Vitamin D and COVID-19: Clinical Evidence and Immunological Insights
Abstract
1. Introduction
2. Methods
3. Immune Mechanisms of Vitamin D in Viral Infections
- ✓
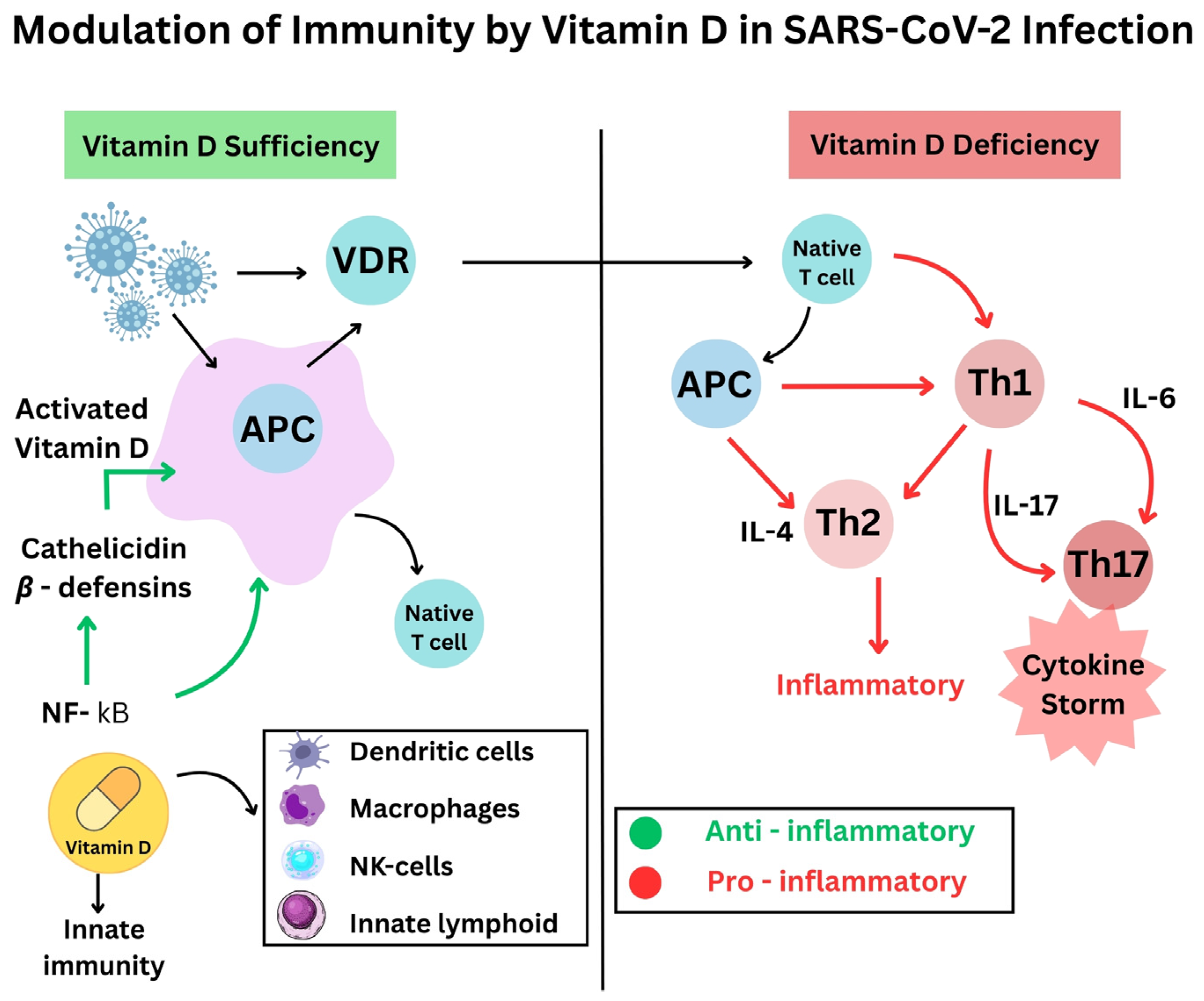
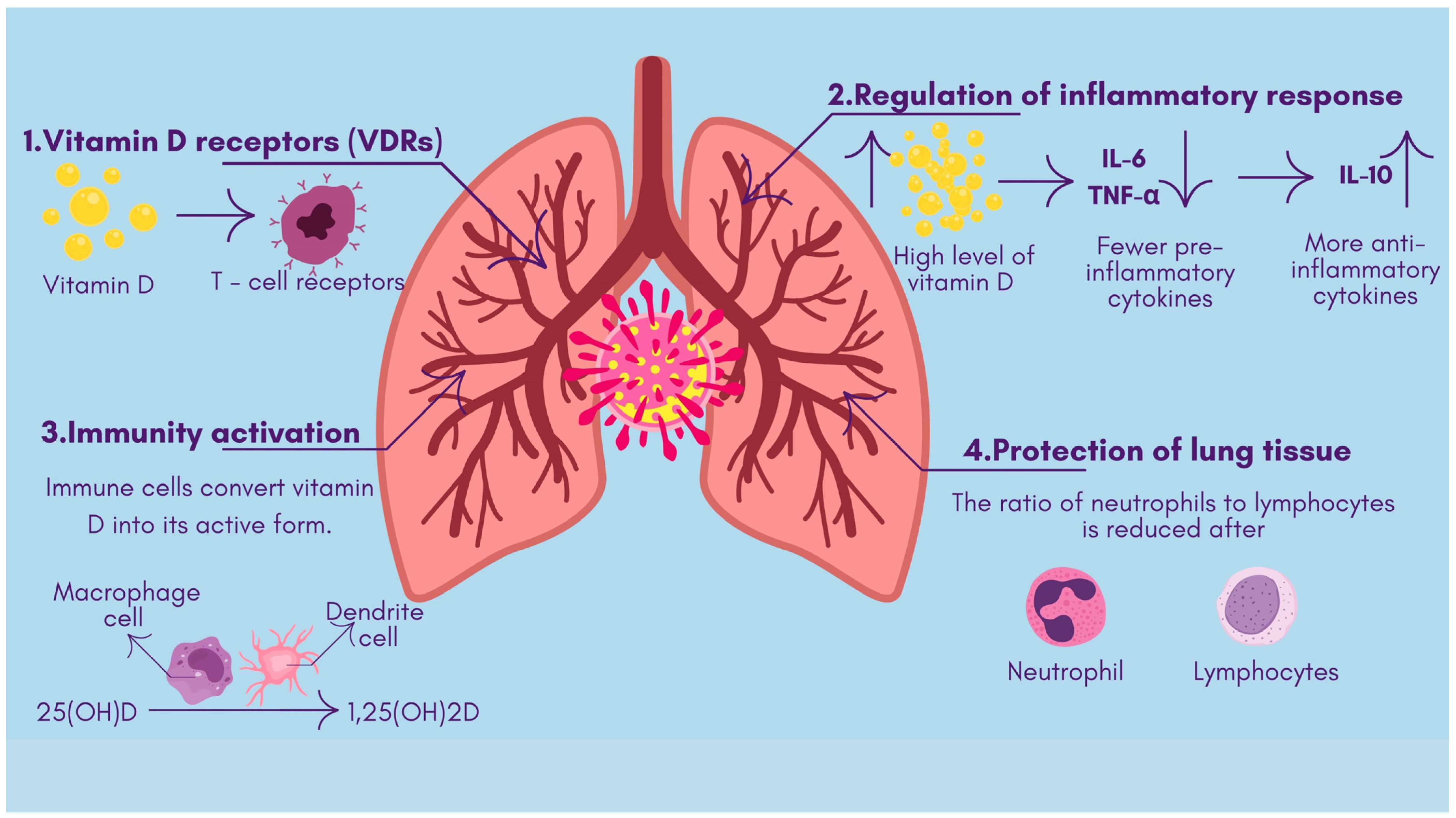
- The antimicrobial effects of vitamin D include both viral replication suppression and protection of respiratory tract epithelial barriers [15]. Antimicrobial peptides, including defensins and cathelicidin, develop from vitamin D activation, improve lung mucosal protection, and directly destroy viruses [16].
- ✓
- Sufficiently high vitamin D levels tend to inhibit overactive inflammatory responses in bodily systems. The use of vitamin D alters T-cell inflammation by turning dangerous pro-inflammatory Th1 and Th17 cells into safer anti-inflammatory Th2 and Treg cell responses [17]. When vitamin D levels rise, the immune system releases fewer pro-inflammatory cytokines, like IL-6 and TNF-α, while increasing the production of anti-inflammatory cytokines, such as IL-10, thus stopping the hyperactive “cytokine storm”. Active vitamin D modifies immune response control mechanisms to stabilize inflammatory processes, thereby preventing dangerous cytokine storm development [18] (Figure 1).
- ✓
- The immune system cells, like macrophages and dendritic cells, that possess a vitamin D receptor undertake the local transformation of 25(OH)D to active 1,25(OH)_2D. Such local activity enables vitamin D to enhance innate immune responses [19]. The signaling mechanisms of vitamin D help macrophages fight pathogens while simultaneously controlling their inflammatory release. Research demonstrates that vitamin D stimulates both regulatory T-cell population magnitude and activity, which helps regulate immune system reactions [20].
- ✓
- When vitamin D controls natural immune activation and helps to resolve inflammatory reactions, it diminishes the level of lung tissue damage. Patients with COVID-19 develop severe illness when their bodies display elevated neutrophil-to-lymphocyte ratios combined with excessive inflammation. A pilot study revealed that patients given vitamin D treatment (calcifediol) had an increased number of lymphocytes and reduced neutrophil-to-lymphocyte ratio values, which showed that they had better immune markers of severe COVID-19 [21]. The immunomodulatory properties of vitamin D potentially allow for milder inflammatory damage to the body while fighting infection (Figure 2).
4. Clinical Trials on Vitamin D and COVID-19 Outcomes
5. Dosage Recommendations and Guidelines
6. Vitamin D Status and Severe COVID-19 Outcomes
7. Role of Vitamin D in Long COVID Patients
8. Highlights
- High-dose vitamin D supplementation has not been recommended as a routine treatment for preventing or treating COVID-19 because researchers lack sufficient proof of its effectiveness.
- The relationship between vitamin D deficiency with levels under 20 ng/mL in serum 25(OH)D and extreme COVID-19 outcomes remains under debate regarding any causal connection.
