Comparative Effects of Tadalafil Cream Versus Oral Tadalafil on Males with Erectile Disfunction Regarding Relationship Dynamics: A Secondary Analysis of Dyadic Adjustment Outcomes in a Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Trial Interventions
2.4. Randomization and Blinding
2.5. Outcome Measures and Data Collection
2.5.1. Data Analysis
2.5.2. Handling Missing Data
2.6. Ethical Considerations
3. Results
3.1. DAS Reliability Assessment
3.2. DAS Subscale Comparison Between Tadalafil Cream and Oral Formulation
3.3. Evolution Between Baseline and Final Values
4. Discussion
4.1. Study Limitations
4.2. Study Strengths
4.3. Future Directions
Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irwin, G.M. Erectile Dysfunction. Prim. Care Clin. Off. Pract. 2019, 46, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Fadzil, M.A.; Sidi, H.; Ismail, Z.; Hassan, M.R.C.; Thuzar, K.; Midin, M.; Jaafar, N.R.N.; Das, S. Socio-Demographic and Psychosocial Correlates of Erectile Dysfunction among Hypertensive Patients. Compr. Psychiatry 2014, 55, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Sexual and Reproductive Health—Uroweb. Available online: https://uroweb.org/guidelines/sexual-and-reproductive-health/chapter/management-of-erectile-dysfunction (accessed on 19 October 2024).
- Bulow, S. Integrating Sex and Couples Therapy: A Multifaceted Case History. Fam. Process 2009, 48, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, S. The Married Couple with Functional Sexual Disorders in the Sexual Learning Therapy. Psychiatr. Neurol. Med. Psychol. 1982, 34, 282–290. [Google Scholar]
- Bockaj, A.; Muise, M.D.; Belu, C.F.; Rosen, N.O.; O’Sullivan, L.F. Under Pressure: Men’s and Women’s Sexual Performance Anxiety in the Sexual Interactions of Adult Couples. J. Sex Res. 2024, 1–13. [Google Scholar] [CrossRef]
- Elterman, D.S.; Bhattacharyya, S.K.; Mafilios, M.; Woodward, E.; Nitschelm, K.; Burnett, A.L. The Quality of Life and Economic Burden of Erectile Dysfunction. Res. Rep. Urol. 2021, 13, 79–86. [Google Scholar] [CrossRef]
- Koon, C.S.; Sidi, H.; Kumar, J.; Xi, O.W.; Das, S.; Hatta, M.H.; Alfonso, C. The Phosphodiasterase 5-Inhibitors (PDE-5i) for Erectile Dysfunction (ED): A Therapeutic Challenge for Psychiatrists. Curr. Drug Targets 2018, 19, 1366–1377. [Google Scholar] [CrossRef]
- Neijenhuijs, K.I.; Holtmaat, K.; Aaronson, N.K.; Holzner, B.; Terwee, C.B.; Cuijpers, P.; Verdonck-de Leeuw, I.M. The International Index of Erectile Function (IIEF)—A Systematic Review of Measurement Properties. J. Sex Med. 2019, 16, 1078–1091. [Google Scholar] [CrossRef]
- Simkins-bullock, J.; Wildman, B.G.; Bullock, W.A.; Sugrue, D.P. Etiological Attributions, Responsibility Attributions, and Marital Adjustment in Erectile Dysfunction Patients. J. Sex Marital. Ther. 1992, 18, 83–103. [Google Scholar] [CrossRef]
- Maroufizadeh, S.; Omani-Samani, R.; Hosseini, M.; Almasi-Hashiani, A.; Sepidarkish, M.; Amini, P. The Persian Version of the Revised Dyadic Adjustment Scale (RDAS): A Validation Study in Infertile Patients. BMC Psychol. 2020, 8, 6. [Google Scholar] [CrossRef]
