Comparison of Postoperative Analgesic Profiles Between Transversus Abdominis Plane Block and Local Wound Infiltration in Living Donor Kidney Transplantation Recipients: A Propensity Score-Matched Analysis
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Considerations
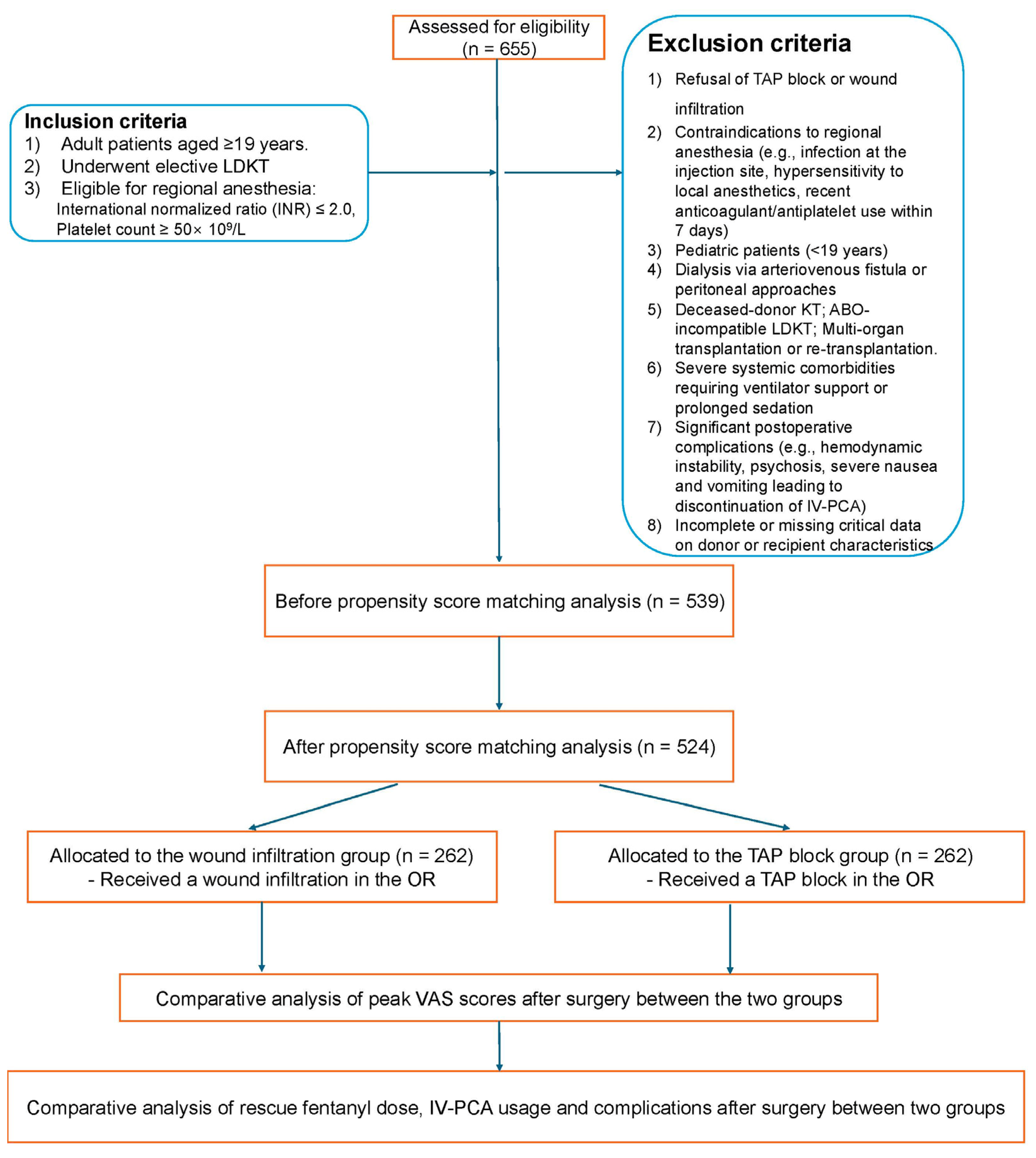
2.2. Study Population
2.3. Surgery and General Anesthesia
2.4. Local Wound Infiltration and TAP Block in the Operating Room
2.5. Adjuvant Strategies for Postoperative Pain Management
2.6. Outcome Measures
2.7. Clinical Variables
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
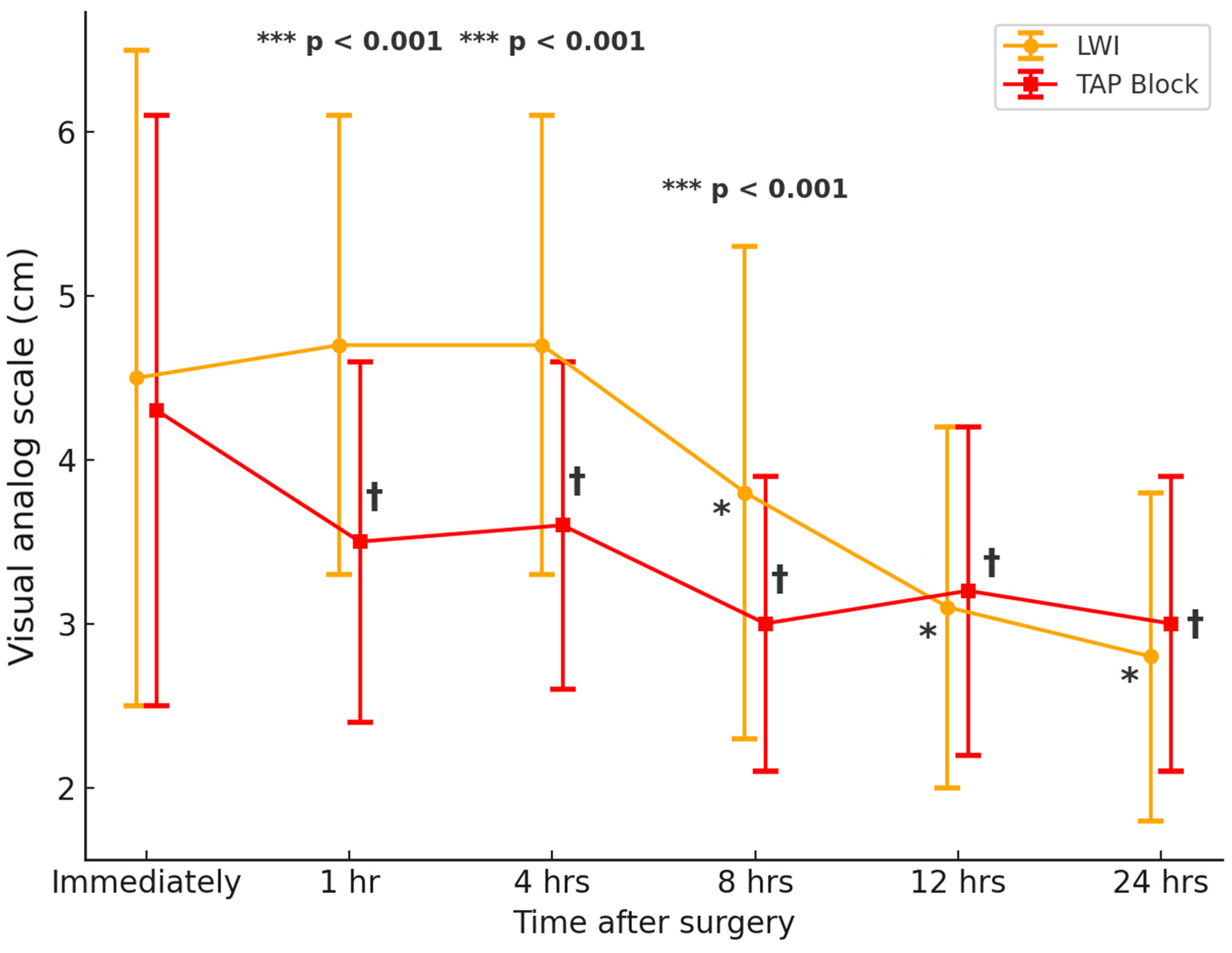
3.2. Postoperative Pain and Opioid Requirements
3.3. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.H.; Hart, A. Global perspective on kidney transplantation: United States. Kidney360 2021, 2, 1836–1839. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Law, L.S.-C.; Gan, T.J. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can. J. Anesth. 2015, 62, 203–218. [Google Scholar] [CrossRef]
- Coussens, N.P.; Sittampalam, G.S.; Jonson, S.G.; Hall, M.D.; Gorby, H.E.; Tamiz, A.P.; McManus, O.B.; Felder, C.C.; Rasmussen, K. The opioid crisis and the future of addiction and pain therapeutics. J. Pharmacol. Exp. Ther. 2019, 371, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Wise, B.; Wilson, L.Z.; Taber, D.J.; Pilch, N.A.; Rohan, V.; Fleming, J.N. The impact of pretransplant opioid exposure on healthcare utilization and costs in kidney transplant. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 6–13. [Google Scholar] [CrossRef]
- Park, J.; Park, S.C.; Chae, M.S.; Hong, S.H.; Shim, J.W. Efficacy of Intraoperative Paracetamol and Nefopam Infusions in Addition to Transversus Abdominis Plane Block in Kidney Transplant Recipients. Medicina 2025, 61, 65. [Google Scholar] [CrossRef]
- Ahlers, O.; Nachtigall, I.; Lenze, J.; Goldmann, A.; Schulte, E.; Höhne, C.; Fritz, G.; Keh, D. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br. J. Anaesth. 2008, 101, 781–787. [Google Scholar] [CrossRef]
- Gonçalves dos Santos, G.; Delay, L.; Yaksh, T.L.; Corr, M. Neuraxial cytokines in pain states. Front. Immunol. 2020, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Kvarnström, A.; Sarbinowski, R.; Bengtson, J.P.; Jacobsson, L.; Bengtsson, A. Complement activation and interleukin response in major abdominal surgery. Scand. J. Immunol. 2012, 75, 510–516. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Jang, J.S.; Hwang, S.M.; Tark, H.; Kim, J.H.; Lee, J.J. Effects of surgery start time on postoperative cortisol, inflammatory cytokines, and postoperative hospital day in hip surgery: Randomized controlled trial. Medicine 2019, 98, e15820. [Google Scholar] [CrossRef]
- Kehlet, H.; Holte, K. Effect of postoperative analgesia on surgical outcome. Br. J. Anaesth. 2001, 87, 62–72. [Google Scholar] [CrossRef]
