Fixation Stability of the Right and Left Eyes Under Binocular and Monocular Viewing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Clinical Measures
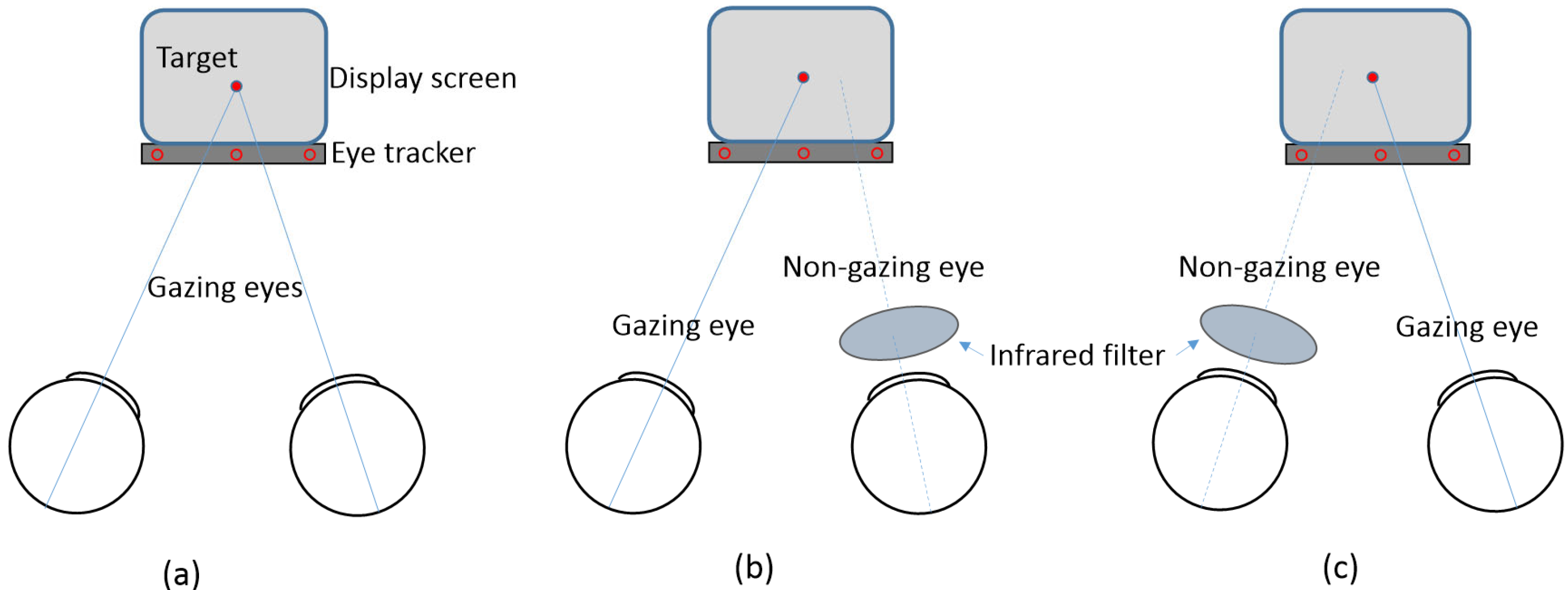
2.2. Apparatus and Procedure
2.3. Statistical Analysis
3. Results
3.1. Participants’ Demographic and Refractive Characteristics
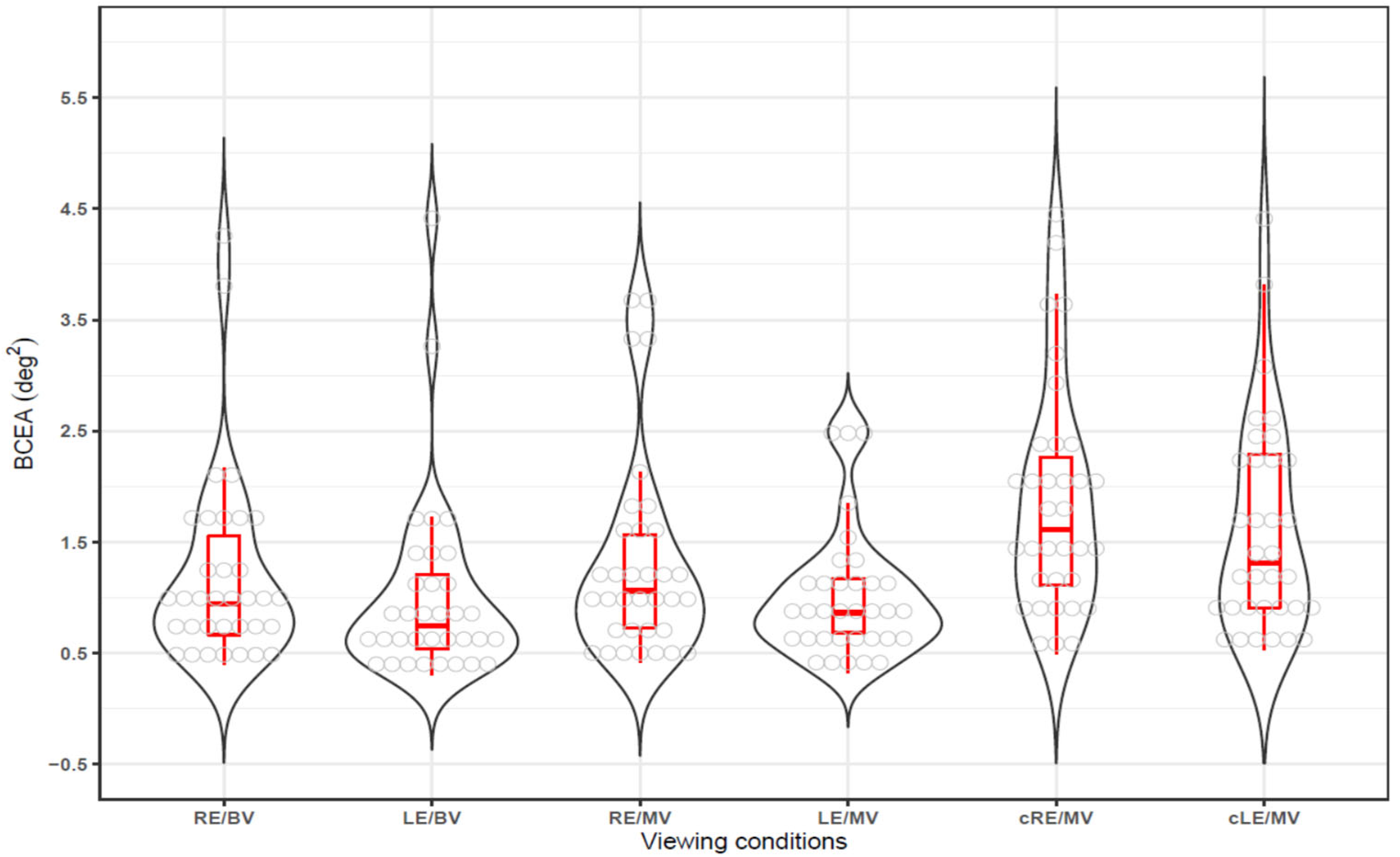
3.2. Fixation Stability in Binocular and Monocualr Viewing
3.3. BCEA in Gazing and Non-Gazing Eyes in Monocualr Viewing
3.4. Comparison of BCEA in Dominant and Non-Dominant Eyes
3.5. Comparison of BCEA Between Doinmant Eyes
3.6. Correction Between BCEA and Phoria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krauzlis, R.J.; Goffart, L.; Hafed, Z.M. Neuronal control of fixation and fixational eye movements. Phil. Trans. R. Soc. B 2017, 372, 20160205. [Google Scholar] [CrossRef]
- Rucci, M.; Poletti, M. Control and functions of fixational eye movements. Annu. Rev. Vis. Sci. 2015, 1, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, R.N.; Bobier, W.R.; Thompson, B. Binocular vision and fixational eye movements. J. Vis. 2019, 19, 9. [Google Scholar] [CrossRef]
- Kumar, G.; Chung, S.T. Characteristics of fixational eye movements in people with macular disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5125–5133. [Google Scholar] [CrossRef] [PubMed]
- Schor, C.; Hallmark, W. Slow control of eye position in strabismic amblyopia. Investig. Ophthalmol. Vis. Sci. 1978, 17, 577–581. [Google Scholar]
- Scaramuzzi, M.; Murray, J.; Otero-Millan, J.; Nucci, P.; Shaikh, A.G.; Ghasia, F.F. Fixation instability in amblyopia: Oculomotor disease biomarkers predictive of treatment effectiveness. Prog. Brain Res. 2019, 249, 235–248. [Google Scholar]
- Mompart-Martínez, R.; Argilés, M.; Cardona, G.; Cavero-Roig, L.; González-Sanchís, L.; Pighin, M.S. The relationship between fixation stability and retinal structural parameters in children with anisometropic, strabismic and mixed amblyopia. Life 2023, 13, 1517. [Google Scholar] [CrossRef]
- Kelly, K.R.; Cheng-Patel, C.S.; Jost, R.M.; Wang, Y.Z.; Birch, E.E. Fixation instability during binocular viewing in anisometropic and strabismic children. Exp. Eye Res. 2019, 183, 29–37. [Google Scholar] [CrossRef]
- Birch, E.E.; Subramanian, V.; Weakley, D.R. Fixation instability in anisometropic children with reduced stereopsis. J. AAPOS 2013, 17, 287–290. [Google Scholar] [CrossRef]