- Hamid, N.; Muhamad, R.; Kueh, Y.C.; Zahari, Z.; Mohamad Nor, N.; Abdullah, N.; Wong, M.S.; Meor Zul Kefli’Auni, S.A.; Ma, Z.F.; Lee, Y.Y. Translation of the Revised Dyadic Adjustment Scale (RDAS) into the Malay Language and Its Psychometric Qualities among Healthy Married Malay Women. J. Pharm. Bioallied Sci. 2020, 12, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Santona, A.; Vismara, L.; Gorla, L.; Tognasso, G.; Ambrosini, C.; Luli, A.; Rollè, L. The Relationship between Attachment, Dyadic Adjustment, and Sexuality: A Comparison between Infertile Men and Women. Int. J. Environ. Res. Public Health 2023, 20, 3020. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D. Psychologic Factors in the Multidisciplinary Evaluation and Treatment of Erectile Dysfunction. Urol. Clin. N. Am. 1988, 15, 41–51. [Google Scholar] [CrossRef]
- Graham, B. DAS, Dyadic Adjustment Scale; Iliescu, D., Petre, L., Eds.; Sinapsis: Cluj-Napoca, Romania, 2009. [Google Scholar]
- Cuenca Montesino, M.; Graña Gómez, J.; Peña Fernández, M.; Andreu Rodríguez, J. Psychometric Properties of the Dyadic Adjustment Scale (DAS) in a Community Sample of Couples. Psicothema 2013, 4, 536–541. [Google Scholar] [CrossRef]
- Peixoto, M.M.; Nobre, P. Distressing Sexual Problems and Dyadic Adjustment in Heterosexuals, Gay Men, and Lesbian Women. J. Sex Marital. Ther. 2016, 42, 369–381. [Google Scholar] [CrossRef]
- Kinkead, A.; Salas, C.; Ewert, C.P. Couples’ Extrinsic Emotion Regulation and Dyadic Adjustment: An Actor-Partner Interdependence Model Analysis. Curr. Psychol. 2022, 42, 31619–31633. [Google Scholar] [CrossRef]
- Villeneuve, L.; Trudel, G.; Préville, M.; Dargis, L.; Boyer, R.; Bégin, J. Dyadic Adjustment Scale: A Validation Study among Older French-Canadians Living in Relationships. Can. J. Aging 2015, 34, 26–35. [Google Scholar] [CrossRef]
- Zeren, F.; Gürsoy, E.; Çolak, E. The Quality of Life and Dyadic Adjustment of Couples Receiving Infertility Treatment. Afr. J. Reprod. Health 2019, 23, 117–127. [Google Scholar] [CrossRef]
- Sabourin, S.; Valois, P.; Lussier, Y. Development and Validation of a Brief Version of the Dyadic Adjustment Scale With a Nonparametric Item Analysis Model. Psychol. Assess. 2005, 17, 15–27. [Google Scholar] [CrossRef]
- Kurdek, L.A. Relationship Stability and Relationship Satisfaction in Cohabiting Gay and Lesbian Couples: A Prospective Longitudinal Test of the Contextual and Interdependence Models. J. Soc. Pers. Relatsh. 1992, 9, 125–142. [Google Scholar] [CrossRef]
- Trifu, D.-M.; Leucuța, D.-C.; Pintea-Trifu, M.-L.; Elec, F.; Crișan, N.; Eniu, D.; Coman, I. The Intra-Meatal Application of Tadalafil Cream Versus Oral Administration Efficacy and Safety: Results from a Randomized, Two-Administration Route, Cross-Over Clinical Trial. J. Clin. Med. 2024, 13, 6557. [Google Scholar] [CrossRef] [PubMed]
- Rathod, A.; Sawant, N.; Bandgar, T. Sexual Dysfunction, Depression, and Marital Adjustment in Diabetic Male Patients. Indian J. Psychiatry 2024, 66, 853–858. [Google Scholar] [CrossRef] [PubMed]
- LEGE 190 18/07/2018—Portal Legislativ. Available online: https://legislatie.just.ro/Public/DetaliiDocument/203151 (accessed on 18 October 2024).
- Protecția Datelor Cu Caracter Personal—GDPR—Ministerul Investițiilor Și Proiectelor Europene. Available online: https://mfe.gov.ro/informatii-de-interes-public/solicitare-informatii-legislatie/protectia-datelor-cu-caracter-personal-gdpr/ (accessed on 18 October 2024).