- Bova, S.; Samet, R.E.; Deering, J.; Gaines, S.; Weinrub, A.; Bhati, C.; Niederhaus, S. Successful Opioid Minimization Following Kidney Transplant: A Quality Improvement Initiative. Cureus 2024, 16, e52917. [Google Scholar] [CrossRef] [PubMed]
- Grape, S.; Kirkham, K.R.; Akiki, L.; Albrecht, E. Transversus abdominis plane block versus local anesthetic wound infiltration for optimal analgesia after laparoscopic cholecystectomy: A systematic review and meta-analysis with trial sequential analysis. J. Clin. Anesth. 2021, 75, 110450. [Google Scholar] [CrossRef] [PubMed]
- Plakhotnik, J.; Zhang, L.; Estrada, M.; Coles, J.G.; Lonnqvist, P.-A.; Maynes, J.T. Local anesthetic cardiac toxicity is mediated by cardiomyocyte calcium dynamics. Anesthesiology 2022, 137, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Mather, L.E. The acute toxicity of local anesthetics. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1313–1332. [Google Scholar] [CrossRef]
- Johns, N.; O’neill, S.; Ventham, N.; Barron, F.; Brady, R.; Daniel, T. Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: A systematic review and meta-analysis. Color. Dis. 2012, 14, e635–e642. [Google Scholar] [CrossRef]
- Stamenkovic, D.M.; Bezmarevic, M.; Bojic, S.; Unic-Stojanovic, D.; Stojkovic, D.; Slavkovic, D.Z.; Bancevic, V.; Maric, N.; Karanikolas, M. Updates on wound infiltration use for postoperative pain management: A narrative review. J. Clin. Med. 2021, 10, 4659. [Google Scholar] [CrossRef]
- Mw, H. Local anesthetics and the inflammatory response: A new therapeutic indication? Anesthesiology 2000, 93, 858–875. [Google Scholar]
- Weinschenk, S.; Weiss, C.; Benrath, J.; von Baehr, V.; Strowitzki, T.; Feißt, M. Anti-inflammatory characteristics of local anesthetics: Inhibition of TNF-α secretion of lipopolysaccharide-stimulated leucocytes in human blood samples. Int. J. Mol. Sci. 2022, 23, 3283. [Google Scholar] [CrossRef]
- Piegeler, T.; Votta-Velis, E.G.; Bakhshi, F.R.; Mao, M.; Carnegie, G.; Bonini, M.G.; Schwartz, D.E.; Borgeat, A.; Beck-Schimmer, B.; Minshall, R.D. Endothelial barrier protection by local anesthetics: Ropivacaine and lidocaine block tumor necrosis factor-α–induced endothelial cell Src activation. Anesthesiology 2014, 120, 1414. [Google Scholar] [CrossRef]
- Petersen, P.; Mathiesen, O.; Torup, H.; Dahl, J. The transversus abdominis plane block: A valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol. Scand. 2010, 54, 529–535. [Google Scholar] [CrossRef]
- Guo, Q.; Li, R.; Wang, L.; Zhang, D.; Ma, Y. Transversus abdominis plane block versus local anaesthetic wound infiltration for postoperative analgesia: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 17343. [Google Scholar] [PubMed]