- Subramanian, P.S. Fixation Instability: A new measure of neurologic dysfunction in multiple sclerosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 202. [Google Scholar] [CrossRef]
- Tarita-Nistor, L.; Brent, M.H.; Steinbach, M.J.; González, E.G. Fixation stability during binocular viewing in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1887–1893. [Google Scholar] [CrossRef]
- Subramanian, V.; Jost, R.M.; Birch, E.E. A quantitative study of fixation stability in amblyopia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Crossland, M.D.; Rubin, G.S. The use of an infrared eyetracker to measure fixation stability. Optom. Vis. Sci. 2002, 79, 735–739. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Liu, N.; Li, S. Investigating the eye movement characteristics of basketball players executing 3-point shot at varied intensities and their correlation with shot accuracy. PeerJ 2024, 12, e17634. [Google Scholar] [CrossRef]
- Krauskopf, J.; Cornsweet, T.N.; Riggs, L.A. Analysis of eye movements during monocular and binocular fixation. J. Opt. Soc. Am. 1960, 50, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Liversedge, S.P.; White, S.J.; Findlay, J.M.; Rayner, K. Binocular coordination of eye movements during reading. Vision Res. 2006, 46, 2363–2374. [Google Scholar] [CrossRef]
- Jakobsen, N.S.; Larsen, D.A.; Bek, T. Binocular fixation reduces fixational eye movements in the worst eye of patients with center-involving diabetic macular edema. Ophthalmic Res. 2017, 58, 142–149. [Google Scholar] [CrossRef]
- Corbett, J.J. The bedside and office neuro-ophthalmology examination. Semin. Neurol. 2003, 23, 63–76. [Google Scholar]
- Wu, C.; Cao, B.; Dali, V.; Gagliardi, C.; Barthelemy, O.J.; Salazar, R.D.; Pomplun, M.; Cronin-Golomb, A.; Yazdanbakhsh, A. Eye movement control during visual pursuit in Parkinson’s disease. PeerJ 2018, 6, e5442. [Google Scholar] [CrossRef]
- Otero-Millan, J.; Macknik, S.L.; Martinez-Conde, S. Fixational eye movements and binocular vision. Front. Integr. Neurosci. 2014, 8, 52. [Google Scholar] [CrossRef]
- González-Vides, L.; Hernández-Verdejo, J.L.; Cañadas-Suárez, P. Eye tracking in optometry: A systematic review. J. Eye Mov. Res. 2023, 16, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Moon, B.Y.; Cho, H.G.; Yu, D.S. Quantitative evaluation of the association between fixation stability and phoria during short-term binocular viewing. Front. Neurosci. 2022, 16, 721665. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Moon, B.Y.; Cho, H.G.; Yu, D.S. Comparison of image-based quantification methods in evaluating fixation stability using a remote eye tracker in abnormal phoria. J. Int. Med. Res. 2022, 50, 3000605221098183. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Moon, B.Y.; Cho, H.G.; Yu, D.S. Comparative analysis of fixation stability before and after blinking during binocular viewing. J. Korean Ophthalmic Opt. Soc. 2023, 28, 9–17. [Google Scholar] [CrossRef]
- Blignaut, P.; van Rensburg, E.J.; Oberholzer, M. Visualization and quantification of eye tracking data for the evaluation of oculomotor function. Heliyon 2019, 5, e01127. [Google Scholar] [CrossRef]
- Saida, S.; Ono, H.; Mapp, A.P. Closed-loop and open-loop accommodative vergence eye movements. Vision Res. 2001, 41, 77–86. [Google Scholar] [CrossRef]
- González, E.G.; Wong, A.M.; Niechwiej-Szwedo, E.; Tarita-Nistor, L.; Steinbach, M.J. Eye position stability in amblyopia and in normal binocular vision. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5386–5394. [Google Scholar] [CrossRef]
- Samet, S.; González, E.G.; Mandelcorn, M.S.; Brent, M.H.; Tarita-Nistor, L. Changes in fixation stability with time during binocular and monocular viewing in maculopathy. Vision 2018, 2, 40. [Google Scholar] [CrossRef]
- Reilly, J.; Gaiser, H.; Young, B. Clinical Procedures for Ocular Examination, 5th ed.; McGraw Hill: New York, NY, USA, 2023; pp. 61–147. [Google Scholar]
- Castet, E.; Crossland, M. Quantifying eye stability during a fixation task: A review of definitions and methods. Seeing Perceiving 2012, 25, 449–469. [Google Scholar] [CrossRef]
- Linke, S.J.; Baviera, J.; Munzer, G.; Steinberg, J.; Richard, G.; Katz, T. Association between ocular dominance and spherical/astigmatic anisometropia, age, and sex: Analysis of 10,264 myopic individuals. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9166–9173. [Google Scholar] [CrossRef]
- Ono, H.; Gonda, G. Apparent movement, eye movements and phoria when two eyes alternate in viewing a stimulus. Perception 1978, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.U.; Saker, S.; Wilde, C.; Pellizzari, C.; Pallikaris, A.; Notaroberto, N.; Rubinstein, M.; Rui, C.; Limoli, P.; Smolek, M.K.; et al. Reference clinical database for fixation stability metrics in normal subjects measured with the MAIA microperimeter. Transl. Vis. Sci. Technol. 2016, 5, 6. [Google Scholar] [CrossRef]
