Abstract
Acute surgical abdomen is characterized by intense, sudden abdominal pain due to intra-abdominal conditions requiring prompt surgical intervention. The coronavirus disease 2019 (COVID-19) pandemic has led to various complications related to the disease’s complex pathophysiological mechanisms, hence the hypothesis of COVID-19-induced acute abdominal surgical pathologies. The connection between acute surgical abdomen and COVID-19 involves two primary mechanisms. First, there is the presence of angiotensin-converting enzyme 2 (ACE2) receptors in multiple abdominal organs. This facilitates the cytokine storm through direct viral injury and inflammation. Second, the hypercoagulable state induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) increases the thrombotic risk within abdominal vessels, which can subsequently lead to ischemia. ACE2 receptors are notably expressed in the gastric, duodenal, and rectal epithelium, with SARS-CoV-2 viral RNA and nucleocapsid proteins detected in these tissues. The inflammatory response results in significant endothelial damage, activating coagulation pathways that cause monocellular infiltration, lymphocytic inflammation, and uncontrolled coagulation. These findings highlight the need for further research to clarify how COVID-19 leads to acute abdominal pathologies. Understanding these mechanisms is vital for improving clinical management and patient outcomes during future health crises and in the aftermath of the pandemic.
1. Introduction
In the late months of the year 2019, an atypical pneumonia outbreak arose in the People’s Republic of China’s town of Wuhan. Later on, the causative agent was identified as being the novel coronavirus, part of the severe acute respiratory syndrome coronavirus family; hence, the name of the disease, coronavirus disease 2019 (COVID-19).
The great number of severe acute respiratory syndrome cases, the rapid global expansion, and the rapidly increasing numbers of deaths due to COVID-19 led the World Health Organization (WHO) to declare a global pandemic, starting in March 2020 [1].
According to data provided by Johns Hopkins University in the United States, as of April 2020, almost 2.5 million total confirmed cases of COVID-19 had been reported in over 205 nations and territories, resulting in about 170,400 fatalities [2].
Early investigations indicated that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) likely originated in bats, while the intermediary host between the bat reservoir and the human remains unknown. COVID-19 is transmitted from person to person mostly through droplets and/or close contact between an infected and a healthy person. Although the virus has been found in affected people’s tears and stool, it is unknown if the disease spreads through the oral, fecal, or conjunctival pathways [3]. Patients with COVID-19 may be asymptomatic carriers or may experience symptoms ranging from mild to severe, including respiratory or multiorgan failure. Patients commonly report fever, dry cough, myalgia, tiredness, and dyspnea. Other symptoms include diarrhea, abdominal pain, altered mental status, active cough, pleuritic chest pain, and hemoptysis [3].
The acute surgical abdomen is a condition characterized by the sudden onset of intense, localized, or diffuse abdominal pain; the persistence of the symptoms ranges from hours to days, and it is one of the most frequent reasons for outpatient presentations in the emergency departments worldwide [4]. Due to its varying clinical presentation, diagnosis poses a real challenge for emergency physicians around the world [5]. The vast array of underlying causes makes it a time-sensitive condition that must be rapidly diagnosed and treated to avoid severe complications, such as sepsis, necrosis, gangrene, and fistula, leading to death. According to literature data, over 6% of acute surgical abdomens are misdiagnosed [4]. Some of the most prevalent pathologies which can be misdiagnosed as an acute surgical abdomen are acute inferior wall myocardial infarction, with a prevalence of 40% and Diabetic Ketoacidosis, with a prevalence of 65% among type I diabetic patients, followed by Sickle Cell Crysis, Pneumonia, Reno-ureteral cholic, Herpes Zoster infection, Irritable Bowel Syndrome, Pelvic Inflammatory Disease, and many more [6]. Previous research has shown that accurate pre- and perioperative diagnosis can dramatically reduce management errors, with lower rates of unnecessary surgical interventions and perioperative complications. Also, the extensive and confirmatory pre-operative diagnosis of acute abdomen ensures optimal decision-making for surgical treatment, hence reducing the incidence of negative laparotomies [7].
The literature suggests that severe COVID-19 increases the risk of acute abdominal pathologies, such as pancreatitis, appendicitis, and cholitis, likely due to inflammation. However, the underlying mechanisms remain unclear [8,9].
The aim of the current study is to provide a better understanding and overview of the involvement of SARS-CoV-2 infection in the pathophysiological mechanism of the acute surgical abdomen.
We performed an extensive literature search using the PubMed, Google Scholar, Web of Science, and EBSCO databases, using the keywords “acute surgical abdomen”, “COVID-19”, “inflammation”, “cytokine storm”, and “thrombosis”.
3. Acute Surgical Abdomen
The acute surgical abdomen is a life-threatening condition that requires immediate care and treatment, usually caused by inflammation, infection, ischemia, intrinsic or extrinsic obstruction, and traumatic events [27]. As a general rule, acute surgical abdomen is consistent with sudden severe abdominal pain, with a rapid evolutive pattern, caused by an intraabdominal pathology, requiring immediate surgical intervention [28].