- Humar, A.; Matas, A.J. Surgical complications after kidney transplantation. In Seminars in Dialysis; Wiley Online Library: Hoboken, NJ, USA, 2005. [Google Scholar]
- Sessa, A.; Esposito, A.; Giliberti, A.; Iavicoli, G.; Costa, C.; Bergallo, M.; Lettieri, E.; Rossano, R.; Capuano, M. Immunosuppressive agents and metabolic factors of cardiovascular risk in renal transplant recipients. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Ren, L.; Qin, P.; Min, S.; Wang, W.; Jin, J. Transversus abdominis plane block versus local wound infiltration for postoperative pain after laparoscopic colorectal cancer resection: A randomized, double-blinded study. J. Gastrointest. Surg. 2022, 26, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Working Party; Harrop-Griffiths, W.; Cook, T.; Gill, H.; Hill, D.; Ingram, M.; Makris, M.; Malhotra, S.; Nicholls, B.; Popat, M. Regional anaesthesia and patients with abnormalities of coagulation: The association of anaesthetists of great britain & ireland the obstetric anaesthetists’ association regional anaesthesia UK. Anaesthesia 2013, 68, 966–972. [Google Scholar]
- Chowdhury, S.; McLure, H. Chronic kidney disease and anaesthesia. BJA Educ. 2022, 22, 321–328. [Google Scholar] [CrossRef]
- Huh, J.; Kwon, H.; Park, H.; Park, S.C.; Yun, S.S.; Chae, M.S. Impact of Norepinephrine and Dopamine Infusion on Renal Arterial Resistive Index during Pre-Emptive Living Donor Kidney Transplantation: Propensity Score Matching Analysis. Medicina 2024, 60, 1066. [Google Scholar] [CrossRef]
- Go, J.; Park, S.-C.; Yun, S.-S.; Ku, J.; Park, J.; Shim, J.-W.; Lee, H.M.; Kim, Y.-S.; Moon, Y.E.; Hong, S.H. Exposure to hyperchloremia is associated with poor early recovery of kidney graft function after living-donor kidney transplantation: A propensity score-matching analysis. J. Clin. Med. 2019, 8, 955. [Google Scholar] [CrossRef]
- Benham-Hermetz, J.; Mitchell, V. Safe tracheal extubation after general anaesthesia. BJA Educ. 2021, 21, 446–454. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Yoshida, T.; Chuang, T.-Y.; Yang, S.-F.; Chang, C.-C.; Yao, H.-Y.; Tai, Y.-T.; Lin, J.-A.; Chen, K.-Y. Transversus abdominis plane block: An updated review of anatomy and techniques. BioMed Res. Int. 2017, 2017, 8284363. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Casati, A.; Putzu, M. Bupivacaine, levobupivacaine and ropivacaine: Are they clinically different? Best Pract. Res. Clin. Anaesthesiol. 2005, 19, 247–268. [Google Scholar] [CrossRef]
- McClellan, K.J.; Faulds, D. Ropivacaine: An update of its use in regional anaesthesia. Drugs 2000, 60, 1065–1093. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.B.; Lee, A.; Fagan, D.; Bowler, G.M.; Bloomfield, P.; Lundh, R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth. Analg. 1989, 69, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Gao, M.-L.; Chen, G.-Y.; Pan, L.-H. Transversus Abdominis Plane Block versus Wound Infiltration with Conventional Local Anesthetics in Adult Patients Underwent Surgery: A Systematic Review and Meta-analysis of Randomized Controlled Trials. BioMed Res. Int. 2020, 2020, 8914953. [Google Scholar] [CrossRef]
