Non-Trileaflet Aortic Valve Aortopathies
Abstract
1. Introduction
2. Anatomy of the Aorta
2.1. Anatomy of the Aortic Valve and Root
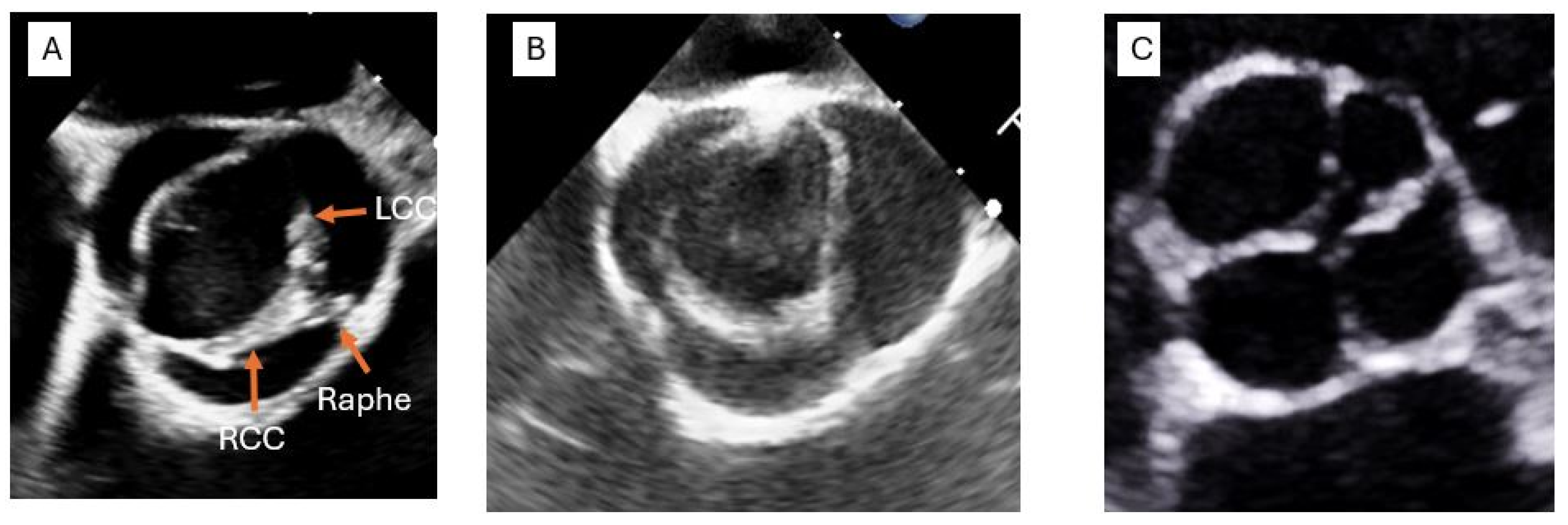
2.2. Bicuspid Aortic Valve: Prevalence, Presentation and Complications
2.3. Unicuspid Aortic Valve
2.4. Quadricuspid Aortic Valve
2.5. Definition and Incidence of Thoracic Aortic Aneurysm
2.6. Pathogenesis of TAA
3. The Role of Multimodality Cardiac Imaging in the Evaluation of Aortic Valve Abnormalities and Aortopathy
3.1. Transthoracic Echocardiography (TTE)
3.2. Transesophageal Echocardiography (TEE)
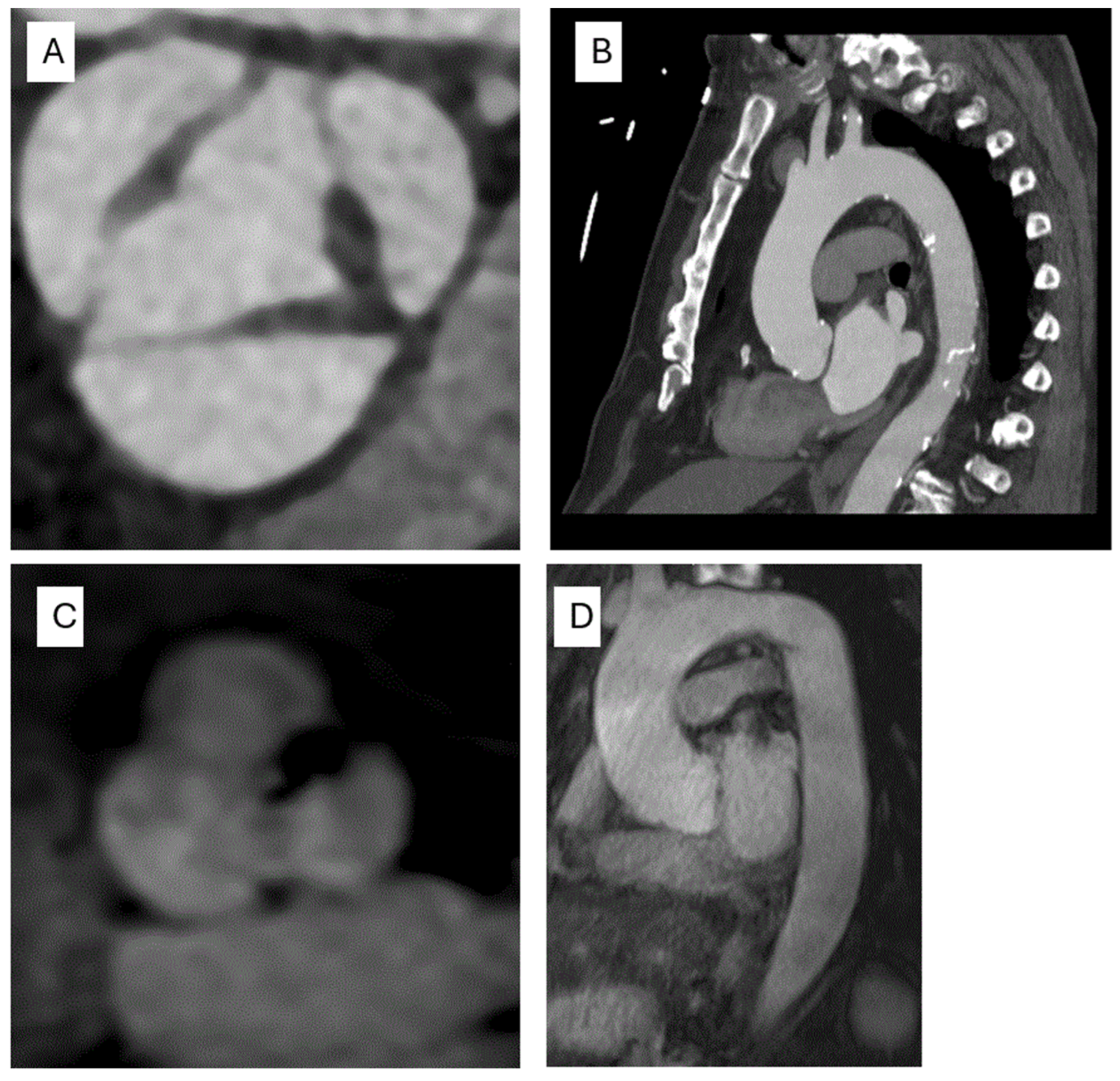
3.3. Computed Tomography (CT)