The usual clinical presentation is abdominal pain associated with a various array of symptoms like nausea, vomiting, bloating, fever, constipation, inappetence, diarrhea, abdominal distension, jaundice, or even shock [29,30].
Abdominal pain accounts for from about 5 to 10% of all emergency department (ED) visits worldwide; in the United States, the number of presentations reaching as high as 3 million patients per year [31,32], out of which from 20 to 25% are diagnosed with a serious intra-abdominal condition requiring hospitalization and immediate surgical intervention [33]. According to current studies, there is no significant difference in the incidence and prevalence of abdominal pain and the occurrence of acute surgical abdomen related to gender [34].
Diagnostics are, nevertheless, a challenge for any physician due to the vast symptoms at presentation, many of which are nonspecific, hence the need for a thorough history taking, clinical examination, paraclinical investigations, and extensive differential diagnosis [33,35].
The most common causes of acute abdominal pain are represented by non-specific abdominal pain, reno-ureteral colic, acute appendicitis, biliary disorders, diverticulitis, bowel obstruction, acute pancreatitis, gastritis, gastroenteritis, inflammatory bowel disease, mesenteric infarction, gynecological disorders, and others [34].
Appendicitis is less common in people over 50 years old, while cholecystitis, bowel obstruction, perforated malignancies, strangulated hernias, and acute mesenteric ischemia are more prevalent [36].
In Asia, findings reveal a high number of perforated gastric tumors, predominantly among the elderly. Ileal perforation as a complication of Typhoid fever, tuberculous peritonitis, obstructive biliary and enteric ascariasis, amoebic liver abscess, and other less frequent abdominal parasitosis are prevalent in endemic tropical and subtropical areas, particularly in low- and middle-income countries. Moreover, high-income countries with an older population and a high-fat, low-fiber diet have a greater incidence of complicated diverticulitis and colon-rectal neoplasia; hence, estimations that around 49 million general surgical emergency procedures are conducted each year worldwide [36].
The range of the mortality rates for all adults with an acute abdomen is 2 to 14%, depending mainly on the primary pathology and age, the elderly being more liable [36]. Moreover, underlying diseases, such as cardiovascular conditions, chronic kidney disease, active malignancies, and the presence of Systemic Inflammatory Response Syndrome (SIRS), sepsis, septic shock, and Multi-System Organ Failure (MSOF) at presentation, were positively correlated with increases in mortality rates [37]. The prediction of the overall outcome in patients with acute surgical abdomen requiring emergency surgical intervention may also be suggested by the increased values of the serum lactate, interleukin 6, C-Reactive Protein, and procalcitonin levels at presentation and their peri- and postoperative evolution [38].
Left untreated, acute surgical abdomen can lead to serious complications, such as bleeding, fistulas, peritonitis, necrosis, sepsis, septic shock, exacerbation of preexisting pathologies, and even death [36].
4. Pathophysiological Mechanisms of COVID-19-Associated Acute Surgical Abdomen
Beginning with the presumption that the mechanism of action in COVID-19 infections involves the virus’s capacity to attach to angiotensin-converting enzyme 2 (ACE2) receptors, it is hypothesized that the manifestation of multiple organ dysfunction is closely linked to COVID-19, owing to the extensive presence of ACE2 across various organs [39]. The confirmation of this supposition came from gastrointestinal biopsies, which identified the coronavirus RNA in the examined samples. One of the mechanisms is attributed to direct damage induced by the virus and an inflammatory response mediated through immunological pathways, which may lead to malabsorption, malfunction of the intestinal mucosa and disruption of intestinal secretion production, and stimulation of the enteric nervous system [40].
A second incriminated mechanism is represented by the COVID-19-induced coagulopathy, which, unlike other coagulopathies, is characterized by elevated fibrinogen levels and extensive fibrinolysis (elevated D-dimers), but with negligible alterations in platelet count, prothrombin time, and antithrombin levels. The extensive inflammatory response causes endothelial damage, which activates coagulation. This leads to endothelial infiltration by monocellular cells, endothelial inflammation with lymphocytic infiltration, platelet activation, and uncontrolled coagulation [41]. Clot formation is accelerated by the decrease in Plasmin activity and an increase in plasminogen inhibitor activator-1 [42]. As stated by previously published articles, hypoxia, which was present in a vast majority of patients with moderate to severe forms of COVID-19, may enhance the procoagulant state by upregulating the function of Tissue Factor in oxygen-deprived vessels [43].
During the COVID-19 pandemic, it was observed that some patients infected with SARS-CoV-2 only had gastrointestinal symptoms as an initial presentation, without pulmonary involvement, hence the hypothesis that direct injury by the virus may be the main cause [44]. Taking into account the two pathophysiological mechanisms, several articles described cases of acute intraabdominal pathologies related to SARS-CoV-2 2infection.
The two main pathways by which COVID-19 can lead to an acute surgical abdomen are illustrated in Figure 1.
