effect of Renal Replacement Therapy and Antibiotic Dose on Antibiotic Concentrations in Critically Ill Patients: Data From the Multinational Sampling Antibiotics in Renal Replacement Therapy Study. Clin. Infect. Dis. 2021, 72, 1369–1378. [Google Scholar] [CrossRef]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef]

| ALL (N = 444) | No AMR (N = 351) | AMR Acquisition (N = 93) | p-Value | |
|---|---|---|---|---|
| Male sex, n (%) | 313 (71) | 258 (74) | 55 (59) | 0.01 |
| Age, mean (SD) | 58 (±15) | 57 (±15) | 56 (±14) | 0.14 |
| APACHE II score on admission, median (IQR) | 23 (17–29) | 23 (17–29) | 25 (17–31) | 0.20 |
| SOFA score, median (IQR) | 10 (7–14) | 10 (7–14) | 10 (7–14) | 0.88 |
| Arterial Hypertension, n (%) | 184 (41) | 151 (43) | 33 (36) | 0.20 |
| Heart disease, n (%) | 221 (50) | 176 (50) | 45 (48) | 0.83 |
| Diabetes Mellitus, n (%) | 113 (26) | 92 (26) | 21 (23) | 0.51 |
| COPD/Asthma, n (%) | 87 (20) | 72 (21) | 15 (16) | 0.38 |
| Immunosuppression, n (%) | 127 (29) | 100 (29) | 27 (29) | 0.90 |
| Cirrhosis, n (%) | 33 (7) | 29 (8) | 4 (4) | 0.27 |
| Chronic Kidney Disease, n (%) | 82 (19) | 67 (19) | 15 (16) | 0.55 |
| Chronic Renal Replacement therapy, n (%) * | 68 (15) | 54 (15) | 14 (15) | 0.99 |
| Malignancy, n (%) | 23 (5) | 19 (5) | 4 (4) | 0.99 |
| Time between admission and antibiotic use, days | 1 (0–5) | 1 (0–5) | 1 (0–4) | 0.19 |
| Time between antibiotic initiation and TDM, days | 2 (1–4) | 2 (1–4) | 2 (1–3) | 0.84 |
| Time at risk, days | 7 (3–12) | 7 (3–13) | 5 (3–11) | 0.17 |
| During ICU stay | ||||
| Vasopressor, n (%) | 230 (52) | 180 (51) | 50 (54) | 0.73 |
| Inotropes, n (%) | 130 (29) | 100 (29) | 30 (32) | 0.52 |
| Mechanical ventilation, n (%) | 234 (53) | 187 (53) | 47 (51) | 0.64 |
| Before MDR acquisition | ||||
| Antibiotic use in the previous 3 months, n (%) | 181 (41) | 142 (41) | 39 (42) | 0.81 |
| Hospitalization in the previous 3 months, n (%) | 202 (46) | 152 (43) | 50 (54) | 0.08 |
| Surgery in the previous 3 months, n (%) | 129 (29) | 96 (27) | 33 (36) | 0.13 |
| Mechanical ventilation, n (%) | 302 (68) | 239 (68) | 63 (68) | 0.99 |
| Central venous catheter, n (%) | 364 (82) | 289 (82) | 75 (81) | 0.76 |
| Urinary tract catheter, n (%) | 329 (74) | 257 (73) | 72 (77) | 0.51 |
| RRT in ICU n, (%) # | 68 (15) | 54 (15) | 14 (15) | 0.99 |
| ECMO, n (%) | 24 (5) | 19 (5) | 5 (5) | 0.99 |
| Previous Colonization by other drug-resistant bacteria, n (%) | 68 (15) | 67 (19) | 27 (29) | 0.05 |
| Source control, n (%) | 95 (21) | 75 (21) | 20 (20) | 0.99 |
| Cmin levels < 1xMIC, n (%) | 65 (15) | 56 (16) | 9 (10) | 0.14 |
| Cmin levels < 4xMIC, n (%) | 305 (69) | 239 (68) | 66 (71) | 0.62 |
| Outcomes | ||||
| ICU length of stay, days, median (IQR) | 14 (10–23) | 14 (9–21) | 18 (12–30) | 0.001 |
| ICU mortality, n (%) | 131 (30) | 110 (31) | 21 (22) | 0.13 |
| Hospital mortality, n (%) | 191 (43) | 156 (44) | 35 (38) | 0.29 |
| Model 1 | Multivariable Analysis sHR (95% CI) | Model 2 | Multivariable Analysis sHR (95% CI) |
|---|---|---|---|
| Insufficient antibiotic levels | 0.84 (0.42–1.68) | Insufficient antibiotic levels | 1.52 (0.95–2.41) |
| Male gender | 0.57 (0.38–0.88) | Male gender | 0.55 (0.36–0.83) |
| APACHE score on admission | 1.02 (0.99–1.05) | APACHE score on admission | 1.03 (0.99–1.06) |
| Previous colonization by other MDR bacteria | 1.05 (0.97–2.33) | Previous colonization by other MDR bacteria | 1.48 (0.96–2.29) |
| Urinary tract catheter | 0.92 (0.54–1.58) | Urinary tract catheter | 0.93 (0.53–1.61) |
| Central venous catheter | 0.86 (0.57–1.45) | Central venous catheter | 0.81 (0.45–1.47) |
| Mechanical ventilation | 0.91 (0.57–1.45) | Mechanical ventilation | 0.90 (0.57–1.45) |
| Previous hospitalization | 1.27 (0.84–1.92) | Previous hospitalization | 1.48 (0.96–2.29) |
| Previous surgery | 1.35 (0.87–2.09) | Previous surgery | 1.39 (0.90–2.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farinella, A.; Salvagno, M.; Minini, A.; Attanasio, L.; Cunha, A.; Menozzi, M.; Saravia, A.; Amado, F.; Gorham, J.; Hites, M.; et al. Beta-Lactam Antibiotic Concentrations and the Acquisition of Multi-Drug Resistant Bacteria in Critically Ill Patients. Life 2025, 15, 739. https://doi.org/10.3390/life15050739
Farinella A, Salvagno M, Minini A, Attanasio L, Cunha A, Menozzi M, Saravia A, Amado F, Gorham J, Hites M, et al. Beta-Lactam Antibiotic Concentrations and the Acquisition of Multi-Drug Resistant Bacteria in Critically Ill Patients. Life. 2025; 15(5):739. https://doi.org/10.3390/life15050739
Chicago/Turabian StyleFarinella, Anita, Michele Salvagno, Andrea Minini, Laila Attanasio, Ana Cunha, Marco Menozzi, Andres Saravia, Filipe Amado, Julie Gorham, Maya Hites, and et al. 2025. "Beta-Lactam Antibiotic Concentrations and the Acquisition of Multi-Drug Resistant Bacteria in Critically Ill Patients" Life 15, no. 5: 739. https://doi.org/10.3390/life15050739
APA StyleFarinella, A., Salvagno, M., Minini, A., Attanasio, L., Cunha, A., Menozzi, M., Saravia, A., Amado, F., Gorham, J., Hites, M., Taccone, F. S., & Gouvêa Bogossian, E. (2025). Beta-Lactam Antibiotic Concentrations and the Acquisition of Multi-Drug Resistant Bacteria in Critically Ill Patients. Life, 15(5), 739. https://doi.org/10.3390/life15050739