- Create a Blocked Randomisation List|Sealed Envelope. Available online: https://www.sealedenvelope.com/simple-randomiser/v1/lists (accessed on 28 August 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Heiman, J.; Talley, D.; Bailen, J.; Oskin, T.; Rosenberg, S.; Pace, C.; Creanga, D.; Bavendam, T. Sexual Function and Satisfaction in Heterosexual Couples When Men Are Administered Sildenafil Citrate (Viagra®) for Erectile Dysfunction: A Multicentre, Randomised, Double-blind, Placebo-controlled Trial. BJOG An Int. J. Obstet. Gynaecol. 2007, 114, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, J.; Esterman, A.; Ben-Tovim, D.; Austin, M.-A.; Dougherty, M. The South Australian Couples Sildenafil Study: Double-Blind, Parallel-Group Randomized Controlled Study to Examine the Psychological and Relationship Consequences of Sildenafil Use in Couples. J. Sex Med. 2007, 4, 1126–1135. [Google Scholar] [CrossRef]
- Roxo, L.; Virgolino, A.; Costa, J.; Alarcao, V. Understanding the Relationship between BMI and Sexual Dysfunction: Can DSM-5 Shed Light into This Topic? Rev. Int. Androl. 2019, 17, 130–137. [Google Scholar] [CrossRef]
- Mollaioli, D.; Ciocca, G.; Limoncin, E.; Di Sante, S.; Gravina, G.L.; Carosa, E.; Lenzi, A.; Jannini, E.A.F. Lifestyles and Sexuality in Men and Women: The Gender Perspective in Sexual Medicine. Reprod. Biol. Endocrinol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Simopoulos, E.F.; Trinidad, A.C. Male Erectile Dysfunction: Integrating Psychopharmacology and Psychotherapy. Gen. Hosp. Psychiatry 2013, 35, 33–38. [Google Scholar] [CrossRef]
- Lee, M.S.; Ziegelmann, M.J.; Ellythy, L.M.; Sax-Bolder, A.N.; Guillen Lozoya, A.H.; Köhler, T.S.; Helo, S.; Yang, D.Y. Discrepancy Between Patient vs Provider Assessment of Erection Quality. Urology 2024, 197, 234–239. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Zhou, Y.; Yu, C.; Pimolsettapun, J.; Yang, L.; Zhao, Y. Study on the Combination of Brief Psychodynamic Psychotherapy with Viagra in the Treatment of Non-Organic ED. Gen. Psychiatry 2020, 33, e100184. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Z.-M. Tadalafil Combined with Behavioral Therapy and Psychological Counseling for Erectile Dysfunction. Zhonghua Nan Ke Xue 2021, 27, 330–333. [Google Scholar]
- Althof, S.E. Role of Psychosexual Counseling as an Adjuvant to Pharmacotherapy in the Treatment of Erectile Dysfunction. J. Sex Med. 2023, 20, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Haydar, A.; Jabbour, T.; Kfoury, P.; Sader, G. The Effectiveness of Psychological Interventions Alone, or in Combination with Phosphodiesterase-5 Inhibitors, for the Treatment of Erectile Dysfunction:A Systematic Review. Arab. J. Urol. 2021, 19, 310–322. [Google Scholar] [CrossRef] [PubMed]