3.4. Cardiac Magnetic Resonance (CMR)
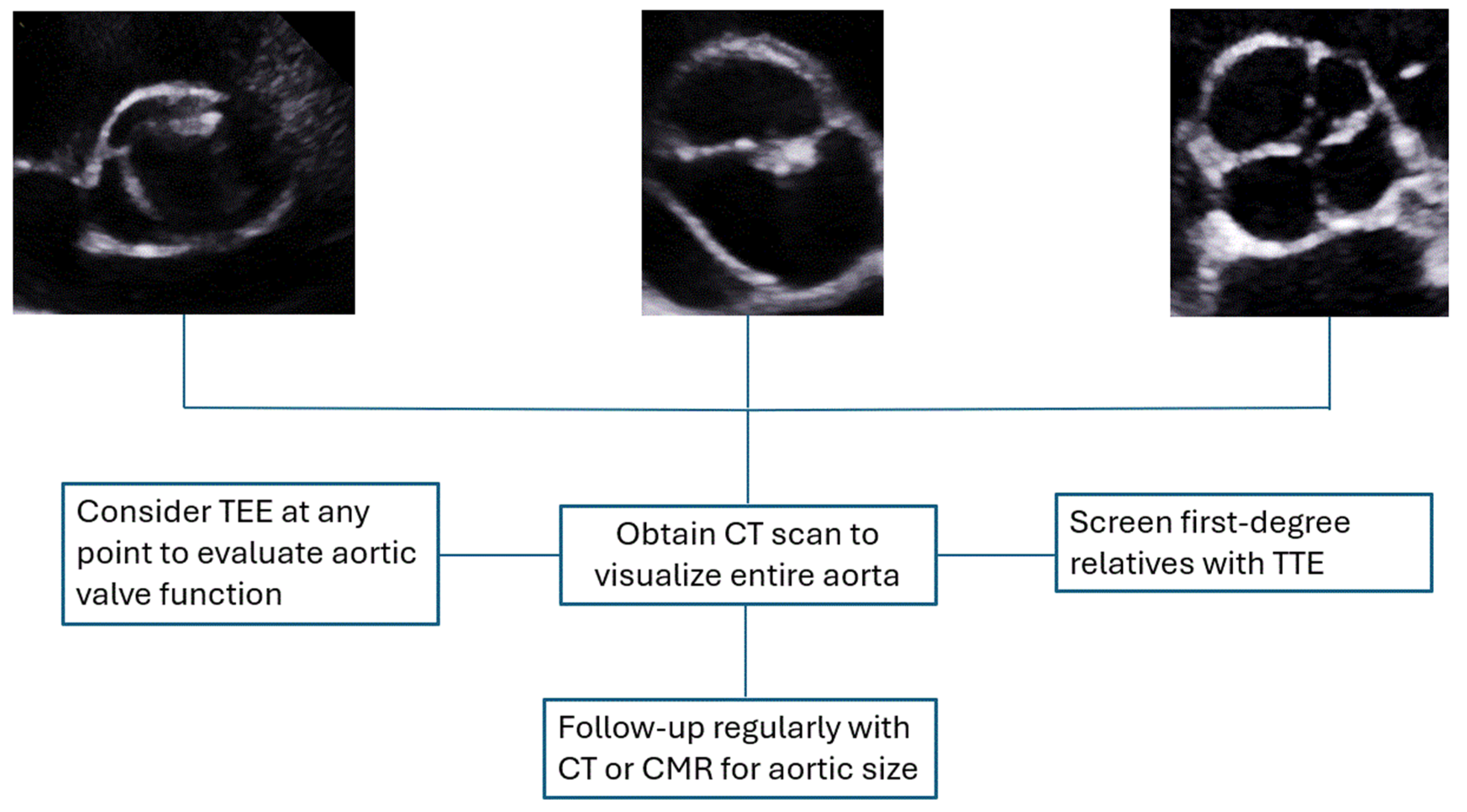
4. Diagnostic Approach
4.1. Surveillance
4.2. Medical Management
4.3. Surgical Intervention
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbour, J.R.; Spinale, F.G.; Ikonomidis, J.S. Proteinase systems and thoracic aortic aneurysm progression. J. Surg. Res. 2007, 139, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Members, W.C.; Isselbacher, E.M.; Preventza, O.; Hamilton Black III, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: A report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Galian-Gay, L.; Evangelista, A. Mechanisms of aortic dilation in patients with bicuspid aortic valve: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2023, 82, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Bohbot, Y.; Michelena, H.I.; Rusinaru, D.; Fay, F.; Elmkies, F.; Sarano, M.E.; Tribouilloy, C. Clinical outcomes of adults with bicuspid aortic valve: A European perspective. In Proceedings of the Mayo Clinic Proceedings; 2021; pp. 648–657. Available online: https://www.sciencedirect.com/science/article/pii/S0025619620308442?casa_token=7zCJVVLVk-IAAAAA:OGoGPfqq9J97dmWoT5SmwyQYla5-1POcPKrzuEfEItEjROEuQungCKNLuF8wplVa2qav1-eKsWtS (accessed on 23 April 2025).