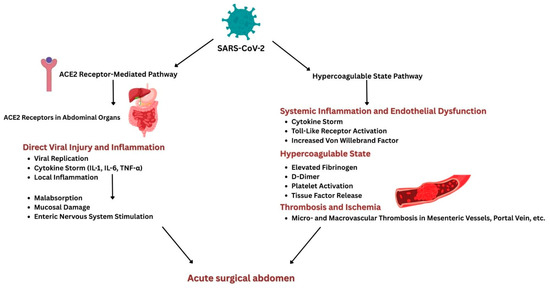
Figure 1.
Schematic illustration of pathophysiological pathways connecting COVID-19 and acute surgical abdomen.
4.1. The ACE2 Receptors Hypothesis
A study published by Xiao et al. identified, by immunofluorescent methods, a high expression of ACE2 receptors in the glandular cells of the gastric, duodenal, and rectal epithelium. Moreover, by RNA detection methods, SARS-CoV-2 viral RNA and nucleocapsid proteins were identified in the above-described epithelia [45].
The detection of SARS-CoV-2 in stool was significant, suggesting the virus can multiply and persist in the digestive tract. A meta-analysis conducted by Cheung et al. states that the persistent finding of viral RNA in feces indicates that infectious virions are excreted by virus-infected gastrointestinal cells. Nonetheless, the percentage of stool samples positive for viral RNA was around 48%; among these, approximately 70% of samples collected after viral clearance from the respiratory specimens also tested positive for the virus (95% CI, 49.6–85.1) [46]. Several studies have proven that viral infections can lead to acute appendicitis by various mechanisms, such as lymphoid hyperplasia, followed by appendiceal obstruction and ulcerations in the appendix mucosa, followed by a secondary bacterial colonization [47]. According to some studies, cases of acute appendicitis as initial complaints of COVID-19 infection were described in some of the patients as being the only symptoms at presentation [48,49]. Moreover, a case report by Kono et al. demonstrated the presence of SARS-CoV-2 RNA in a specimen of phlegmonous appendicitis by RT-PCR techniques, proving an association between the occurrence of acute appendicitis and COVID-19 infection [50]. Furthermore, another study conducted by Georgakopoulou et al. sustains the above-mentioned hypothesis by identifying peculiar histological displays, such as sparse microthrombi, fibrinoid necrosis of appendicular vasculature, and lymphocytic infiltrates around the vessels, which are consistent with coronavirus infection [51].
Another study of Burkett et al., performed on four patients, identified that the specimens harvested by hemicolectomy from COVID-19-infected patients showed ischemic alterations, acute and chronic inflammation, and fibrinous microthrombosis in small blood vessels in the regions where mucosal ulceration was present. Nonetheless, by means of immunofluorescence and In Situ Hybridization performed on the specimens from the affected bowel areas, the presence of SARS-CoV-2 RNA was established [52]. Cases of acute diffuse colitis, ulcerative colitis, lymphocytic colitis, and other types of Inflammatory Bowel Diseases (IBDs) were described; in all cases, viral RNA was detected by RT-PCR in the stool [53,54,55].
Several patients with COVID-19 infection developed newly diagnosed type I diabetes melitus (DM), hence the hypothesis of the COVID-induced diabetes, which was also confirmed by a metanalysis conducted by Shrestha et al., which stated that the prevalence of COVID-induced type I DM is as high as 20%, also implying higher death rates than in patients previously diagnosed with DM [56,57]. This can be attributed to the high extent of ACE2 receptors in the pancreatic tissue, within the pancreatic islets and exocrine glands, which is even higher than in the lung tissue, hence the capacity of SARS-CoV-2 to directly bind to the pancreas, inducing pancreatic damage and subsequent distress [58]. According to Hadi et al., pancreatic destruction occurs by direct viral injury of the acinar cells due to inflammation and edema, while another theory is that, by injuring the acinar cells, the leakage of pancreatic enzymes leads to autodigestion of the pancreas [59]. Hegi et al. identified that increased cytokine levels, especially those of IL-6, 8, and 10, were present in both severe cases of COVID-19 and acute pancreatitis, explaining the effect of the cytokine storm in the development of acute pancreatitis [60]. A similar theory is sustained by Wang et al., who state that the ontogeny of acute pancreatitis may be caused through an exaggerated systemic response to the Acute Respiratory Distress Syndrome (ARDS) or by the cytokine storm induced by the COVID-19 infection, which leads to multisystem organ failure [61]. A literature review conducted by Jabłońska et al. revealed a considerably greater incidence of “idiopathic” AP in patients infected with COVID-19 than in non-COVID-19 individuals, hence the conclusion that SARS-CoV-2 may represent a novel infectious element for the development of acute pancreatitis [62].
Nevertheless, hepatic injury was described in several studies, while the primary identified histological abnormalities were the lymphocytic infiltration of the sinusoidal vessels and their dilation, hepatic steatosis, and extensive liver necrosis assigned to direct viral injury, due to the high expression of ACE2 receptors [63]. Also, some studies correlate the presence of liver involvement with the severity of COVID-19 infection [64].