- Altemir, I.; Alejandre, A.; Fanlo-Zarazaga, A.; Ortín, M.; Pérez, T.; Masiá, B.; Pueyo, V. Evaluation of fixational behavior throughout life. Brain Sci. 2021, 12, 19. [Google Scholar] [CrossRef]
- Ghasia, F.; Tychsen, L. Inter-ocular fixation instability of amblyopia: Relationship to visual acuity, strabismus, nystagmus, stereopsis, vergence, and age. Am. J. Ophthalmol. 2024, 267, 230–248. [Google Scholar] [CrossRef]
- Vikesdal, G.H.; Langaas, T. Optically induced refractive errors reduces fixation stability but saccade latency remains stable. J. Eye Mov. Res. 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Hirasawa, K.; Oka, K.; Koshiji, R.; Funaki, W.; Shoji, N. Smaller fixation target size is associated with more stable fixation and less variance in threshold sensitivity. PLoS ONE 2016, 11, e0165046. [Google Scholar] [CrossRef]
- Liu, H.; Bittencourt, M.G.; Sophie, R.; Sepah, Y.J.; Hanout, M.; Rentiya, Z.; Annam, R.; Scholl, H.P.; Nguyen, Q.D. Fixation stability measurement using two types of microperimetry devices. Transl. Vis. Sci. Technol. 2015, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Argilés, M.; Cardona, G. The interaction between vergence and accommodation cues in the assessment of fusional vergence range. Life 2024, 14, 1185. [Google Scholar] [CrossRef]
- Watamaniuk, S.N.J.; Heinen, S.J.; Singh, D.; Chandna, A. Occluding one eye during fixation increases wandering of both eyes. J. Vision 2023, 23, 5670. [Google Scholar] [CrossRef]
- Chandna, A.; Badler, J.; Singh, D.; Watamaniuk, S.; Heinen, S. A covered eye fails to follow an object moving in depth. Sci. Rep. 2021, 11, 10983. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, P.; Menon, V. Saccadic underaction in concomitant strabismus and Hering’s law: A new neurophysiologic model for binocular motor correspondence. Indian J. Ophthalmol. 1993, 41, 117–120. [Google Scholar]
- King, W.M. Binocular coordination of eye movements-Hering’s Law of equal innervation or uniocular control? Eur. J. Neurosci. 2011, 33, 2139–2146. [Google Scholar] [CrossRef]
- Peli, E.; McCormack, G. Dynamics of cover test eye movements. Am. J. Optom. Physiol. Opt. 1983, 60, 712–724. [Google Scholar] [CrossRef]
- Lopes-Ferreira, D.; Neves, H.; Queiros, A.; Faria-Ribeiro, M.; Peixoto-de-Matos, S.C.; González-Méijome, J.M. Ocular dominance and visual function testing. Biomed. Res. Int. 2013, 2013, 238943. [Google Scholar] [CrossRef] [PubMed]