- Nam, S.W.; Do, S.-H.; Hwang, J.-W.; Park, I.; Hwang, I.; Na, H.-S. Effects of opioid-sparing general anesthesia on postoperative nausea and vomiting in laparoscopic gynecological surgery. Korean J. Anesthesiol. 2024, 77, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Tolchard, S.; Davies, R.; Martindale, S. Efficacy of the subcostal transversus abdominis plane block in laparoscopic cholecystectomy: Comparison with conventional port-site infiltration. J. Anaesthesiol. Clin. Pharmacol. 2012, 28, 339–343. [Google Scholar] [CrossRef]
- Kissin, I. Preemptive analgesia. Anesthesiology 2000, 93, 1138–1144. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, D.; Lang, B.; Zang, C.; Sun, Z.; Ren, S.; Chen, H. Effect of opioid-free anesthesia on the incidence of postoperative nausea and vomiting: A meta-analysis of randomized controlled studies. Medicine 2023, 102, e35126. [Google Scholar] [CrossRef]
- Jai, D.; Handscombe, M.; Brooke, M.; Karena, S.; Arune, S.; Leslie, K. Interpretation of the four risk factors for postoperative nausea and vomiting in the Apfel simplified risk score: An analysis of published studies. Can. J. Anesth. 2021, 68, 1057–1063. [Google Scholar]
- Schlesinger, T.; Meybohm, P.; Kranke, P. Postoperative nausea and vomiting: Risk factors, prediction tools, and algorithms. Curr. Opin. Anesthesiol. 2023, 36, 117–123. [Google Scholar] [CrossRef]
- Joshi, G.P. Rational multimodal analgesia for perioperative pain management. Curr. Pain Headache Rep. 2023, 27, 227–237. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.-T.; Yang, S.-M.; Kim, W.H.; Han, A.; Ha, J.; Min, S.; Park, S.-K. Anterior quadratus lumborum block for analgesia after living-donor renal transplantation: A double-blinded randomized controlled trial. Reg. Anesth. Pain Med. 2024, 49, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R., Jr.; Pergolizzi, J.V.; Sinclair, A.; Raffa, R.B.; Aldington, D.; Plavin, S.; Apfel, C.C. Transversus abdominis block: Clinical uses, side effects, and future perspectives. Pain Pract. 2013, 13, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.; Guirguis, M.N.; Helou, M.; Dalton, J.E.; Ngo, F.; Ghobrial, M.; O’Hara, J.; Seif, J.; Krishnamurthi, V.; Goldfarb, D. Continuous transversus abdominis plane block catheter analgesia for postoperative pain control in renal transplant. J. Anesth. 2015, 29, 4–8. [Google Scholar] [CrossRef]
- Jankovic, Z.B.; Pollard, S.G.; Nachiappan, M.M. Continuous transversus abdominis plane block for renal transplant recipients. Anesth. Analg. 2009, 109, 1710–1711. [Google Scholar] [CrossRef] [PubMed]