4.2. The Hypercoagulable State Hypothesis
As stated by Avila et al., previous research has explored the involvement of inflammation in causing a hypercoagulable status, probably due to stimulation of endothelial cells, platelets, and leukocytes, inducing tissue factor (TF), which, by binding to clotting factor VIIa, initiates the coagulation process [65]. A study conducted by Lippi et al. determined that the hypercoagulable state in COVID-19 is dominated by the elements of Disseminated Intravascular Coagulation (DIC), such as elevated D-Dimer values, prolonged prothrombin time, and hyperfibrinogenemia [66].
A potential mechanism proposed by Abou-Ismail et al. states that the production of proinflammatory cytokines may enhance epithelial cells and monocyte and macrophage activity, which, superimposed with the activation of endothelial cells by direct infection through the ACE2 receptors, leads to the formation of the fibrin clot by thrombin overproduction. Moreover, this mechanism is enhanced by the overproduction of Tissue Factor (TF), activation of thrombocytes, and increased production of Von Willebrand Factor and coagulation factor VIII.
Further on, thrombin sustains inflammation by triggering platelet activation, which further leads to the production of Neutrophil Extracellular Traps [67].
Considering these mechanisms, several case reports have described cases of ischemia and thrombosis in different intraabdominal organs and vessels, both microvascular and large vessels, as a consequence of concomitant or recent COVID-19 infection.
Several cases of abdominal thromboembolic complications of COVID-19 infection have been reported, as shown in Table 1. All patients included in these studies had a positive RT-PCR test for COVID-19 previously (maximum 14 days prior) or concomitant with the current presentation.

Table 1.
Case reports of thromboembolic complications of COVID-19 infection.
5. Discussions and Limitations
While existing literature has explored the individual aspects of COVID-19 and acute surgical abdomen, this review distinguishes itself by providing a comprehensive and integrated analysis of the pathophysiological mechanisms linking the two. Unlike previous studies that primarily focus on specific complications or individual case reports, our work synthesizes the current understanding of how COVID-19-induced inflammation, hypercoagulability, and ACE2 receptor involvement contribute to the development of various acute surgical abdominal pathologies. Furthermore, this review offers a consolidated overview of the thromboembolic complications associated with COVID-19 in the context of acute surgical abdomen, providing a valuable resource for clinicians and researchers seeking a holistic understanding of this complex interplay. The present work must also be viewed in light of some limitations. The investigation was limited to literature published in English, which could result in bias by excluding relevant studies published in other languages. The selection of studies for inclusion in the present review involved a degree of subjective judgment based on the inclusion and exclusion criteria. Furthermore, the included studies may have varied significantly in terms of study design, patient populations, interventions, and outcome measures. This heterogeneity could limit the ability to draw firm conclusions or make strong recommendations.
6. Conclusions
Infection with the novel coronavirus may have a major role in the pathophysiological mechanism of the acute surgical abdomen, mainly through the two described mechanisms involving the ACE2 inhibitors widely spread into the gastrointestinal tract and the thrombogenic effects leading to vessel occlusion. Thus, further studies are needed to elucidate the complex pathophysiological mechanism leading to acute abdominal pathology and whether the abdominal complications are directly related to COVID-19 infection.
Author Contributions