- Sillesen, A.-S.; Vøgg, O.; Pihl, C.; Raja, A.A.; Sundberg, K.; Vedel, C.; Zingenberg, H.; Jørgensen, F.S.; Vejlstrup, N.; Iversen, K. Prevalence of bicuspid aortic valve and associated aortopathy in newborns in Copenhagen, Denmark. JAMA 2021, 325, 561–567. [Google Scholar] [CrossRef]
- Losenno, K.L.; Goodman, R.L.; Chu, M.W. Bicuspid aortic valve disease and ascending aortic aneurysms: Gaps in knowledge. Cardiol. Res. Pract. 2012, 2012, 145202. [Google Scholar] [CrossRef]
- Masri, A.; Svensson, L.G.; Griffin, B.P.; Desai, M.Y. Contemporary natural history of bicuspid aortic valve disease: A systematic review. Heart 2017, 103, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Jaimes, K.; Prakash, S.K. Genetics in bicuspid aortic valve disease: Where are we? Prog. Cardiovasc. Dis. 2020, 63, 398–406. [Google Scholar] [CrossRef]
- Sievers, H.-H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef]
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur. J. Cardio-Thorac. Surg. 2021, 60, 448–476. [Google Scholar] [CrossRef]
- Michelena, H.I.; Khanna, A.D.; Mahoney, D.; Margaryan, E.; Topilsky, Y.; Suri, R.M.; Eidem, B.; Edwards, W.D.; Sundt, T.M.; Enriquez-Sarano, M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011, 306, 1104–1112. [Google Scholar] [CrossRef]
- Tzemos, N.; Therrien, J.; Yip, J.; Thanassoulis, G.; Tremblay, S.; Jamorski, M.T.; Webb, G.D.; Siu, S.C. Outcomes in adults with bicuspid aortic valves. JAMA 2008, 300, 1317–1325. [Google Scholar] [CrossRef]
- Slostad, B.D.; Witt, C.M.; O’Leary, P.W.; Maleszewski, J.J.; Scott, C.G.; Dearani, J.A.; Pellikka, P.A. Unicuspid aortic valve: Demographics, comorbidities, echocardiographic features, and long-term outcomes. Circulation 2019, 140, 1853–1855. [Google Scholar] [CrossRef]
- Slostad, B.D.; Witt, C.M.; O’Leary, P.W.; Maleszewski, J.J.; Scott, C.G.; Dearani, J.A.; Pellikka, P.A. Diagnostic accuracy of echocardiography and intraoperative surgical inspection of the unicuspid aortic valve. Am. J. Cardiol. 2019, 123, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Mookadam, F.; Thota, V.R.; Garcia-Lopez, A.M.; Emani, U.R.; Alharthi, M.S.; Zamorano, J.; Khandheria, B.K. Unicuspid aortic valve in adults: A systematic review. J. Heart Valve Dis. 2010, 19, 79–85. [Google Scholar] [PubMed]
- Feldman, B.J.; Khandheria, B.K.; Warnes, C.A.; Seward, J.B.; Taylor, C.L.; Tajik, A.J. Incidence, description and functional assessment of isolated quadricuspid aortic valves. Am. J. Cardiol. 1990, 65, 937–938. [Google Scholar] [CrossRef]
- Hurwitz, L.E.; Roberts, W.C. Quadricuspid semilunar valve. Am. J. Cardiol. 1973, 31, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.Y.C.; Abudiab, M.M.; Ammash, N.M.; Naqvi, T.Z.; Edwards, W.D.; Nkomo, V.T.; Pellikka, P.A. Quadricuspid Aortic Valve. Circulation 2016, 133, 312–319. [Google Scholar] [CrossRef]
- Paruchuri, V.; Salhab, K.F.; Kuzmik, G.; Gubernikoff, G.; Fang, H.; Rizzo, J.A.; Ziganshin, B.A.; Elefteriades, J.A. Aortic size distribution in the general population: Explaining the size paradox in aortic dissection. Cardiology 2015, 131, 265–272. [Google Scholar] [CrossRef]
- Clouse, W.D.; Hallett Jr, J.W.; Schaff, H.V.; Gayari, M.M.; Ilstrup, D.M.; Melton III, L.J. Improved prognosis of thoracic aortic aneurysms: A population-based study. JAMA 1998, 280, 1926–1929. [Google Scholar] [CrossRef]