- Serpa, E.; Alecka, M.; Ceple, I.; Krumina, G.; Svede, A.; Kassaliete, E.; Goliskina, V.; Volberga, L.; Berzina, A.; Mikelsone, R.; et al. The impact of eye dominance on fixation stability in school-aged children. J. Eye Mov. Res. 2023, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.B.; Shim, H.S. Direction changes in the dominant eye and comparison of single-eye inset amounts depending on the view distance. J. Korean Ophthalmic Opt. Soc. 2020, 25, 19–24. [Google Scholar] [CrossRef]
- Kim, J.S.; Shim, J.B. Comparison of amount of at distance and near phoria in dominant eye and non-dominant eye by von Graefe method. J. Korean Ophthalmic Opt. Soc. 2018, 23, 111–116. [Google Scholar] [CrossRef]
- Vikesdal, G.H.; Langaas, T. Saccade latency and fixation stability: Repeatability and reliability. J. Eye Mov. Res. 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Mestre, C.; Otero, C.; Díaz-Doutón, F.; Gautier, J.; Pujol, J. An automated and objective cover test to measure heterophoria. PLoS ONE 2018, 13, e0206674. [Google Scholar] [CrossRef]
- Park, K.; Shebilske, W.L. Phoria, Hering’s laws, and monocular perception of direction. J. Exp. Psychol. Hum. Percept. Perform. 1991, 17, 219–231. [Google Scholar] [CrossRef]
| Mean ± SD | Range | |
|---|---|---|
| Male/female (n) | 15/19 | |
| Age (years) | 22.4 ± 2.1 | 20—27 |
| Refractive correction/no correction (n) | 23/11 | |
| Decimal visual acuity (logMAR) | 0.07 ± 0.16 | −0.08—0.70 |
| Better than 0.15/worse than 0.20 (eyes) | 61/7 | no correction: 3 |
| Refractive errors (n) | ||
| Myopia/emmetropia/not confirmed (n) | 23/6/5 | |
| Corrected refractive power (D, diopters) | ||
| Spherical power | −2.88 ± 1.42 | 0.25—−5.75 |
| Cylindrical power | −1.24 ± 0.93 | −0.25—−3.50 |
| Spherical equivalent | −3.32 ± 1.53 | −0.25—−6.50 |
| Phoria (△, prism diopters) | ||
| Distance | −2.24 ± 2.35 | 1.50—−9.50 |
| Near | −5.91 ± 5.42 | 1.00—−24.00 |
| Dominant eyes: right/left eyes (n) | ||
| Distance | 21/13 | Distance ≠ near: 3 |
| Near | 20/14 |
| n | Wilcoxon Signed-Rank Test | Correlation Coefficient | |||
|---|---|---|---|---|---|
| Z-Score | p-Value | rs 1 | p-Value | ||
| RE/BV vs. RE/MV | 34 | −0.563 | 0.573 | 0.484 | 0.005 * |
| RE/BV vs. LE/MV | 34 | 0.427 | 0.669 | 0.007 | 0.966 |
| LE/BV vs. RE/MV | 34 | −2.188 | 0.029 * | 0.308 | 0.077 |
| LE/BV vs. LE/MV | 34 | −0.241 | 0.809 | 0.345 | 0.047 * |