- von Büren, M.; Rodler, S.; Wiesenhütter, I.; Schröder, F.; Buchner, A.; Stief, C.; Gratzke, C.; Wülfing, C.; von Büren, J. Digital Real-World Data Suggest Patient Preference for Tadalafil over Sildenafil in Patients with Erectile Dysfunction. Eur. Urol. Focus 2022, 8, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Rice, T.; Eggenberger, L. Precarious Manhood Beliefs Are Positively Associated with Erectile Dysfunction in Cisgender Men. Arch. Sex Behav. 2023, 52, 3123–3138. [Google Scholar] [CrossRef]
- Herkommer, K.; Meissner, V.H.; Dinkel, A.; Jahnen, M.; Schiele, S.; Kron, M.; Ankerst, D.P.; Gschwend, J.E. Prevalence, Lifestyle, and Risk Factors of Erectile Dysfunction, Premature Ejaculation, and Low Libido in Middle-aged Men: First Results of the Bavarian Men’s Health-Study. Andrology 2024, 12, 801–808. [Google Scholar] [CrossRef]
- Selice, L.; Morris, K.L. Mindfulness and Sexual Dysfunction: A Systematic Research Synthesis. J. Sex Marital. Ther. 2022, 48, 323–342. [Google Scholar] [CrossRef]
- Kell, P. Patient Preference and Satisfaction in Erectile Dysfunction Therapy: A Comparison of the Three Phosphodiesterase-5 Inhibitors Sildenafil, Vardenafil and Tadalafil. Patient Prefer. Adherence 2009, 3, 99–104. [Google Scholar] [CrossRef]
- Gong, B.-S. ED Patients and Their Female Partners Prefer Tadalafil. Zhonghua Nan Ke Xue 2011, 17, 571–575. [Google Scholar]
- Rubio-Aurioles, E.; Reyes, L.A.; Borregales, L.; Cairoli, C.; Sorsaburu, S. A 6 Month, Prospective, Observational Study of PDE5 Inhibitor Treatment Persistence and Adherence in Latin American Men with Erectile Dysfunction. Curr. Med. Res. Opin. 2013, 29, 695–706. [Google Scholar] [CrossRef]
- Ströberg, P.; Murphy, A.; Costigan, T. Switching Patients with Erectile Dysfunction from Sildenafil Citrate to Tadalafil: Results of a European Multicenter, Open-Label Study of Patient Preference. Clin. Ther. 2003, 25, 2724–2737. [Google Scholar] [CrossRef]
- Mirone, V.; Fusco, F.; Rossi, A.; Sicuteri, R.; Montorsi, F. Tadalafil and Vardenafil vs Sildenafil: A Review of Patient-preference Studies. BJU Int. 2009, 103, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Pyrgidis, N.; Mykoniatis, I.; Haidich, A.-B.; Tirta, M.; Talimtzi, P.; Kalyvianakis, D.; Ouranidis, A.; Hatzichristou, D. The Effect of Phosphodiesterase-Type 5 Inhibitors on Erectile Function: An Overview of Systematic Reviews. Front. Pharmacol. 2021, 12, 735708. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Aurioles, E.; Porst, H.; Eardley, I.; Goldstein, I. Comparing Vardenafil and Sildenafil in the Treatment of Men with Erectile Dysfunction and Risk Factors for Cardiovascular Disease: A Randomized, Double-Blind, Pooled Crossover Study. J. Sex Med. 2006, 3, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