- Patients with long COVID show widespread vitamin D deficiency patterns, which concurrently worsen all their persistent symptoms, including fatigue, muscle weakness, cognitive impairment, and mood disturbances.
- Evidence is insufficient to draw firm conclusions on whether vitamin D deficiency correction through supplementation helps long COVID patients, irrespective of muscle pain symptoms and fatigue, yet research using randomized controlled trials continues to provide insights into this topic.
9. Limitations of This Review
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IU | International Unit |
| ICU | Intensive Care Unit |
| RCT | Randomized Controlled Trial |
References
- Katz, J.; Yue, S.; Xue, W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition 2021, 84, 111106. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, H.M.A.; Kvietys, P.R.; Shakir, I.; Shams, H.; Grant, W.B.; Alkattan, K. Lung-Centric Inflammation of COVID-19: Potential Modulation by Vitamin D. Nutrients 2021, 13, 2216. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.L.; Golovatyuk, K.A.; Kudryavtsev, I.V.; Chernikova, A.T.; Mikhaylova, A.A.; Aquino, A.D.; Lagutina, D.I.; Zaikova, E.K.; Kalinina, O.V.; Golovkin, A.S.; et al. Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study. Nutrients 2022, 14, 2602. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Takase, T.; Tsugawa, N.; Sugiyama, T.; Ikesue, H.; Eto, M.; Hashida, T.; Tomii, K.; Muroi, N. Association between 25-hydroxyvitamin D levels and COVID-19 severity. Clin. Nutr. ESPEN 2022, 49, 256–263. [Google Scholar] [CrossRef]
- Akbar, M.R.; Wibowo, A.; Pranata, R.; Setiabudiawan, B. Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated With Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 660420. [Google Scholar] [CrossRef]
- Sartini, M.; Del Puente, F.; Oliva, M.; Carbone, A.; Bobbio, N.; Schinca, E.; Giribone, L.; Cristina, M.L. Preventive Vitamin D Supplementation and Risk for COVID-19 Infection: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 679. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Martineau, A.R.; Curtis, E.M.; Moon, R.J.; Darling, A.; Lanham-New, S.; Ward, K.A.; Cooper, C.; Munroe, P.B.; Petersen, S.E.; et al. Vitamin D and coronavirus disease 2019 (COVID-19): Rapid evidence review. Aging Clin. Exp. Res. 2021, 33, 2031–2041. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Bhalekar, P.; Shambharkar, P. Effect of vitamin D supplementation on the immune response to respiratory tract infections and inflammatory conditions: A systematic review and meta-analysis. Hum. Nutr. Metab. 2024, 37, 200272. [Google Scholar] [CrossRef]
- Gibbons, J.B.; Norton, E.C.; McCullough, J.S.; Meltzer, D.O.; Lavigne, J.; Fiedler, V.C.; Gibbons, R.D. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci. Rep. 2022, 12, 19397. [Google Scholar] [CrossRef]
- Vitamin D—Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 24 March 2025).