- Evangelista, A. Aneurysm of the ascending aorta. Heart 2010, 96, 979–985. [Google Scholar] [CrossRef]
- Dobrin, P.; Mrkvicka, R. Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc. Surg. 1994, 2, 484–488. [Google Scholar] [CrossRef]
- Newman, K.M.; Jean-Claude, J.; Li, H.; Ramey, W.G.; Tilson, M.D. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation 1994, 90, II224–II227. [Google Scholar] [PubMed]
- Taketani, T.; Imai, Y.; Morota, T.; Maemura, K.; Morita, H.; Hayashi, D.; Yamazaki, T.; Nagai, R.; Takamoto, S. Altered Patterns of Gene Expression Specific to Thoracic Aortic Aneurysms Microarray Analysis of Surgically Resected Specimens. Int. Heart J. 2005, 46, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, S.; Tedgui, A. Cellular mechanics and gene expression in blood vessels. J. Biomech. 2003, 36, 631–643. [Google Scholar] [CrossRef]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhäll, C.-J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D. 4-D flow cardiovascular magnetic resonance consensus statement. J. Cardiovasc. Magn. Reson. 2015, 17, 72. [Google Scholar] [CrossRef]
- Van Ooij, P.; Powell, A.L.; Potters, W.V.; Carr, J.C.; Markl, M.; Barker, A.J. Reproducibility and interobserver variability of systolic blood flow velocity and 3D wall shear stress derived from 4-D flow MRI in the healthy aorta. J. Magn. Reson. Imaging 2016, 43, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Guzzardi, D.G.; Barker, A.J.; Van Ooij, P.; Malaisrie, S.C.; Puthumana, J.J.; Belke, D.D.; Mewhort, H.E.; Svystonyuk, D.A.; Kang, S.; Verma, S. Valve-related hemodynamics mediate human bicuspid aortopathy: Insights from wall shear stress mapping. J. Am. Coll. Cardiol. 2015, 66, 892–900. [Google Scholar] [CrossRef]
- Bossone, E.; Eagle, K.A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 2021, 18, 331–348. [Google Scholar] [CrossRef]
- Tsai, T.T.; Nienaber, C.A.; Eagle, K.A. Acute aortic syndromes. Circulation 2005, 112, 3802–3813. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Pol. Heart J. (Kardiol. Pol.) 2014, 72, 1169–1252. [Google Scholar] [CrossRef]
- Goldstein, S.A.; Evangelista, A.; Abbara, S.; Arai, A.; Asch, F.M.; Badano, L.P.; Bolen, M.A.; Connolly, H.M.; Cuéllar-Calàbria, H.; Czerny, M. Multimodality imaging of diseases of the thoracic aorta in adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging: Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2015, 28, 119–182. [Google Scholar]
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute aortic syndromes: Diagnosis and management, an update. Eur. Heart J. 2018, 39, 739–749d. [Google Scholar] [CrossRef] [PubMed]
- Harky, A.; Bashir, M.; Antoniou, A.; Francis, N.; Alhamdan, L.; Uppal, R. Size and dissection: What is the relation? Indian J. Thorac. Cardiovasc. Surg. 2019, 35, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Spotnitz, M.; Lindsay, M.E.; MacGillivray, T.E.; Isselbacher, E.M.; Sundt, T.M. Risk of aortic dissection in the moderately dilated ascending aorta. J. Am. Coll. Cardiol. 2016, 68, 1209–1219. [Google Scholar] [CrossRef]
- Yiu, R.S.; Cheng, S.W. Natural history and risk factors for rupture of thoracic aortic arch aneurysms. J. Vasc. Surg. 2016, 63, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, A.; Bancone, C.; Buonocore, M.; Dialetto, G.; Covino, F.E.; Manduca, S.; Scognamiglio, G.; D’Oria, V.; De Feo, M. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc. Imaging 2013, 6, 1301–1310. [Google Scholar] [CrossRef]