Conceptualization, A.M., V.-O.B. and C.M.; methodology, A.M., I.-P.R., V.-B.H., V.-O.B. and C.M.; validation, C.M., C.M.B., T.S.T. and V.-O.B.; formal analysis, A.M., T.S.T., C.M.B. and E.T.; investigation, A.M., E.T., T.S.T., C.M.B., M.C.G. and P.C.R.; resources, A.M., V.-B.H., P.C.R., V.-O.B. and I.-P.R.; data curation, A.M., I.-P.R., V.-B.H. and V.-O.B.; writing—original draft preparation, A.M., I.-P.R., V.-B.H. and V.-O.B.; writing—review and editing, A.M., I.-P.R., V.-B.H., V.-O.B., C.M.B., T.S.T. and E.T.; visualization, A.M., I.-P.R., V.-B.H. and V.-O.B.; supervision, C.M., C.M.B., T.S.T., E.T., P.C.R. and M.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Acter, T.; Uddin, N.; Das, J.; Akhter, A.; Choudhury, T.R.; Kim, S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020, 730, 138996. [Google Scholar] [CrossRef] [PubMed]
- Al-Balas, M.; Al-Balas, H.I.; Al-Balas, H. Surgery during the COVID-19 pandemic: A comprehensive overview and perioperative care. Am. J. Surg. 2020, 219, 903–906. [Google Scholar] [CrossRef]
- Al-Faifi, J.J.; Alruwaili, K.A.; Alkhenizan, A.H.; Alharbi, M.F.; Alammar, F.N. Level of Knowledge and Attitude Toward Acute Abdomen Among the Public: A Nationwide Study. Cureus 2024, 16, e52416. [Google Scholar] [CrossRef] [PubMed]
- Aamir, J.; Imran, A.; Muhammad, H.L. Etiological Spectrum of Surgical Acute Abdomen Among Patients Attending Emergency Department. Pak. J. Med. Health Sci. 2023, 17, 94. [Google Scholar] [CrossRef]
- Sapmaz, F.; Başyiğit, S.; Başaran, M.; Demirci, S. Non-Surgical Causes of Acute Abdominal Pain. In Actual Problems of Emergency Abdominal Surgery; BoD: Hamburg, Germany, 2016. [Google Scholar] [CrossRef]
- Chhetri, R.K.; Shrestha, M.L. A comparative study of pre-operative with operative diagnosis in acute abdomen. Kathmandu Univ. Med. J. KUMJ 2005, 3, 107–110. [Google Scholar]
- Lima, M.I.D.; Fonseca, O.C.L.D. Acute abdomen in patients with COVID-19: An integrative review. Rev. Col. Bras. Cir. 2023, 50, e20233576. [Google Scholar] [CrossRef]
- Orthopoulos, G.; Santone, E.; Izzo, F.; Tirabassi, M.; Pérez-Caraballo, A.M.; Corriveau, N.; Jabbour, N. Increasing incidence of complicated appendicitis during COVID-19 pandemic. Am. J. Surg. 2020, 221, 1056–1060. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jha, P.; Brown, P.E.; Ansumana, R. Counting the global COVID-19 dead. Lancet 2022, 399, 1937–1938. [Google Scholar] [CrossRef]
- Han, E.; Tan, M.M.J.; Turk, E.; Sridhar, D.; Leung, G.M.; Shibuya, K.; Legido-Quigley, H. Lessons learnt from easing COVID-19 restrictions: An analysis of countries and regions in Asia Pacific and Europe. Lancet 2020, 396, 1525–1534. [Google Scholar] [CrossRef]
- Hoffmann, C.; Wolf, E. Older age groups and country-specific case fatality rates of COVID-19 in Europe, USA and Canada. Infection 2021, 49, 111–116. [Google Scholar] [CrossRef]
- Nicola, M.; O’Neill, N.; Sohrabi, C.; Khan, M.; Agha, M.; Agha, R. Evidence based management guideline for the COVID-19 pandemic—Review article. Int. J. Surg. 2020, 77, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Anant, P. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Sun, J.; He, W.T.; Wang, L.; Lai, A.; Ji, X.; Zhai, X.; Li, G.; Suchard, M.A.; Tian, J.; Zhou, J.; et al. COVID-19: Epidemiology, Evolution, and Cross-Disciplinary Perspectives. Trends Mol. Med. 2020, 26, 483–495. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Gąsecka, A.; Borovac, J.A.; Guerreiro, R.A.; Giustozzi, M.; Parker, W.; Caldeira, D.; Chiva-Blanch, G. Thrombotic Complications in Patients with COVID-19: Pathophysiological Mechanisms, Diagnosis, and Treatment. Cardiovasc. Drugs Ther. 2021, 35, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Acherjee, T.; Behara, A.; Saad, M.; Vittorio, T.J. Mechanisms and management of prothrombotic state in COVID-19 disease. Ther. Adv. Cardiovasc. Dis. 2021, 15, 17539447211053470. [Google Scholar] [CrossRef]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sinha, A.; Banach, M.; Mittoo, S.; Weissert, R.; Kass, J.S.; Rajagopal, S.; Pai, A.R.; Kutty, S. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front. Immunol. 2020, 11, 1648. [Google Scholar] [CrossRef]
- Dharra, R.; Kumar Sharma, A.; Datta, S. Emerging aspects of cytokine storm in COVID-19: The role of proinflammatory cytokines and therapeutic prospects. Cytokine 2023, 169, 156287. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.W.; Kashyap, S.; Dominique, E. Acute Abdomen. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Malviya, A.; Hussain, A.; Bulchandani, H.P.; Bhardwaj, G.; Kataria, S. A comprehensive study on acute non-traumatic abdominal emergencies. Int. Surg. J. 2017, 4, 2297–2302. [Google Scholar] [CrossRef]