- Hariyanto, T.I.; Intan, D.; Hananto, J.E.; Harapan, H.; Kurniawan, A. Vitamin D supplementation and COVID-19 outcomes: A systematic review, meta-analysis and meta-regression. Rev. Med. Virol. 2022, 32, e2269. [Google Scholar] [CrossRef]
- Shah, K.; Varna, V.P.; Sharma, U.; Mavalankar, D. Does vitamin D supplementation reduce COVID-19 severity?: A systematic review. Qjm Int. J. Med. 2022, 115, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Díaz, J.A.; Julve, J.; Vlacho, B.; Corcoy, R.; Ponte, P.; Román, E.; Navas-Méndez, E.; Llauradó, G.; Franch-Nadal, J.; Domingo, P.; et al. Previous Vitamin D Supplementation and Morbidity and Mortality Outcomes in People Hospitalised for COVID19: A Cross-Sectional Study. Front. Public Health 2021, 9, 758347. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Rustecka, A.; Lipińska-Opałka, A.; Piprek, R.P.; Kloc, M.; Kalicki, B.; Kubiak, J.Z. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front. Pharmacol. 2022, 13, 836738. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.K.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2020, 5, e10405. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Griffin, G.; Kenny, R.A. Perspective: Vitamin D deficiency and COVID-19 severity–plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J. Intern. Med. 2021, 289, 97–115. [Google Scholar] [CrossRef]
- Grant, W.B.; Lordan, R. Vitamin D for COVID-19 on Trial: An Update on Prevention and Therapeutic Application. Endocr. Pract. 2021, 27, 1266–1268. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Holt, H.; Greenig, M.; Talaei, M.; Perdek, N.; Pfeffer, P.; Vivaldi, G.; Maltby, S.; Symons, J.; Barlow, N.L.; et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and COVID-19: Phase 3 randomised controlled trial (CORONAVIT). BMJ 2022, 378, e071230. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Entrenas Castillo, M.E.; Entrenas Costa, L.M.E.; Vaquero Barrios, J.M.V.; Alcalá Díaz, J.F.A.; López Miranda, J.L.; Bouillon, R.; Quesada Gomez, J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R. Notable Developments for Vitamin D Amid the COVID-19 Pandemic, but Caution Warranted Overall: A Narrative Review. Nutrients 2021, 13, 740. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Gonsard, J.; Boucher, S.; Chapelet, G.; Darsonval, A.; Fougère, B.; Guérin, O.; Houvet, M.; et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med. 2022, 19, e1003999. [Google Scholar] [CrossRef]
- Villasis-Keever, M.A.; López-Alarcón, M.G.; Miranda-Novales, G.; Zurita-Cruz, J.N.; Barrada-Vázquez, A.S.; González-Ibarra, J.; Martínez-Reyes, M.; Grajales-Muñiz, C.; Santacruz-Tinoco, C.E.; Martínez-Miguel, B.; et al. Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial. Arch. Med. Res. 2022, 53, 423–430. [Google Scholar] [CrossRef]
- Mariani, J.; Antonietti, L.; Tajer, C.; Ferder, L.; Inserra, F.; Cunto, M.S.; Brosio, D.; Ross, F.; Zylberman, M.; López, D.E.; et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial. PLoS ONE 2022, 17, e0267918. [Google Scholar] [CrossRef]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef]
- Petrelli, F.; Oldani, S.; Borgonovo, K.; Cabiddu, M.; Dognini, G.; Ghilardi, M.; Parati, M.C.; Petro’, D.; Dottorini, L.; Rea, C.; et al. Vitamin D3 and COVID-19 Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Antioxidants 2023, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment With 25-Hydroxyvitamin D3 (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Register—Search for COVID-19 and Vitamin D. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=covid-19+and+Vitamin+D (accessed on 24 April 2025).
- Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001717-20/ES (accessed on 24 April 2025).
- Study Details | Oral 25-Hydroxyvitamin D3 and COVID-19 | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04386850 (accessed on 24 April 2025).
- Study Details | Evaluation of the Relationship Between Zinc Vitamin D and b12 Levels in the COVID-19 Positive Pregnant Women | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04407572 (accessed on 24 April 2025).
- Bahat, P.Y.; Talmac, M.A.; Bestel, A.; Selcuki, N.F.T.; Aydın, Z.; Polat, I. Micronutrients in COVID-19 Positive Pregnancies. Cureus 2020, 12, e10609. [Google Scholar] [CrossRef]
- Ried, K.; BinJemain, T.; Sali, A. Therapies to Prevent Progression of COVID-19, Including Hydroxychloroquine, Azithromycin, Zinc, and Vitamin D3 With or Without Intravenous Vitamin C: An International, Multicenter, Randomized Trial. Cureus 2021, 13, e19902. [Google Scholar] [CrossRef]
- Study Details | International ALLIANCE Study of Therapies to Prevent Progression of COVID-19 | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04395768 (accessed on 24 April 2025).
- Wang, R.; DeGruttola, V.; Lei, Q.; Mayer, K.H.; Redline, S.; Hazra, A.; Mora, S.; Willett, W.C.; Ganmaa, D.; Manson, J.E. The vitamin D for COVID-19 (VIVID) trial: A pragmatic cluster-randomized design. Contemp. Clin. Trials 2021, 100, 106176. [Google Scholar] [CrossRef]
- Study Details | Effect of Vitamin D on Morbidity and Mortality of the COVID-19 | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04552951 (accessed on 24 April 2025).