- Admassie, B.M.; Debas, S.A.; Admass, B.A. Prevention and management of rebound pain after resolution of regional block: A systematic review. Ann. Med. Surg. 2024, 86, 4732–4737. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Peters, J.H.; Heesen, M.; Scheel, J.; Kunz, M. Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci. Biobehav. Rev. 2017, 75, 104–113. [Google Scholar] [CrossRef]
- Singh, J.A.; Lewallen, D. Age, gender, obesity, and depression are associated with patient-related pain and function outcome after revision total hip arthroplasty. Clin. Rheumatol. 2009, 28, 1419–1430. [Google Scholar] [CrossRef]
- Kivrak, Y.; Kose-Ozlece, H.; Ustundag, M.F.; Asoglu, M. Pain perception: Predictive value of sex, depression, anxiety, somatosensory amplification, obesity, and age. Neuropsychiatr. Dis. Treat. 2016, 12, 1913–1918. [Google Scholar]
- Wassef, M.; Lee, D.Y.; Levine, J.L.; Ross, R.E.; Guend, H.; Vandepitte, C.; Hadzic, A.; Teixeira, J. Feasibility and analgesic efficacy of the transversus abdominis plane block after single-port laparoscopy in patients having bariatric surgery. J. Pain Res. 2013, 6, 837–841. [Google Scholar] [CrossRef]
- Shah, A.A.; Alnajib, A.M.A.; Baldaniya, L.; Hassan, H.; Kaur, P.; Sharma, R.; Ramzan, H.S.; Sami, W. Investigating the Effectiveness of Enhanced Recovery after Surgery (ERAS) Protocols in Improving Postoperative Outcomes and Reducing Hospital Readmission Rates in Patients Undergoing abdominal Surgery. J. Pharm. Bioallied. Sci. 2024, 16, S3534–S3537. [Google Scholar] [CrossRef]
- Chen, Y.K.; Boden, K.A.; Schreiber, K.L. The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: A narrative review. Anaesthesia 2021, 76 (Suppl. 1), 8–17. [Google Scholar] [CrossRef] [PubMed]
- Mancel, L.; Van Loon, K.; Lopez, A.M. Role of regional anesthesia in Enhanced Recovery After Surgery (ERAS) protocols. Curr. Opin. Anaesthesiol. 2021, 34, 616–625. [Google Scholar] [CrossRef]
- Crumplin, M.K. Hand-held surgical retractors. Br. J. Surg. 2023, 110, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Hinkson, L.; Siedentopf, J.-P.; Weichert, A.; Henrich, W. Surgical site infection in cesarean sections with the use of a plastic sheath wound retractor compared to the traditional self-retaining metal retractor. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 232–238. [Google Scholar] [CrossRef]
- Hardy-Fairbanks, A.J.; Mackenzie, T.; McCarthy, M., Jr.; Goldman, M.B.; Lauria, M.R. A randomized controlled trial comparing two types of retractors at caesarean delivery. J. Obstet. Gynaecol. 2017, 37, 1009–1014. [Google Scholar] [CrossRef]
- Yamada, S.; Hotta, K.; Takahata, M.; Iwami, D.; Sugito, Y.; Tanabe, T.; Iwahara, N.; Shinohara, N. Femoral nerve palsy following kidney transplantation: A case report and review of the literature. IJU Case Rep. 2020, 3, 248–251. [Google Scholar] [CrossRef]