- Chanana, L.; Jegaraj, M.A.; Kalyaniwala, K.; Yadav, B.; Abilash, K. Clinical profile of non-traumatic acute abdominal pain presenting to an adult emergency department. J. Fam. Med. Prim. Care 2015, 4, 422–425. [Google Scholar] [CrossRef]
- Bailey, I. The acute abdomen. Medicine 2021, 49, 103–105. [Google Scholar] [CrossRef]
- Wolfe, C.; Halsey-Nichols, M.; Ritter, K.; McCoin, N. Abdominal Pain in the Emergency Department: How to Select the Correct Imaging for Diagnosis. Open Access Emerg. Med. 2022, 14, 335–345. [Google Scholar] [CrossRef]
- Sebbane, M.; Dumont, R.; Jreige, R.; Eledjam, J.-J. Epidemiology of Acute Abdominal Pain in Adults in the Emergency Department Setting. Med. Radiol. 2011, 3, 13. [Google Scholar] [CrossRef]
- Graff, L.G.; Robinson, D. Abdominal Pain and Emergency Department Evaluation. Emerg. Med. Clin. N. Am. 2001, 19, 123–136. [Google Scholar] [CrossRef]
- Cervellin, G.; Mora, R.; Ticinesi, A.; Meschi, T.; Comelli, I.; Catena, F.; Lippi, G. Epidemiology and outcomes of acute abdominal pain in a large urban Emergency Department: Retrospective analysis of 5,340 cases. Ann. Transl. Med. 2016, 4, 362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ray, M.S. Pattern of illnesses presenting as acute abdomen: Surgical study in 118 patients. Int. Surg. J. 2021, 8, 1705–1711. [Google Scholar] [CrossRef]
- Stewart, B.; Khanduri, P.; McCord, C.; Ohene-Yeboah, M.; Uranues, S.; Vega Rivera, F.; Mock, C. Global disease burden of conditions requiring emergency surgery. Br. J. Surg. 2013, 101, e9–e22. [Google Scholar] [CrossRef]
- Proaño-Zamudio, J.A.; Argandykov, D.; Gebran, A.; Renne, A.; Paranjape, C.N.; Maroney, S.J.; Onyewadume, L.; Kaafarani, H.M.; King, D.R.; Velmahos, G.C.; et al. Open Abdomen in Elderly Patients with Surgical Sepsis: Predictors of Mortality. J. Surg. Res. 2023, 287, 160–167. [Google Scholar] [CrossRef]
- Ravishankaran, P.; Shah, A.M.; Bhat, R. Correlation of interleukin-6, serum lactate, and C-reactive protein to inflammation, complication, and outcome during the surgical course of patients with acute abdomen. J. Interferon Cytokine Res. 2011, 31, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Rodean, I.-P.; Biriș, C.-I.; Halațiu, V.-B.; Modiga, A.; Lazăr, L.; Benedek, I.; Benedek, T. Is There a Link between COVID-19 Infection, Periodontal Disease and Acute Myocardial Infarction? Life 2021, 11, 1050. [Google Scholar] [CrossRef]
- Kohansal Vajari, M.; Shirin, M.; Pourbagheri-Sigaroodi, A.; Akbari, M.E.; Abolghasemi, H.; Bashash, D. COVID-19-related coagulopathy: A review of pathophysiology and pharmaceutical management. Cell Biol. Int. 2021, 45, 1832–1850. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Mentzer, S.J.; Fontenay, M.; Laffan, M.A.; Ackermann, M.; Helms, J.; Jonigk, D.; Chocron, R.; Pier, G.B.; Gendron, N.; et al. COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis 2021, 24, 755–788. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Yan, S.F.; Mackman, N.; Kisiel, W.; Stern, D.M.; Pinsky, D.J. Hypoxia/Hypoxemia-Induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis. Arter. Thromb. Vasc. Biol. 1999, 19, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Cong, Y.; Zhang, H. COVID-19 and the Digestive System. Am. J. Gastroenterol. 2020, 115, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Hung, I.F.; Chan, P.P.; Lung, K.C.; Tso, E.; Liu, R.; Leung, W.K. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Abdalhadi, A.; Alkhatib, M.; Mismar, A.Y.; Awouda, W.; Albarqouni, L. Can COVID 19 present like appendicitis? IDCases 2020, 2, e00860. [Google Scholar] [CrossRef]
- Elbakouri, A.; El Azhary, A.; Bouali, M.; Bensardi, F.; Elhattabi, K.; Fadil, A. Gastrointestinal manifestations related to infection with SARS-CoV-2: Appendicular syndrome (A case report). Ann. Med. Surg. 2021, 65, 102288. [Google Scholar] [CrossRef]
- Malbul, K.; Katwal, S.; Maharjan, S.; Shrestha, S.; Dhital, R.; Rajbhandari, A.P. Appendicitis as a presentation of COVID-19: A case report. Ann. Med. Surg. 2021, 69, 102719. [Google Scholar] [CrossRef]