- Cannata-Andía, J.B.; Díaz-Sottolano, A.; Fernández, P.; Palomo-Antequera, C.; Herrero-Puente, P.; Mouzo, R.; Carrillo-López, N.; Panizo, S.; Ibañez, G.H.; Cusumano, C.A.; et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: The COVID-VIT-D—A randomised multicentre international clinical trial. BMC Med. 2022, 20, 83. [Google Scholar] [CrossRef]
- Trial | NCT04579640. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT04579640/ (accessed on 24 April 2025).
- Study Details | COVID-19 and Vitamin D Supplementation: A Multicenter Randomized Controlled Trial of High Dose Versus Standard Dose Vitamin D3 in High-Risk COVID-19 Patients (CoVitTrial) | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04344041?cond=NCT04344041&rank=1 (accessed on 24 April 2025).
- Study Details | The LEAD COVID-19 Trial: Low-Risk, Early Aspirin and Vitamin D to Reduce COVID-19 Hospitalizations | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04363840 (accessed on 24 April 2025).
- Stroehlein, J.K.; Wallqvist, J.; Iannizzi, C.; Mikolajewska, A.; Metzendorf, M.-I.; Benstoem, C.; Meybohm, P.; Becker, M.; Skoetz, N.; Stegemann, M.; et al. Vitamin D supplementation for the treatment of COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 2021, CD015043. [Google Scholar] [CrossRef]
- Study Details | Vitamin D Supplementation in the Prevention and Mitigation of COVID-19 Infection | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04482673?cond=NCT04482673&rank=1 (accessed on 24 April 2025).
- Study Details | Vitamin D Testing and Treatment for COVID 19 | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04407286 (accessed on 24 April 2025).
- Babajani, F.; Kakavand, A.; Mohammadi, H.; Sharifi, A.; Zakeri, S.; Asadi, S.; Afshar, Z.M.; Rahimi, Z.; Sayad, B. COVID-19 and renin angiotensin aldosterone system: Pathogenesis and therapy. Health Sci. Rep. 2021, 4, e440. [Google Scholar] [CrossRef]
- Study Details | Trial of Combination Therapy to Treat COVID-19 Infection | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04482686 (accessed on 24 April 2025).
- Study Details | Vitamin D Supplementation in Patients with COVID-19 | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04449718 (accessed on 24 April 2025).
- Fernandes, A.L.; Sales, L.P.; Santos, M.D.; Caparbo, V.F.; Murai, I.H.; Pereira, R.M.R. Persistent or new symptoms 1 year after a single high dose of vitamin D3 in patients with moderate to severe COVID-19. Front. Nutr. 2022, 9, 979667. [Google Scholar] [CrossRef]
- Fernandes, A.L.; Murai, I.H.; Reis, B.Z.; Sales, L.P.; Santos, M.D.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; et al. Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19. Am. J. Clin. Nutr. 2022, 115, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Antonangelo, L.; Gualano, B.; Pereira, R.M.R. Effect of a Single High-Dose Vitamin D3 on the Length of Hospital Stay of Severely 25-Hydroxyvitamin D-Deficient Patients with COVID-19. Clinics 2021, 76, e3549. [Google Scholar] [CrossRef] [PubMed]
- Study Details | High Dose Vitamin-D Substitution in Patients with COVID-19: A Randomized Controlled, Multi Center Study | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04525820 (accessed on 24 April 2025).
- Jaun, F.; Boesing, M.; Lüthi-Corridori, G.; Abig, K.; Makhdoomi, A.; Bloch, N.; Lins, C.; Raess, A.; Grillmayr, V.; Haas, P.; et al. High-dose vitamin D substitution in patients with COVID-19: Study protocol for a randomized, double-blind, placebo-controlled, multi-center study—VitCov Trial. Trials 2022, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Study Details | Efficacy of Vitamin D Supplementation to Prevent the Risk of Acquiring COVID-19 in Healthcare Workers | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04535791 (accessed on 24 April 2025).
- Study Details | Efficacy of Vitamin D Treatment in Pediatric Patients Hospitalized by COVID-19 | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04502667 (accessed on 24 April 2025).
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79–80, 111017. [Google Scholar] [CrossRef]
- Study Details | A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04335084 (accessed on 24 April 2025).
- Speakman, L.; Michienzi, S.; Badowski, M. Vitamins, supplements and COVID-19: A review of currently available evidence. Drugs Context 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Study Details | Prevention of COVID-19 With Oral Vitamin D Supplemental Therapy in Essential healthCare Teams | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04483635 (accessed on 24 April 2025).
- Ducharme, F.M.; Tremblay, C.; Golchi, S.; Hosseini, B.; Longo, C.; White, J.H.; Coviello, D.; Quach, C.; Ste-Marie, L.-G.; Platt, R.W. Prevention of COVID-19 with oral vitamin D supplemental therapy in essential healthcare teams (PROTECT): Protocol for a multicentre, triple-blind, randomised, placebo-controlled trial. BMJ Open 2023, 13, e064058. [Google Scholar] [CrossRef]
- Study Details | Vitamin D and Zinc Supplementation for Improving Treatment Outcomes Among COVID-19 Patients in India | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04641195 (accessed on 24 April 2025).
- Sharma, K.K.; Partap, U.; Mistry, N.; Marathe, Y.; Wang, M.; Shaikh, S.; D’Costa, P.; Gupta, G.; Bromage, S.; Hemler, E.; et al. Randomised trial to determine the effect of vitamin D and zinc supplementation for improving treatment outcomes among patients with COVID-19 in India: Trial protocol. BMJ Open 2022, 12, e061301. [Google Scholar] [CrossRef]
- Study Details | Vitamin D and COVID-19 Management | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04385940 (accessed on 24 April 2025).
- Study Details | Investigating the Role of Vitamin D in the Morbidity of COVID-19 Patients | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04386044 (accessed on 24 April 2025).
- Study Details | Baseline Vitamin D Deficiency and COVID-19 Disease Severity | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04628000 (accessed on 24 April 2025).
- Study Details | Increased Risk of Severe Coronavirus Disease 2019 in Patients with Vitamin D Deficiency | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04403932 (accessed on 24 April 2025).