| n | Wilcoxon Signed-Rank Test | Correlation Coefficient | |||
|---|---|---|---|---|---|
| Z-Score | p-Value | rs 1 | p-Value | ||
| RE/MV vs. cRE/MV | 34 | −3.274 | 0.001 * | 0.646 | <0.001 * |
| RE/MV vs. cLE/MV | 34 | −1.903 | 0.057 | 0.580 | 0.001 * |
| LE/MV vs. cRE/MV | 34 | −4.197 | <0.001 * | 0.401 | 0.021 * |
| LE/MV vs. cLE/MV | 34 | −3.830 | 0.001 * | 0.444 | 0.011 * |
| Dominant Eye (n = 34) | Non-Dominant Eye (n = 34) | Wilcoxon Signed-Rank Test | ||||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Z-Score | p-Value | |
| BV | 0.97 | 0.56–1.62 | 0.73 | 0.57–0.99 | 1.864 | 0.062 |
| MV | 1.01 | 0.69–1.85 | 1.06 | 0.77–1.35 | 0.060 | 0.952 |
| cMV | 1.65 | 0.96–2.38 | 1.11 | 0.71–2.14 | 2.653 | 0.008 * |
| RE Dominant (n = 20) | LE Dominant (n = 14) | Mann–Whitney U Test | ||||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Z-Score | p-Value | |
| RE/BV | 1.12 | 0.84–1.73 | 0.73 | 0.58–0.96 | 1.995 | 0.046 * |
| LE/BV | 0.71 | 0.57–1.26 | 0.83 | 0.50–1.22 | 0.157 | 0.875 |
| RE/MV | 1.17 | 0.76–1.63 | 0.97 | 0.63–1.28 | 0.980 | 0.327 |
| LE/MV | 0.77 | 0.65–1.22 | 0.94 | 0.77–1.18 | −0.490 | 0.624 |
| cRE/MV | 2.03 | 1.21–2.70 | 1.41 | 0.94–1.95 | 1.505 | 0.132 |
| cLE/MV | 1.26 | 0.80–2.42 | 1.44 | 0.91–1.80 | 0.192 | 0.847 |
| rs 1 | p-Value | |
|---|---|---|
| RE/BV | 0.091 | 0.600 |
| LE/BV | 0.413 | 0.018 * |
| RE/MV | −0.020 | 0.908 |
| LE/MV | −0.075 | 0.665 |
| cRE/MV | −0.179 | 0.305 |
| cLE/MV | −0.314 | 0.072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, J.-H.; Kim, S.-Y.; Moon, B.-Y.; Cho, H.G.; Yu, D.-S. Fixation Stability of the Right and Left Eyes Under Binocular and Monocular Viewing Conditions. Life 2025, 15, 703. https://doi.org/10.3390/life15050703
Noh J-H, Kim S-Y, Moon B-Y, Cho HG, Yu D-S. Fixation Stability of the Right and Left Eyes Under Binocular and Monocular Viewing Conditions. Life. 2025; 15(5):703. https://doi.org/10.3390/life15050703
Chicago/Turabian StyleNoh, Jae-Hyeon, Sang-Yeob Kim, Byeong-Yeon Moon, Hyun Gug Cho, and Dong-Sik Yu. 2025. "Fixation Stability of the Right and Left Eyes Under Binocular and Monocular Viewing Conditions" Life 15, no. 5: 703. https://doi.org/10.3390/life15050703
APA StyleNoh, J.-H., Kim, S.-Y., Moon, B.-Y., Cho, H. G., & Yu, D.-S. (2025). Fixation Stability of the Right and Left Eyes Under Binocular and Monocular Viewing Conditions. Life, 15(5), 703. https://doi.org/10.3390/life15050703