- Kono, J.; Yoshimaru, K.; Matsuura, T.; Tamaki, A.; Takemoto, J.; Matsumoto, S.; Hotta, T.; Kohashi, K.; Oda, Y.; Tajiri, T. COVID19 detection in appendix of acute appendicitis in a child: A case report and review of literature. Surg. Case Rep. 2023, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, V.E.; Gkoufa, A.; Damaskos, C.; Papalexis, P.; Pierrakou, A.; Makrodimitri, S.; Sypsa, G.; Apostolou, A.; Asimakopoulou, S.; Chlapoutakis, S.; et al. COVID-19-associated acute appendicitis in adults. A report of five cases and a review of the literature. Exp. Ther. Med. 2022, 24, 482. [Google Scholar] [CrossRef]
- Burkett, A.E.; Sher, S.B.; Patel, C.R.; Ildin-Eltoum, I.; Dhall, D.; Margaroli, C.; Peter, S.; Lee, G.; Bajpai, P.; Benson, P.V.; et al. Gastrointestinal Manifestations of COVID-19 Infection: Clinicopathologic Findings in Intestinal Resections Performed at Single Institution. Front. Med. 2022, 9, 811546. [Google Scholar] [CrossRef]
- Asil, R.S.; Mahmoodi, S.; Zamani, A.; Hakakzadeh, A.; Jamali, E. Abdominal Pain and Diffuse Colitis Following COVID-19 Infection: Report of a Case. Int. J. Surg. Case Rep. 2021, 88, 106473. [Google Scholar] [CrossRef]
- Gill, R.; Siau, E. Colitis After SARS-CoV-2 Infection. Cureus 2022, 14, e26532. [Google Scholar] [CrossRef] [PubMed]
- Rutigliani, M.; Bozzo, M.; Barberis, A.; Greppi, M.; Anelli, E.; Castellaro, L.; Bonsignore, A.; Azzinnaro, A.; Pesce, S.; Filauro, M.; et al. Case Report: A Peculiar Case of Inflammatory Colitis After SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 849140. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C.; Pozzilli, P. COVID-19 induced Diabetes: A novel presentation. Diabetes Res. Clin. Pract. 2022, 191, 110034. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Budhathoki, P.; Raut, S.; Adhikari, S.; Ghimire, P.; Thapaliya, S.; Rabaan, A.A.; Karki, B.J. New-onset diabetes in COVID-19 and clinical outcomes: A systematic review and meta-analysis. World J. Virol. 2021, 10, 275–287. [Google Scholar] [CrossRef]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128–2130.e2. [Google Scholar] [CrossRef]
- Hadi, A.; Werge, M.; Kristiansen, K.T.; Pedersen, U.G.; Karstensen, J.G.; Novovic, S.; Gluud, L.L. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology 2020, 20, 665–667. [Google Scholar] [CrossRef]
- Hegyi, P.; Szakács, Z.; Sahin-Tóth, M. Lipotoxicity and Cytokine Storm in Severe Acute Pancreatitis and COVID-19. Gastroenterology 2020, 159, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Fan, J.; Zhang, Y.; Wang, H.; Zhao, Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology 2020, 159, 367–370. [Google Scholar] [CrossRef]
- Jabłońska, B.; Olakowski, M.; Mrowiec, S. Association between acute pancreatitis and COVID-19 infection: What do we know? World J. Gastrointest. Surg. 2021, 13, 548–562. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, S.Y. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J. Med. Virol. 2020, 92, 1491–1494. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Liver injury in the era of COVID-19. World J. Gastroenterol. 2021, 27, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Long, B.; Holladay, D.; Gottlieb, M. Thrombotic complications of COVID-19. Am. J. Emerg. Med. 2021, 39, 213–218. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb. Haemost. 2020, 120, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ismail, M.Y.; Diamond, A.; Kapoor, S.; Arafah, Y.; Nayak, L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020, 194, 101–115, Erratum in Thromb. Res. 2021, 204, 146. [Google Scholar] [CrossRef]
- Borazjani, R.; Seraj, S.R.; Fallahi, M.J.; Rahmanian, Z. Acute portal vein thrombosis secondary to COVID-19: A case report. BMC Gastroenterol. 2020, 20, 386. [Google Scholar] [CrossRef]
- Cheung, S.; Quiwa, J.C.; Pillai, A.; Onwu, C.; Tharayil, Z.J.; Gupta, R. Superior Mesenteric Artery Thrombosis and Acute Intestinal Ischemia as a Consequence of COVID-19 Infection. Am. J. Case Rep. 2020, 21, e925753. [Google Scholar] [CrossRef]
- Posada-Arango, A.M.; García-Madrigal, J.; Echeverri-Isaza, S.; Alberto-Castrillón, G.; Martínez, D.; Gómez, A.C.; Pinto, J.A.; Pinillos, L. Thrombosis in abdominal vessels associated with COVID-19 Infection: A report of three cases. Radiol. Case Rep. 2021, 16, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Badrawi, N.; Abdulghaffar, S. Ovarian vein thrombosis as a first manifestation of COVID-19 infection. Radiol. Case Rep. 2021, 16, 3491–3493. [Google Scholar] [CrossRef]