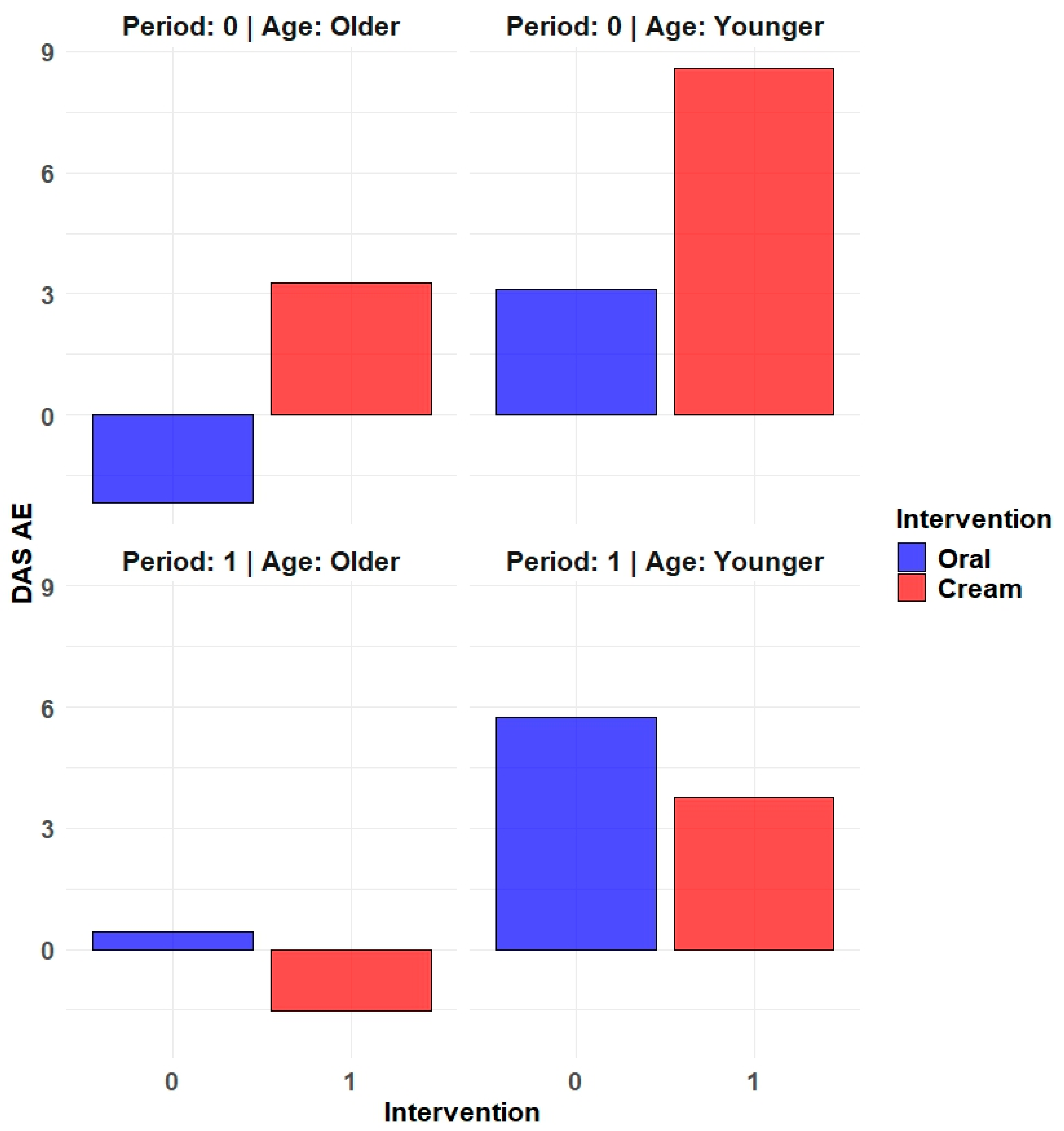
| Period 1 | Period 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| DAS, Mean (SD) | Baseline | Final | Baseline | Final | ||||
| Tadalafil Cream | Oral Tadalafil | Tadalafil Cream | Oral Tadalafil | Tadalafil Cream | Oral Tadalafil | Tadalafil Cream | Oral Tadalafil | |
| (n = 18) | (n = 17) | (n = 18) | (n = 17) | (n = 17) | (n = 18) | (n = 17) | (n = 18) | |
| Affective Expression | 47.56 (10.79) | 47.29 (6.75) | 52.89 (9.02) | 48.24 (7.49) | 49.12 (7.59) | 48.83 (10.72) | 50.71 (6.68) | 51.33 (8.48) |
| Dyadic Consensus | 55.22 (15.1) | 56 (7.38) | 56.78 (12.11) | 56.06 (7.55) | 57.29 (7.1) | 55.28 (14.81) | 57.24 (6.97) | 55.06 (13.88) |
| Dyadic Cohesion | 49.67 (12.62) | 53.82 (11.63) | 52.89 (8.98) | 54.29 (9.95) | 52.24 (11.05) | 50.33 (13.43) | 53.12 (10.9) | 50.28 (13.46) |
| Dyadic Satisfaction | 50.83 (10.04) | 54.88 (8.08) | 52.61 (5.62) | 55.88 (7.87) | 56.53 (7.33) | 51.44 (10.13) | 56.29 (8.1) | 52.89 (6.99) |
| Dyadic Adjustment | 52.78 (12.98) | 55.47 (5.49) | 55.28 (9.06) | 56.06 (6.21) | 56.53 (5.77) | 52.83 (13.39) | 56.88 (5.78) | 53.67 (11.14) |
| First Period | Second Period | |||
|---|---|---|---|---|
| DAS, Mean (SD) | Tadalafil Cream | Oral Tadalafil | Tadalafil Cream | Oral Tadalafil |
| (n = 18) | (n = 17) | (n = 17) | (n = 18) | |
| Affective Expression | 5.33 (12.78) | 0.94 (3.27) | 1.59 (6.15) | 2.5 (6.26) |
| Dyadic Consensus | 1.56 (5.76) | 0.06 (1.03) | −0.06 (0.97) | −0.22 (3.72) |
| Dyadic Cohesion | 3.22 (6.58) | 0.47 (4.57) | 0.88 (2.5) | −0.06 (6.55) |
| Dyadic Satisfaction | 1.78 (6.59) | 1 (4.26) | −0.24 (2.66) | 1.44 (4.76) |
| Dyadic Adjustment | 2.5 (6.44) | 0.59 (2.45) | 0.35 (1.66) | 0.83 (5.06) |
| DAS Change, Mean (SD) | Tadalafil Cream (n = 35) | Oral Tadalafil (n = 35) | Difference (95% CI) |
|---|---|---|---|
| All | |||
| Affective Expression | 3.51 (10.16) | 1.74 (5.03) | 1.77 (−1.1–4.64) |
| Dyadic Consensus | 0.77 (4.21) | −0.09 (2.73) | 0.86 (−0.02–1.74) |
| Dyadic Cohesion | 2.09 (5.1) | 0.2 (5.6) | 1.89 (−0.53–4.3) |
| Dyadic Satisfaction | 0.8 (5.11) | 1.23 (4.46) | −0.43 (−1.8–0.94) |
| Dyadic Adjustment | 1.46 (4.82) | 0.71 (3.95) | 0.74 (−0.32–1.8) |
| Age ≥ 51 years | (n = 18) | (n = 18) | |
| Affective Expression | 0.06 (7.91) | 0.78 (3.81) | −0.72 (−4.45–3.01) |
| Dyadic Consensus | −0.44 (3.43) | −0.94 (3.24) | 0.5 (−0.34–1.34) |
| Dyadic Cohesion | 2.44 (5.03) | −1.28 (5.78) | 3.72 (−0.42–7.86) |
| Dyadic Satisfaction | −0.89 (2.72) | 1.11 (4.19) | −2 (−4.29–0.29) |
| Dyadic Adjustment | 0.06 (3.33) | −0.22 (3.69) | 0.28 (−1.11–1.66) |