- Geneletti, S.; Richardson, S.; Best, N. Adjusting for selection bias in retrospective, case-control studies. Biostatistics 2009, 10, 17–31. [Google Scholar] [CrossRef]
- Pirie, K.; Traer, E.; Finniss, D.; Myles, P.S.; Riedel, B. Current approaches to acute postoperative pain management after major abdominal surgery: A narrative review and future directions. Br. J. Anaesth. 2022, 129, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Warrillow, S.J.; Reade, M.C. Why we should be wary of single-center trials. Crit. Care Med. 2009, 37, 3114–3119. [Google Scholar] [CrossRef]
- Munoz-Leyva, F.; Cubillos, J.; Chin, K.J. Managing rebound pain after regional anesthesia. Korean J. Anesthesiol. 2020, 73, 372–383. [Google Scholar] [CrossRef]
| Before PS Matching | After PS Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | TAP Block (n = 269) | LWI (n = 270) | p Value | SD | TAP Block (n = 262) | LWI (n = 262) | p Value | SD |
| Preoperative variables | ||||||||
| Sex; n (%) | 138 (51.3%) | 124 (45.9%) | 0.212 | 0.107 | 128 (48.9%) | 144 (55.0%) | 0.162 | 0.122 |
| Age; years | 52.0 (41.5–59.0) | 50.0 (40.0–57.0) | 0.124 | 0.121 | 51.5 (41.8–59.0) | 50.0 (40.8–57.0) | 0.141 | 0.117 |
| BMI; kg/m2 | 22.8 (20.6–26.0) | 22.9 (20.4–25.4) | 0.676 | 0.071 | 22.9 (20.6–26.1) | 23.0 (20.4–25.5) | 0.643 | 0.073 |
| Diabetes mellitus; n (%) | 112 (41.6%) | 84 (31.1%) | 0.011 | 0.213 | 105 (40.1%) | 84 (32.1%) | 0.056 | 0.193 |
| Hypertension; n (%) | 146 (54.3%) | 132 (48.9%) | 0.211 | 0.108 | 142 (54.2%) | 128 (48.9%) | 0.221 | 0.107 |
| Dialysis period; day | 1.0 (0.0–7.5) | 1.0 (0.0–11.0) | 0.900 | −0.081 | 1.0 (0.0–6.0) | 0.5 (0.0–10.0) | 0.937 | −0.082 |
| Echocardiography | ||||||||
| Ejection fraction; % | 62.0 (58.7–64.8) | 62.0 (57.3–64.6) | 0.314 | 0.086 | 62.0 (58.8–64.9) | 62.0 (57.9–64.7) | 0.481 | 0.038 |
| LVMI; g/m2 | 119.1 (101.0–140.3) | 119.1 (102.0–144.0) | 0.376 | −0.109 | 119.0 (99.1–140.2) | 119.1 (101.1–142.6) | 0.435 | −0.103 |
| E/e’ ratio | 10.3 (8.7–12.9) | 10.0 (7.8–12.7) | 0.170 | 0.005 | 10.3 (8.7–12.6) | 10.0 (7.8–12.5) | 0.156 | 0.004 |
| Corrected QT interval; ms | 450.0 (431.0–469.5) | 452.0 (432.0–475.0) | 0.431 | −0.069 | 450.0 (430.8–469.3) | 452.0 (432.0–473.3) | 0.456 | −0.067 |
| Hourly urine output; mL/kg/h | 18.8 (12.5–25.0) | 17.1 (10.4–25.0) | 0.150 | 0.131 | 18.8 (12.5–25.0) | 17.9 (10.4–25.0) | 0.187 | 0.119 |
| Laboratory variables | ||||||||
| WBC; ×109/L | 6.2 (4.8–8.1) | 6.3 (4.8–7.8) | 0.983 | −0.026 | 6.2 (4.8–8.1) | 6.3 (4.8–7.8) | 0.942 | −0.025 |
| Neutrophil; % | 70.5 (61.6–85.4) | 67.2 (60.7–82.9) | 0.284 | 0.099 | 70.3 (61.5–85.2) | 67.4 (60.7–82.9) | 0.419 | 0.079 |