- Study Details | Vitamin D Status and Immune-Inflammatory Status in Different UK Populations with COVID-19 Infection | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04519034 (accessed on 24 April 2025).
- Study Details | Should Ranges of Vitamin D be Redefined to Prevent or Treat Viral Infections? | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04394390 (accessed on 24 April 2025).
- Study Details | Cholecalciferol to Improve the Outcomes of COVID-19 Patients | ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04411446#study-overview (accessed on 24 April 2025).
- Study Details | Vitamin D on Prevention and Treatment of COVID-19 | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04334005 (accessed on 24 April 2025).
- Zhang, Y.; Li, J.; Yang, M.; Wang, Q. Effect of vitamin D supplementation on COVID-19 patients: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1131103. [Google Scholar] [CrossRef]
- Grant, W.B.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R.Z. Vitamin D: Evidence-Based Health Benefits and Recommendations for Population Guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef]
- Vitamin D—NHS. Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-d/ (accessed on 25 March 2025).
- SACN Vitamin D and Health Report—GOV.UK. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 25 March 2025).
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Dramé, M.; Cofais, C.; Hentzien, M.; Proye, E.; Coulibaly, P.S.; Demoustier-Tampère, D.; Destailleur, M.-H.; Lotin, M.; Cantagrit, E.; Cebille, A.; et al. Relation between Vitamin D and COVID-19 in Aged People: A Systematic Review. Nutrients 2021, 13, 1339. [Google Scholar] [CrossRef]
- COVID-19 Rapid Guideline: Vitamin D | Guidance | NICE. Available online: https://www.nice.org.uk/guidance/ng191/chapter/Recommendations-for-research (accessed on 14 March 2025).
- COVID-19 Rapid Guideline: Vitamin, D. COVID-19 Rapid Guideline: Vitamin D, December 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK566063/ (accessed on 25 March 2025).
- Nitulescu, G.M.; Paunescu, H.; Moschos, S.A.; Petrakis, D.; Nitulescu, G.; Ion, G.N.D.; Spandidos, D.A.; Nikolouzakis, T.K.; Drakoulis, N.; Tsatsakis, A. Comprehensive analysis of drugs to treat SARS-CoV-2 infection: Mechanistic insights into current COVID-19 therapies (Review). Int. J. Mol. Med. 2020, 46, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Ginde, A.A. Vitamin D3 to Treat COVID-19. JAMA 2021, 325, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- SACN Rapid Review: Vitamin D and Acute Respiratory Tract Infections—GOV.UK. Available online: https://www.gov.uk/government/publications/sacn-rapid-review-vitamin-d-and-acute-respiratory-tract-infections (accessed on 25 March 2025).
- De Smet, D.; De Smet, K.; Herroelen, P.; Gryspeerdt, S.; A Martens, G. Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality. Am. J. Clin. Pathol. 2021, 155, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalised with COVID-19 are associated with greater disease severity: Results of a local audit of practice. Clin. Endocrinol. 2020, 93, 508–511. [Google Scholar] [CrossRef]
- Hernández, J.L.; Nan, D.; Fernandez-Ayala, M.; García-Unzueta, M.; Hernández-Hernández, M.A.; López-Hoyos, M.; Muñoz-Cacho, P.; Olmos, J.M.; Gutiérrez-Cuadra, M.; Ruiz-Cubillán, J.J.; et al. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2021, 106, e1343–e1353. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Purkart, T.U.; Waltensdorfer, A.; Münch, A.; Warnkross, H.; et al. Effect of high-dose vitamin D3on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA J. Am. Med. Assoc. 2014, 312, 1520–1530. [Google Scholar] [CrossRef]
- Vogiatzi, M.G.; Jacobson-Dickman, E.; DeBoer, M.D. Vitamin D supplementation and risk of toxicity in pediatrics: A review of current literature. J. Clin. Endocrinol. Metab. 2014, 99, 1132–1141. [Google Scholar] [CrossRef]
- Auguste, B.L.; Avila-Casado, C.; Bargman, J.M. Use of vitamin D drops leading to kidney failure in a 54-year-old man. Can. Med. Assoc. J. 2019, 191, E395. [Google Scholar] [CrossRef]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef]
- Luo, X.; Liao, Q.; Shen, Y.; Li, H.; Cheng, L. Vitamin D Deficiency Is Associated with COVID-19 Incidence and Disease Severity in Chinese People [corrected]. J. Nutr. 2021, 151, 98–103. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, A.; Leopold, K.; Jackson, S.; Christensen, S.; Nayfeh, T.; Mohammed, K.; Creo, A.; Tebben, P.; Kumar, S. Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2022, 96, 281–287. [Google Scholar] [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F.; Jude, E.B. High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study. Nutrients 2020, 12, 3799. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Dantas Damascena, A.D.; Galvão Azevedo, L.M.G.; de Almeida Oliveira, T.D.A.; da Mota Santana, J.D.M. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Yisak, H.; Ewunetei, A.; Kefale, B.; Mamuye, M.; Teshome, F.; Ambaw, B.; Yitbarek, G.Y. Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review. Risk Manag. Health Policy 2021, 14, 31–38. [Google Scholar] [CrossRef]
- Herrera-Quintana, L.; Gamarra-Morales, Y.; Vázquez-Lorente, H.; Molina-López, J.; Castaño-Pérez, J.; Machado-Casas, J.F.; Coca-Zúñiga, R.; Pérez-Villares, J.M.; Planells, E. Bad Prognosis in Critical Ill Patients with COVID-19 during Short-Term ICU Stay regarding Vitamin D Levels. Nutrients 2021, 13, 1988. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.S.; Sucosky, M.S.; Lange, S.J.; Gundlapalli, A.V.; Boehmer, T.K.; Blanck, H.M. Body Mass Index and Risk for COVID-19–Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death—United States, March–December 2020. Mmwr-Morb. Mortal. Wkly. Rep. 2021, 70, 355–361. [Google Scholar] [CrossRef]