| Age < 51 years | (n = 17) | (n = 17) | |
| Affective Expression | 7.18 (11.18) | 2.76 (6.01) | 4.41 (−0.02–8.85) |
| Dyadic Consensus | 2.06 (4.66) | 0.82 (1.7) | 1.24 (−0.44–2.91) |
| Dyadic Cohesion | 1.71 (5.3) | 1.76 (5.11) | −0.06 (−2.57–2.45) |
| Dyadic Satisfaction | 2.59 (6.4) | 1.35 (4.86) | 1.24 (0.04–2.43) |
| Dyadic Adjustment | 2.94 (5.74) | 1.71 (4.09) | 1.24 (−0.51–2.98) |
| DAS Predicted Subscale | Treatment (95% CI) | p | Period (95% CI) | p | Age ≥ 51 Years (95% CI) | p | Treatment:Period (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| Affective Expression | 5.45 (0.22–10.67) | 0.041 | 2.61 (−2.61–7.84) | 0.321 | −5.29 (−9.67–−0.91) | 0.019 | −7.41 (−16.18–1.35) | 0.096 |
| Dyadic Consensus | 1.97 (−0.36–4.3) | 0.097 | 0.19 (−2.14–2.52) | 0.871 | −2.36 (−4.55–−0.18) | 0.035 | −2.28 (−6.64–2.09) | 0.302 |
| Dyadic Cohesion | 3.03 (−0.64–6.7) | 0.104 | −0.25 (−3.92–3.42) | 0.892 | −1.39 (−4.2–1.42) | 0.327 | −2.37 (−7.99–3.25) | 0.403 |
| Dyadic Satisfaction | 1.21 (−2.04–4.47) | 0.459 | 0.88 (−2.38–4.14) | 0.591 | −2.19 (−5.16–0.78) | 0.145 | −3.33 (−9.27–2.61) | 0.267 |
| Dyadic Adjustment | 2.46 (−0.45–5.37) | 0.096 | 0.79 (−2.12–3.71) | 0.588 | −2.75 (−5.49–−0.02) | 0.048 | −3.49 (−8.96–1.98) | 0.207 |
| Intervention | Period | Age ≥ 51 Years | Treatment: Period | |
|---|---|---|---|---|
| Affective Expression | 0.56 | 0.27 | −0.85 | −0.6 |
| Dyadic Consensus | 0.53 | 0.05 | −0.76 | −0.37 |
| Dyadic Cohesion | 0.42 | −0.03 | −0.35 | −0.3 |
| Dyadic Satisfaction | 0.22 | 0.16 | −0.52 | −0.4 |
| Dyadic Adjustment | 0.53 | 0.17 | −0.71 | −0.45 |
| DAS, Mean (SD) | Baseline | Final | Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Oral (n = 35) | ||||
| Affective Expression | 48.09 (8.92) | 49.83 (8.05) | 1.74 (0.02; 3.47) | 0.047 |
| Dyadic Consensus | 55.63 (11.64) | 55.54 (11.11) | −0.09 (−1.02; 0.85) | 0.854 |
| Dyadic Cohesion | 52.03 (12.53) | 52.23 (11.89) | 0.2 (−1.72; 2.12) | 0.834 |
| Dyadic Satisfaction | 53.11 (9.22) | 54.34 (7.48) | 1.23 (−0.3; 2.76) | 0.112 |
| Dyadic Adjustment | 54.11 (10.27) | 54.83 (9.03) | 0.71 (−0.64; 2.07) | 0.293 |
| Oral, age ≥ 51 years (n = 18) | ||||
| Affective Expression | 51.11 (9.16) | 51.89 (8.78) | 0.78 (−1.12; 2.67) | 0.399 |
| Dyadic Consensus | 58 (8.32) | 57.06 (8.97) | −0.94 (−2.56; 0.67) | 0.234 |
| Dyadic Cohesion | 52.11 (12.9) | 50.83 (13.4) | −1.28 (−4.15; 1.6) | 0.361 |
| Dyadic Satisfaction | 54 (6.1) | 55.11 (6.04) | 1.11 (−0.97; 3.19) | 0.276 |
| Dyadic Adjustment | 55.89 (6.84) | 55.67 (7.78) | −0.22 (−2.06; 1.61) | 0.801 |
| Oral, age < 51 years (n = 17) | ||||
| Affective Expression | 44.88 (7.66) | 47.65 (6.78) | 2.76 (−0.32; 5.85) | 0.076 |