| Lymophocyte; % | 18.4 (10.9–25.2) | 19.7 (11.6–26.3) | 0.211 | −0.106 | 18.7 (10.9–25.1) | 19.7 (11.6–26.2) | 0.313 | −0.087 |
| Hemoglobin; g/dL | 10.7 (9.6–11.5) | 10.6 (9.4–11.6) | 0.508 | 0.025 | 10.7 (9.6–11.5) | 10.6 (9.5–11.6) | 0.653 | 0.016 |
| Glucose; mg/dL | 121.0 (95.5–155.0) | 118.0 (95.0–147.0) | 0.599 | 0.042 | 120.0 (95.0–151.5) | 119.0 (95.0–147.3) | 0.762 | 0.024 |
| Albumin; g/dL | 4.1 (3.8–4.3) | 4.1 (3.8–4.3) | 0.540 | 0.053 | 4.1 (3.8–4.3) | 4.1 (3.8–4.3) | 0.772 | 0.026 |
| AST; U/L | 17.0 (14.0–23.0) | 17.0 (14.0–22.0) | 0.826 | 0.018 | 17.0 (14.0–23.0) | 17.0 (14.0–22.0) | 0.914 | 0.019 |
| ALT; U/L | 14.0 (10.0–20.0) | 13.0 (10.0–19.0) | 0.235 | 0.057 | 14.0 (10.0–20.0) | 13.0 (10.0–18.3) | 0.161 | 0.068 |
| Sodium; mmol/L | 138.0 (135.0–140.0) | 138.0 (135.0–140.0) | 0.891 | 0.021 | 138.0 (135.0–140.0) | 138.0 (135.0–140.0) | 0.906 | 0.015 |
| Potassium; mmol/L | 4.7 (4.2–5.2) | 4.7 (4.3–5.2) | 0.577 | −0.025 | 4.7 (4.2–5.2) | 4.7 (4.3–5.2) | 0.382 | −0.055 |
| Chloride; mmol/L | 100.0 (97.0–104.0) | 100.0 (96.0–104.3) | 0.967 | 0.011 | 100.0 (97.0–104.3) | 100.0 (96.0–105.0) | 0.978 | 0.006 |
| Platelet count; ×109/L | 178.0 (140.5–218.0) | 178.5 (141.0–230.3) | 0.466 | −0.114 | 178.0 (140.8–218.0) | 180.0 (141.0–230.3) | 0.394 | −0.122 |
| INR | 1.00 (0.96–1.05) | 1.01 (0.97–1.07) | 0.117 | −0.173 | 1.00 (0.96–1.05) | 1.01 (0.96–1.07) | 0.200 | −0.172 |
| Creatinine; mg/dL | 7.1 (6.0–9.1) | 7.6 (5.9–9.3) | 0.371 | −0.06 | 7.1 (6.0–9.1) | 7.5 (5.9–9.2) | 0.508 | −0.037 |
| BNP; pg/mL | 78.7 (31.6–177.2) | 82.4 (36.1–218.7) | 0.166 | −0.391 | 75.9 (31.2–176.1) | 77.3 (34.8–197.9) | 0.302 | −0.193 |
| Troponin I; pg/mL | 21.4 (11.1–44.6) | 20.5 (10.4–46.3) | 0.631 | −0.15 | 21.1 (11.0–44.5) | 20.4 (10.4–46.3) | 0.588 | −0.056 |
| Troponin T; ng/mL | 0.04 (0.02–0.06) | 0.03 (0.02–0.05) | 0.237 | 0.014 | 0.04 (0.02–0.06) | 0.03 (0.02–0.05) | 0.247 | 0.006 |
| Intraoperative variables | ||||||||
| Operation time; min | 225.0 (195.0–255.0) | 225.0 (190.0–260.0) | 0.677 | 0.015 | 225.0 (195.0–255.0) | 221.0 (190.0–260.0) | 0.482 | 0.043 |
| Hourly fluid infusion; mL/kg/h | 9.0 (7.4–11.4) | 9.3 (7.3–11.6) | 0.827 | −0.062 | 9.0 (7.2–11.4) | 9.3 (7.3–11.7) | 0.417 | −0.091 |
| Average of vital signs | ||||||||
| SBP; mmHg | 132.0 (124.0–141.0) | 130.0 (120.0–140.0) | 0.033 | 0.178 | 131.0 (123.8–140.0) | 130.0 (120.0–140.0) | 0.069 | 0.147 |
| DBP; mmHg | 80.0 (80.0–90.0) | 80.0 (79.0–90.0) | 0.256 | 0.092 | 80.0 (80.0–90.0) | 80.0 (80.0–90.0) | 0.411 | 0.061 |
| Heart rate beats/min | 80.0 (73.0–88.0) | 80.0 (73.0–88.0) | 0.583 | 0.042 | 80.0 (73.0–88.0) | 80.0 (73.0–88.3) | 0.491 | 0.039 |
| Donor/graft variables | ||||||||
| Sex; n (%) | 163 (60.6%) | 176 (65.2%) | 0.270 | −0.094 | 161 (61.5%) | 169 (64.5%) | 0.469 | −0.062 |
| Age; years | 51.0 (38.0–59.0) | 51.0 (41.0–57.0) | 0.941 | −0.023 | 51.0 (38.0–59.0) | 51.0 (41.0–57.0) | 0.991 | −0.026 |