- Brunvoll, S.H.; Nygaard, A.B.; Ellingjord-Dale, M.; Holland, P.; Istre, M.S.; Kalleberg, K.T.; Søraas, C.L.; Holven, K.B.; Ulven, S.M.; Hjartåker, A.; et al. Prevention of COVID-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: Quadruple blinded, randomised placebo controlled trial. BMJ 2022, 378, e071245. [Google Scholar] [CrossRef]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef]
- Romero-Ibarguengoitia, M.E.; Gutiérrez-González, D.; Cantú-López, C.; Sanz-Sánchez, M.Á.; González-Cantú, A. Effect of Vitamin D3 Supplementation vs. Dietary–Hygienic Measures on SARS-CoV-2 Infection Rates in Hospital Workers with 25-Hydroxyvitamin D3 [25(OH)D3] Levels ≥20 ng/mL. Microorganisms 2023, 11, 282. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, T.; Heianza, Y.; Qi, L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: A prospective study in UK Biobank. Am. J. Clin. Nutr. 2021, 113, 1275–1281. [Google Scholar] [CrossRef]
- Parant, F.; Bouloy, J.; Haesebaert, J.; Bendim’red, L.; Goldet, K.; Vanhems, P.; Henaff, L.; Gilbert, T.; Cuerq, C.; Blond, E.; et al. Vitamin D and COVID-19 Severity in Hospitalized Older Patients: Potential Benefit of Prehospital Vitamin D Supplementation. Nutrients 2022, 14, 1641. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, B.; Fatti, L.M.; Danesi, L.; Gazzano, G.; Croci, M.; Vitale, G.; Gilardini, L.; Bonadonna, S.; Chiodini, I.; Caparello, C.F.; et al. Mortality in an Italian nursing home during COVID-19 pandemic: Correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging 2020, 12, 24522–24534. [Google Scholar] [CrossRef] [PubMed]
- Oristrell, J.; Oliva, J.C.; Subirana, I.; Casado, E.; Domínguez, D.; Toloba, A.; Aguilera, P.; Esplugues, J.; Fafián, P.; Grau, M. Association of calcitriol supplementation with reduced COVID-19 mortality in patients with chronic kidney disease: A population-based study. Biomedicines 2021, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Bogliolo, L.; Lobascio, F.; Barichella, M.; Zecchinelli, A.L.; Pezzoli, G.; Caccialanza, R. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition 2021, 82, 111055. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 561–565. [Google Scholar] [CrossRef]
- Hosseini, B.; El Abd, A.; Ducharme, F.M. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2134. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism 2021, 115, 154434. [Google Scholar] [CrossRef]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Cohen, A.G.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Di Lecce, V.; Quaranta, V.N.; Zito, A.; Buonamico, E.; Capozza, E.; Palumbo, A.; Di Gioia, G.; Valerio, V.N.; Resta, O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 2021, 44, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Abbeele, K.V.D.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2021, 97, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Je, Y.S.; Baek, J.; Chung, M.-H.; Kwon, H.Y.; Lee, J.-S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020, 100, 390–393. [Google Scholar] [CrossRef]
- Taha, R.; Abureesh, S.; Alghamdi, S.; Hassan, R.Y.; Cheikh, M.M.; Bagabir, R.A.; Almoallim, H.; Abdulkhaliq, A. The Relationship Between Vitamin D and Infections Including COVID-19: Any Hopes? Int. J. Gen. Med. 2021, 14, 3849–3870. [Google Scholar] [CrossRef]
- Alcala-Diaz, J.F.; Limia-Perez, L.; Gomez-Huelgas, R.; Martin-Escalante, M.D.; Cortes-Rodriguez, B.; Zambrana-Garcia, J.L.; Entrenas-Castillo, M.; Perez-Caballero, A.I.; López-Carmona, M.D.; Garcia-Alegria, J.; et al. Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Nikolakopoulou, S.; Konstantinou, A.; Mascha, O.; Siarkos, E.; Samaras, C.; Athanassiou, P.; Shoenfeld, Y. Vitamin D Levels as a Marker of Severe SARS-CoV-2 Infection. Life 2024, 14, 210. [Google Scholar] [CrossRef]
- Weir, E.K.; Thenappan, T.; Bhargava, M.; Chen, Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. 2020, 20, E107–E108. [Google Scholar] [CrossRef]
- Albergamo, A.; Apprato, G.; Silvagno, F. The Role of Vitamin D in Supporting Health in the COVID-19 Era. Int. J. Mol. Sci. 2022, 23, 3621. [Google Scholar] [CrossRef]
- Huang, L.; Song, Z.; Lu, C.; Wang, S.; Guo, C.; Lai, X.-H.; Zhao, Z. A narrative review focusing on randomized clinical trials of vitamin D supplementation for COVID-19 disease. Front. Nutr. 2024, 11, 1461485. [Google Scholar] [CrossRef]
- Schoenmakers, I.; Fraser, W.D.; Forbes, A. Vitamin D and acute and severe illness—A mechanistic and pharmacokinetic perspective. Nutr. Res. Rev. 2023, 36, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Atieh, O.; Daher, J.; Durieux, J.C.; Abboud, M.; Labbato, D.; Baissary, J.; Koberssy, Z.; Ailstock, K.; Cummings, M.; Funderburg, N.T.; et al. Vitamins K2 and D3 Improve Long COVID, Fungal Translocation, and Inflammation: Randomized Controlled Trial. Nutrients 2025, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Chadda, K.R.; Roberts, S.A.; Lugg, S.T.; Faniyi, A.A.; Faustini, S.E.; Webster, C.; Duffy, J.E.; Hewison, M.; Shields, A.; Richter, A.G.; et al. Vitamin D deficiency and duration of COVID-19 symptoms in UK healthcare workers. Front. Med. 2024, 11, 1494129. [Google Scholar] [CrossRef]