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardio-Thorac. Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef]
- Danyi, P.; Elefteriades, J.A.; Jovin, I.S. Medical therapy of thoracic aortic aneurysms: Are we there yet? Circulation 2011, 124, 1469–1476. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- MacCarrick, G.; Black, J.H.; Bowdin, S.; El-Hamamsy, I.; Frischmeyer-Guerrerio, P.A.; Guerrerio, A.L.; Sponseller, P.D.; Loeys, B.; Dietz, H.C. Loeys–Dietz syndrome: A primer for diagnosis and management. Genet. Med. 2014, 16, 576–587. [Google Scholar] [CrossRef]
- Svensson, L.G.; Kim, K.-H.; Lytle, B.W.; Cosgrove, D.M. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J. Thorac. Cardiovasc. Surg. 2003, 126, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Kalahasti, V.; Svensson, L.G.; Alashi, A.; Schoenhagen, P.; Roselli, E.E.; Johnston, D.R.; Rodriguez, L.L.; Griffin, B.P.; Desai, M.Y. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ. Cardiovasc. Imaging 2017, 10, e006249. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.A.; Fedak, P.W.; Stephens, E.H.; Gleason, T.G.; Girdauskas, E.; Ikonomidis, J.S.; Khoynezhad, A.; Siu, S.C.; Verma, S.; Hope, M.D. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve–related aortopathy: Full online-only version. J. Thorac. Cardiovasc. Surg. 2018, 156, e41–e74. [Google Scholar] [CrossRef] [PubMed]
- Makaroun, M.S.; Dillavou, E.D.; Kee, S.T.; Sicard, G.; Chaikof, E.; Bavaria, J.; Williams, D.; Cambria, R.P.; Mitchell, R.S.; Investigators, G.T. Endovascular treatment of thoracic aortic aneurysms: Results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J. Vasc. Surg. 2005, 41, 1–9. [Google Scholar] [CrossRef]
- Archie, M.M.; Archie, M.M.; Khoynezhand, A. Techniques in hybrid repair of aortic arch and thoracoabdominal aortic pathologies. Expert Rev. Med. Devices, 2025; accepted. [Google Scholar] [CrossRef]
- Elhelali, A.; Hynes, N.; Devane, D.; Sultan, S.; Kavanagh, E.P.; Morris, L.; Veerasingam, D.; Jordan, F. Hybrid repair versus conventional open repair for thoracic aortic arch aneurysms. Cochrane Database Syst. Rev. 2021, 6, Cd012923. [Google Scholar] [CrossRef]
- Antoniou, G.; El Sakka, K.; Hamady, M.; Wolfe, J. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 683–690. [Google Scholar] [CrossRef]
- Acharya, M.; Sherzad, H.; Bashir, M.; Mariscalco, G. The frozen elephant trunk procedure: Indications, outcomes and future directions. Cardiovasc. Diagn. Ther. 2022, 12, 708–721. [Google Scholar] [CrossRef]
| TTE | TEE | CT | CMR | |
|---|---|---|---|---|
| Indication | ||||
| Techniques for assessing the aorta |
|
|
|
|
| Techniques for assessing the AV |
|
|
|
|
| TTE | TEE | CT | CMR | |
|---|---|---|---|---|
| Strengths |
|
|
|
|
| Limitations |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Wang, T.K.M. Non-Trileaflet Aortic Valve Aortopathies. Life 2025, 15, 713. https://doi.org/10.3390/life15050713
Ahmed A, Wang TKM. Non-Trileaflet Aortic Valve Aortopathies. Life. 2025; 15(5):713. https://doi.org/10.3390/life15050713
Chicago/Turabian StyleAhmed, Abdelrahman, and Tom Kai Ming Wang. 2025. "Non-Trileaflet Aortic Valve Aortopathies" Life 15, no. 5: 713. https://doi.org/10.3390/life15050713
APA StyleAhmed, A., & Wang, T. K. M. (2025). Non-Trileaflet Aortic Valve Aortopathies. Life, 15(5), 713. https://doi.org/10.3390/life15050713