| BMI; kg/m2 | 23.5 (21.9–26.1) | 23.6 (21.7–25.6) | 0.602 | 0.068 | 23.5 (21.9–26.1) | 23.7 (21.8–25.7) | 0.811 | 0.049 |
| Left kidney graft; n (%) | 174 (64.7%) | 162 (60.0%) | 0.262 | −0.098 | 93 (35.5%) | 105 (40.1%) | 0.280 | −0.096 |
| Graft weight; g | 174.0 (150.0–208.0) | 178.0 (150.0–204.5) | 0.810 | −0.001 | 174.0 (150.0–208.0) | 178.0 (152.0–206.5) | 0.601 | −0.022 |
| Graft ischemic time; min | 54.0 (45.0–67.5) | 55.0 (43.8–67.3) | 0.885 | −0.041 | 54.5 (45.0–68.0) | 55.0 (43.0–67.0) | 0.803 | 0.005 |
| Hemoglobin; g/dL | 13.9 (12.9–15.1) | 13.7 (12.8–14.9) | 0.540 | 0.035 | 13.8 (12.9–15.1) | 13.7 (12.8–14.9) | 0.626 | 0.027 |
| Group | TAP Block (n = 262) | LWI (n = 262) | p Value |
|---|---|---|---|
| Visual analog scale (cm) | |||
| Immediately after surgery | 4.3 ± 1.8 | 4.5 ± 2.0 | 0.202 |
| 1 h after surgery | 3.5 ± 1.1 † | 4.7 ± 1.4 | <0.001 *** |
| 4 h after surgery | 3.6 ± 1.0 † | 4.7 ± 1.4 | <0.001 *** |
| 8 h after surgery | 3.0 ± 0.9 † | 3.8 ± 1.5 * | <0.001 *** |
| 12 h after surgery | 3.2 ± 1.0 † | 3.1 ± 1.1 * | 0.481 |
| 24 h after surgery | 3.0 ± 0.9 † | 2.8 ± 1.0 * | 0.052 |
| Fentanyl infusion for POD 1 | |||
| Rescue dose (μg) | 67.7 ± 30.6 | 119.1 ± 71.8 | <0.001 *** |
| IV-PCA (mL) | 55.9 ± 10.2 | 69.7 ± 18.2 | <0.001 *** |
| Opioid-related PONV | 35 (13.4%) | 50 (19.1%) | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, M.S.; Lee, K.K.; Jeong, J.-O.; Jeong, W.; Moon, Y.W.; Min, J.Y. Comparison of Postoperative Analgesic Profiles Between Transversus Abdominis Plane Block and Local Wound Infiltration in Living Donor Kidney Transplantation Recipients: A Propensity Score-Matched Analysis. Life 2025, 15, 687. https://doi.org/10.3390/life15050687
Chae MS, Lee KK, Jeong J-O, Jeong W, Moon YW, Min JY. Comparison of Postoperative Analgesic Profiles Between Transversus Abdominis Plane Block and Local Wound Infiltration in Living Donor Kidney Transplantation Recipients: A Propensity Score-Matched Analysis. Life. 2025; 15(5):687. https://doi.org/10.3390/life15050687
Chicago/Turabian StyleChae, Min Suk, Kyung Kwan Lee, Jin-Oh Jeong, Wonwoo Jeong, Young Wook Moon, and Ji Young Min. 2025. "Comparison of Postoperative Analgesic Profiles Between Transversus Abdominis Plane Block and Local Wound Infiltration in Living Donor Kidney Transplantation Recipients: A Propensity Score-Matched Analysis" Life 15, no. 5: 687. https://doi.org/10.3390/life15050687
APA StyleChae, M. S., Lee, K. K., Jeong, J.-O., Jeong, W., Moon, Y. W., & Min, J. Y. (2025). Comparison of Postoperative Analgesic Profiles Between Transversus Abdominis Plane Block and Local Wound Infiltration in Living Donor Kidney Transplantation Recipients: A Propensity Score-Matched Analysis. Life, 15(5), 687. https://doi.org/10.3390/life15050687