- Baeza, C.; González, A.; Torres, P.; Pizzamiglio, M.; Arribas, A.; Aparicio, C. Acute aortic thrombosis in COVID-19. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 483–486. [Google Scholar] [CrossRef]
- Segovia, F.D.; Ream, S.; Dang, T.; Chaganti, B.T.; Ortega, A.J.; Rhee, S.; Borges, J.C. COVID-19-Associated Superior Mesenteric Artery Thrombosis and Acute Intestinal Ischemia. Cureus 2022, 14, e27722. [Google Scholar] [CrossRef]
- Hanif, M.; Ahmad, Z.; Khan, A.W.; Naz, S.; Sundas, F. COVID-19-Induced Mesenteric Thrombosis. Cureus 2021, 13, e12953. [Google Scholar] [CrossRef]
- Morioka, H.; Goto, M.; Tanaka, H.; Momose, H.; Fujino, K.; Hagiwara, T.; Aoki, J.; Orihata, M.; Kaneko, K. Acute intestinal necrosis due to multiple thrombosis in COVID-19 patient. Report of a case. Surg. Case Rep. 2022, 8, 136. [Google Scholar] [CrossRef]
- de Barry, O.; Mekki, A.; Diffre, C.; Seror, M.; El Hajjam, M.; Carlier, R.-Y. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol. Case Rep. 2020, 15, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Levolger, S.; Bokkers, R.P.H.; Wille, J.; Kropman, R.H.J.; de Vries, J.P.M. Arterial thrombotic complications in COVID-19 patients. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 454–459. [Google Scholar] [CrossRef]
- Sinz, S.; Glaser-Gallion, F.; Steffen, T. Portal vein thrombosis in COVID-19 infection. Surg. Case Rep. 2021, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nakamura, R.M.; Gonzalez-Calatayud, M.; Martinez Martinez, A.R. Acute mesenteric thrombosis in two patients with COVID-19. Two cases report and literature review. Int. J. Surg. Case Rep. 2020, 76, 409–414. [Google Scholar] [CrossRef]
- Alemán, W.; Cevallos, L.C. Subacute mesenteric venous thrombosis secondary to COVID-19: A late thrombotic complication in a nonsevere patient. Radiol. Case Rep. 2021, 16, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Philipponnet, C.; Aniort, J.; Chabrot, P.; Souweine, B.; Heng, A.E. Renal artery thrombosis induced by COVID-19. Clin. Kidney J. 2020, 13, 713. [Google Scholar] [CrossRef]
- Qasim Agha, O.; Berryman, R. Acute Splenic Artery Thrombosis and Infarction Associated with COVID-19 Disease. Case Rep. Crit. Care 2020, 2020, 8880143. [Google Scholar] [CrossRef]
- Carmo Filho, A.; Cunha, B.D.S. Inferior mesenteric vein thrombosis and COVID-19. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200412. [Google Scholar] [CrossRef]
- Sarkardeh, M.; Dalili, A.; Tayyebi Meibodi, N.; Izanlu, M.; Davari-Sani, S.J.; Moghaddamzade, S.; Jamalinik, M.; Hosseini, S.J.; Koushki, J.; Abedi, A. Intestinal Infarction in COVID-19 Pandemic: A Case Series. Iran. J. Pathol. 2022, 17, 85–90. [Google Scholar] [CrossRef]
- Hashim, Z.; Khan, A.; Areekkara, P.; Neyaz, Z.; Nath, A.; Jaiswal, S.; Mohindra, S. Thrombosis leading to acute abdomen in corona virus disease-19: A case series. Indian. J. Gastroenterol. 2022, 41, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Voci, D.; Micieli, E.; Johner, F.A.; Kucher, N.; Barco, S. Thrombosis and Dissection of the Abdominal Arteries Associated with Infarcts of Solid Organs in a Patient with COVID-19: A Novel Clinical Entity. Hamostaseologie 2022, 42, 195–197. [Google Scholar] [CrossRef]
- Veyseh, M.; Pophali, P.; Jayarangaiah, A.; Kumar, A. Left gonadal vein thrombosis in a patient with COVID-19-associated coagulopathy. BMJ Case Rep. 2020, 13, e236786. [Google Scholar] [CrossRef] [PubMed]
- Antunes de Brito, C.A.; de Oliveira Filho, J.R.B.; Marques, D.T.; Lencastre, M.D.C.; de Almeida, J.R.; Lopes, E.P. COVID-19 and Hepatic Artery Thrombosis: A Case Report. Am. J. Case Rep. 2021, 22, e932531-1–e932531-5. [Google Scholar] [CrossRef] [PubMed]
- Del Hoyo, J.; López-Muñoz, P.; Fernández-de la Varga, M.; Garrido-Marín, A.; Valero-Pérez, E.; Prieto, M.; Aguilera, V. Hepatobiliary and Pancreatic: A fatal case of extensive splanchnic vein thrombosis in a patient with COVID-19. J. Gastroenterol. Hepatol. 2020, 35, 1853. [Google Scholar] [CrossRef]
- Besutti, G.; Bonacini, R.; Iotti, V.; Marini, G.; Riva, N.; Dolci, G.; Maiorana, M.; Spaggiari, L.; Monelli, F.; Ligabue, G.; et al. Abdominal Visceral Infarction in 3 Patients with COVID-19. Emerg. Infect. Dis. 2020, 26, 1926–1928. [Google Scholar] [CrossRef]
- Azouz, E.; Yang, S.; Monnier-Cholley, L.; Arrivé, L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med. 2020, 46, 1464–1465. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).