| Dyadic Adjustment | 52.24 (12.94) | 53.94 (10.37) | 1.71 (−0.4; 3.81) | 0.105 |
| Dyadic Consensus | 53.12 (14.19) | 53.94 (13.09) | 0.82 (−0.05; 1.7) | 0.064 |
| Dyadic Cohesion | 51.94 (12.52) | 53.71 (10.26) | 1.76 (−0.86; 4.39) | 0.173 |
| Dyadic Satisfaction | 52.18 (11.81) | 53.53 (8.87) | 1.35 (−1.15; 3.85) | 0.268 |
| Cream (n = 35) | ||||
| Affective Expression | 48.31 (9.27) | 51.83 (7.93) | 3.51 (0.03; 7) | 0.048 |
| Dyadic Consensus | 56.23 (11.79) | 57 (9.81) | 0.77 (−0.67; 2.22) | 0.286 |
| Dyadic Cohesion | 50.91 (11.78) | 53 (9.81) | 2.09 (0.33; 3.84) | 0.021 |
| Dyadic Satisfaction | 53.6 (9.17) | 54.4 (7.08) | 0.8 (−0.96; 2.56) | 0.361 |
| Dyadic Adjustment | 54.6 (10.18) | 56.06 (7.58) | 1.46 (−0.2; 3.11) | 0.082 |
| Cream, age ≥ 51 years (n = 18) | ||||
| Affective Expression | 51.89 (8.22) | 51.94 (9.63) | 0.06 (−3.88; 3.99) | 0.977 |
| Dyadic Consensus | 57.39 (8.51) | 56.94 (9.07) | −0.44 (−2.15; 1.26) | 0.590 |
| Dyadic Cohesion | 50.22 (9.94) | 52.67 (9.39) | 2.44 (−0.06; 4.95) | 0.055 |
| Dyadic Satisfaction | 54.83 (5.7) | 53.94 (6.27) | −0.89 (−2.24; 0.46) | 0.184 |
| Dyadic Adjustment | 55.67 (6.94) | 55.72 (7.47) | 0.06 (−1.6; 1.71) | 0.944 |
| Cream, age < 51 years (n = 17) | ||||
| Affective Expression | 44.53 (9.01) | 51.71 (5.91) | 7.18 (1.43; 12.92) | 0.018 |
| Dyadic Consensus | 55 (14.67) | 57.06 (10.83) | 2.06 (−0.34; 4.45) | 0.087 |
| Dyadic Cohesion | 51.65 (13.74) | 53.35 (10.52) | 1.71 (−1.02; 4.43) | 0.203 |
| Dyadic Satisfaction | 52.29 (11.85) | 54.88 (8.01) | 2.59 (−0.7; 5.88) | 0.115 |
| Dyadic Adjustment | 53.47 (12.89) | 56.41 (7.9) | 2.94 (−0.01; 5.89) | 0.051 |
| Study/Reference | Therapy/PDE5i | Sample/Design | Relationship or ED Measures | Key Findings |
|---|---|---|---|---|
| Raheem & Kell et al., 2009 [43] | Sildenafil, Vardenafil, and tadalafil | n = 60 men with non-organic ED; randomized controlled trial | IIEF; patient and partner satisfaction (custom questionnaires) | Patients who tried all three drugs generally reported better satisfaction when given the choice. The study highlights differences in preference among PDE5 inhibitors. |
| Gong et al., 2011 [44] | Tadalafil | Survey-based evaluation in men with ED (and partner feedback) | IIEF; partner-reported satisfaction | Both patients and their female partners significantly preferred tadalafil due to its flexible dosing window and perceived ease of use. |
| Rubio-Aurioles et al., 2013 [45] | PDE5 inhibitors (various) | Prospective observational study in Latin American men with ED | IIEF; treatment persistence/adherence data (indirectly linked to relationship outcomes) | Reported adherence patterns over 6 months. The findings underscore the importance of sustained treatment for long-term satisfaction, though direct relationship measures were not the focus. |