- Menéndez, S.G.; Giménez, V.M.M.; Holick, M.F.; Barrantes, F.J.; Manucha, W. COVID-19 and neurological sequelae: Vitamin D as a possible neuroprotective and/or neuroreparative agent. Life Sci. 2022, 297, 120464. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, R.G.; Wejse, C.; Agergaard, J. Effect of Vitamin D in Long COVID Patients. Int. J. Environ. Res. Public Health 2023, 20, 7058. [Google Scholar] [CrossRef]
- di Filippo, L.; Frara, S.; Nannipieri, F.; Cotellessa, A.; Locatelli, M.; Querini, P.R.; Giustina, A. Low Vitamin D Levels Are Associated With Long COVID Syndrome in COVID-19 Survivors. J. Clin. Endocrinol. Metab. 2023, 108, e1106–e1116. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Grant, W.B.; Frias-Toral, E.; Sarno, G.; Vetrani, C.; Ceriani, F.; Garcia-Velasquez, E.; Contreras-Briceño, J.; Savastano, S.; et al. Vitamin D: A Role Also in Long COVID-19? Nutrients 2022, 14, 1625. [Google Scholar] [CrossRef]
- Hussein, A.A.R.M.; Galal, I.; Amin, M.T.; Moshnib, A.A.; Makhlouf, N.A.; Makhlouf, H.A.; Abd-Elaal, H.K.; Kholief, K.M.S.; Tawab, D.A.A.; Eldin, K.A.K.; et al. Prevalence of vitamin D deficiency among patients attending Post COVID-19 follow-up clinic: A cross-sectional study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3038–3045. [Google Scholar] [CrossRef]
- O’donovan, J.; Cheong, J.; Chambler, D. Vitamin D Levels in COVID-19 Patients Admitted to Intensive Care. Health 2023, 15, 845–860. [Google Scholar] [CrossRef]
- Sabit, H.; Abdel-Ghany, S.; Abdallah, M.S.; Abul-Maaty, O.; Khoder, A.I.; Shoman, N.A.; Farrag, M.S.; Martasek, P.; Noreddin, A.M.; Nazih, M. Vitamin D: A key player in COVID-19 immunity and lessons from the pandemic to combat immune-evasive variants. Inflammopharmacology 2024, 32, 3631–3652. [Google Scholar] [CrossRef]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Vitamin D and COVID-19: Evidence and recommendations for supplementation. R. Soc. Open Sci. 2020, 7, 201912. [Google Scholar] [CrossRef]
| Study Title | Registry ID | Phase | Study Type | Recruitment Status | Results Posted | Registry Link |
|---|---|---|---|---|---|---|
| Efficacy of Vitamin D Treatment in Patients Diagnosed with Pneumonia who Require Hospital Admission and have Vitamin D Deficiency and a Positive Diagnosis for SARS-CoV-2 (COVID-19) | EudraCT 2020-001960-28 | – (Not stated) | I | Ongoing | No | EU CTR [35] |
| Preventing Disease Aggravation in COVID-19 by High Dose Vitamin D: a Randomized Trial (COVIT-D) | EudraCT 2020-001793-30 | – (Not stated) | I | Prematurely Ended | No | EU CTR [35] |
| Usefulness of Vitamin D on Morbidity and Mortality of SARS-CoV-2 Infection (COVID-19) at the Central University Hospital of Asturias | EudraCT 2020-002274-28 | – (Not stated) | I | Completed | No | EU CTR [35] |
| COVID-19 and Vitamin D Supplementation: a Multicenter Randomized Controlled Trial of High Dose versus Standard Dose Vitamin 3 in High-Risk COVID-19 Patients | EudraCT 2020-001602-34 | – (Not stated) | I | Completed | No | EU CTR [35] |
| COVID-19 Prophylaxis with Hydroxychloroquine, Vitamin D, and Zinc Supplementation in Danish Nursing Home Residents—a Randomized Controlled Trial | EudraCT 2020-001363-85 | – (Not stated) | I | Prematurely Ended | Yes (Posted) | EU CTR [35] |
| A Randomized Clinical Trial (IIIb) of Efficacy of a Single Dose of Tocilizumab or a Combination of Tocilizumab plus Vitamin D for the Treatment of COVID-19 Hyperimmune Complications | EudraCT 2020-001903-17 | IIIb | I | Ongoing | No | EU CTR [35] |
| Phase III Randomized Open-Label Trial to Evaluate High-Dose Cholecalciferol (Vitamin D3) in Patients with COVID-19 Pneumonia | EudraCT 2020-002312-43 | III | I | Completed | No | EU CTR [35] |
| COVitaminD Trial: Prevention of Complications from COVID-19 in Cancer Patients Under Active Treatment | EudraCT 2020-002119-23 | – (Not stated) | I | Prematurely Ended | No | EU CTR [35] |
| Multicenter, Double-blind, Randomized Trial to Evaluate the Efficacy of Calcifediol Soft Capsules versus Placebo in Reducing Hospital Admissions in Patients with COVID-19 | EudraCT 2021-000316-31 | – (Not stated) | I | Prematurely Ended | Yes (Posted) | EU CTR [35] |
| Prevention and Treatment with Calcifediol of Coronavirus COVID-19–Induced Acute Respiratory Syndrome (COVIDIOL) | EudraCT 2020-001717-20 | – (Not stated) | I | Prematurely Ended | No | EU CTR [35] |
| Prevention and Treatment with Calcifediol of COVID-19–Induced Acute Respiratory Syndrome (COVIDIOL trial, Spain) [24] | NCT04366908 | 2 | I | Recruiting (ongoing) | No | CT.gov [36] |
| Oral 25-hydroxyvitamin D3 and COVID-19 (Iran) | NCT04386850 | 2/3 | I | Recruiting (ongoing) | No | CT.gov [37] |
| Evaluation of the Relationship Between Zinc, Vitamin D, and B12 Levels in COVID-19-Positive Pregnant Women (Turkey) | NCT04407572 | N/A | O | Completed | No | CT.gov [38,39] |
| International ALLIANCE Study of Therapies to Prevent Progression of COVID-19 (includes Vitamin D3 arm) | NCT04395768 | 2 | I | Recruiting ongoing | No | CT.gov [40,41] |
| Vitamin D for COVID-19 Trial (VIVID—U.S.) | NCT04536298 | 3 | I | Completed | No (No results posted) | CT.gov [42] |
| Effect of Vitamin D on Morbidity and Mortality of the COVID-19 (COVID-VIT-D Trial) (Spain/Argentina) | NCT04552951 | 3 | I | Completed (status last known) | No (No results posted) | CT.gov [43,44] |
| Prevention of COVID-19 With Oral Vitamin D Supplementation (CORONAVIT trial, UK) | NCT04579640 | 3 | I | Completed | No (No results posted) | CT.gov [22,45] |
| High-Dose Vitamin D Supplementation in COVID-19 Patients (Angers trial, France) | EudraCT Number: 2020-001602-34 | 3 | I | Completed (status last known) | No | CT.gov [46] |
| The LEAD COVID-19 Trial: Low-risk, Early Aspirin, and Vitamin D to Reduce COVID-19 Hospitalizations (LEAD COVID-19, USA) | NCT04363840 | 2 | I | Withdrawn (lack of funding) | No | CT.gov [47,48] |
| COVID-19 and High-dose Vitamin D Supplementation in High-Risk Older Patients (COVIT-TRIAL, Europe) | NCT04344041 | 3 | I | Completed (status last known) | No | CT.gov [27,46,49] |
| Pilot Study of Vitamin D in COVID-19 Patients (single-arm trial, USA) | NCT04407286 | N/A | I (single-arm) | Completed (status last known) | No | CT.gov [50,51] |
| Trial of Combination Therapy to Treat COVID-19 Infection ProgenaBiome | NCT04482686 | 1 | I | Completed (status last known) | No | CT.gov [52] |
| Vitamin D Supplementation in Patients With COVID-19 (Brazil) | NCT04449718 | 3 | I | Completed (status last known) | No | CT.gov [53,54,55,56] |
| High Dose Vitamin-D Substitution in Patients With COVID-19: a Randomized Controlled, Multi-Center Study (VitCov) | NCT04525820 | N/A | I | Completed (status last known) | No | CT.gov [57,58] |
| Efficacy of Vitamin D Supplementation to Prevent the Risk of Acquiring COVID-19 in Healthcare Workers (COVID-19) | NCT04535791 | 3 | I | Completed (status last known) | No | CT.gov [28,59] |
| Efficacy of Vitamin D Treatment in Pediatric Patients Hospitalized by COVID-19 (Mexico) | NCT04502667 | 3 | I | Completed (status last known) | No | CT.gov [60] |
| Vitamin D, Magnesium, and B12 in COVID-19 (DMB) (Singapore cohort study) | – (No NCT, local study) | N/A | O | Completed | – | [61] |
| Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for COVID-19 Prevention (HELP COVID-19 Trial, USA) | NCT04335084 | 2 | I | Active, not recruiting | No | CT.gov [62] |
| High-Dose vs. Standard-Dose Vitamin D3 in Patients with COVID-19 (SHADE trial, India) | CTRI/2020/06/026189 (no NCT) | – | I | Completed | Published only | CT.gov [26,63] |
| Prevention of COVID-19 With Oral Vitamin D Supplemental Therapy in Essential Healthcare Teams (PROTECT) | NCT04483635 | 3 | I | Completed | Published | CT.gov [64,65] |
| Vitamin D and Zinc Supplementation for Improving Treatment Outcomes Among COVID-19 Patients in India | NCT04641195 | 3 | I | Completed | Published | CT.gov [66,67] |
| Vitamin D Supplementation in Patients With COVID-19 | NCT04449718 | N/A | I | Completed | Published | CT.gov [23,53] |
| Vitamin D and COVID-19 Management (Canada) | NCT04385940 | 3 | I | Completed | No | CT.gov [68] |
| Investigating the Role of Vitamin D in the Morbidity of COVID-19 Patients (UK) | NCT04386044 | N/A | I | Completed | No | CT.gov [69] |
| Baseline Vitamin D Deficiency and COVID-19 Disease Severity (USA) | NCT04628000 | N/A | O | Completed | No | CT.gov [70] |
| Increased Risk of Severe Coronavirus Disease 2019 in Patients with Vitamin D Deficiency (COVIT-D, Spain) | NCT04403932 | N/A | O | Completed | No | CT.gov [71] |
| Vitamin D Status and Immune-inflammatory Status in Different UK Populations With COVID-19 Infection | NCT04519034 | N/A | O | Active, not recruiting | No | CT.gov [72] |
| Should Ranges of Vitamin D be Redefined to Prevent or Treat Viral Infections? (Turkey) | NCT04394390 | N/A | O | Completed | No | CT.gov [73] |
| Cholecalciferol to Improve the Outcomes of COVID-19 Patients (CARED) | NCT04411446 | 4 | O | Unknown status | No | CT.gov [29,74] |
| Vitamin D on Prevention and Treatment of COVID-19 (COVITD-19) | NCT04334005 | N/A | I | Unknown status | No | CT.gov [75] |
| Population | Recommended Vitamin D Intake | Purpose/Comments | Evidence Level/ Guideline Source |
|---|---|---|---|
| General population | 400–800 IU (10–20 µg) daily | Maintain general health and immune support | CDC, WHO, NHS guidelines |
| At-risk groups (elderly, limited sun exposure, chronic illnesses) | 800–2000 IU (20–50 µg) daily | Ensure adequate vitamin D status, potentially reducing severe respiratory infections | NICE (UK), NIH guidelines |
| Hospitalized COVID-19 patients | No routine high-dose supplementation recommended | Correct vitamin D deficiency if present (typically 1000–2000 IU/day or higher doses under medical supervision) | NIH COVID-19 Treatment Guidelines |
| COVID-19 prevention (general) | No evidence-based recommendation beyond general intake | Maintaining adequate vitamin D levels might indirectly support immune health | CDC, NIH COVID-19 Treatment Guidelines |
| Safe upper limit for adults | 4000 IU (100 µg) per day | Avoid potential toxicity and hypercalcemia | Institute of Medicine (IOM), NIH guidelines |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caliman-Sturdza, O.A.; Gheorghita, R.E.; Soldanescu, I. Vitamin D and COVID-19: Clinical Evidence and Immunological Insights. Life 2025, 15, 733. https://doi.org/10.3390/life15050733
Caliman-Sturdza OA, Gheorghita RE, Soldanescu I. Vitamin D and COVID-19: Clinical Evidence and Immunological Insights. Life. 2025; 15(5):733. https://doi.org/10.3390/life15050733
Chicago/Turabian StyleCaliman-Sturdza, Olga Adriana, Roxana Elena Gheorghita, and Iuliana Soldanescu. 2025. "Vitamin D and COVID-19: Clinical Evidence and Immunological Insights" Life 15, no. 5: 733. https://doi.org/10.3390/life15050733
APA StyleCaliman-Sturdza, O. A., Gheorghita, R. E., & Soldanescu, I. (2025). Vitamin D and COVID-19: Clinical Evidence and Immunological Insights. Life, 15(5), 733. https://doi.org/10.3390/life15050733