| Ströberg P et al., 2003 [46] | Transition from sildenafil to tadalafil | European multicenter, open-label, crossover study in men with ED | IIEF; patient-reported preference | The majority of patients switched from sildenafil to tadalafil, indicating a clear preference for the latter, based on improved convenience and satisfaction. |
| Mirone V et al., 2009 [47] | Comparison among tadalafil, Vardenafil, and sildenafil | Narrative review of multiple patient preference studies | IIEF; satisfaction and preference metrics | Review indicates that, while all agents improve ED, differences in onset, duration, and side effects may drive preference—young men often favor tadalafil. |
| Pyrgidis N et al., 2021 [48] | Tadalafil | Meta-analysis of randomized controlled trials in men with ED | IIEF; safety and efficacy outcomes | Confirmed that tadalafil is effective and safe for treating ED, with benefits reflected in both erectile function and patient-reported satisfaction. |
| Rubio-Aurioles et al., 2006 [49] | Sildenafil vs. Vardenafil | Double-blind, randomized, crossover study in men with ED | IIEF | Vardenafil demonstrated nominal statistical superiority over sildenafil across several commonly used efficacy measures, while both medications were generally well tolerated. |
| Zhang et al., 2020 [35] | Sildenafil + brief psychodynamic psychotherapy | n = 63 men with non-organic ED; Randomized controlled trial | IIEF; marital/relationship satisfaction (custom questionnaires) | Combination therapy produced significantly better improvements in erectile function and relationship satisfaction compared to PDE5i alone. |
| Atallah et al., 2021 [38] | PDE5i (various) + psychological interventions (systematic review) | Systematic review of 8 studies (mixed designs) | IIEF; partner satisfaction; other psychometric scales | Studies indicated that adding psychotherapy (e.g., CBT) to PDE5i therapy enhanced improvements in ED severity and relationship functioning compared to PDE5i monotherapy. |
| Elterman et al., 2021 [7] | PDE5i (sildenafil/Vardenafil/tadalafil) | Narrative review of clinical and economic burden | ED questionnaires (IIEF-5/15); QoL measures (SF-36) | This review highlights the significant quality of life and economic burden of ED, underscoring the need for greater awareness and improved access to effective treatments. |
| Althof et al., 2023 [37] | PDE5i + psychosexual counseling | Commentary/expert opinion | Focus on integrated ED management | Emphasizes that combining PDE5i with counseling may lead to superior couple outcomes, although data comparing specific PDE5i on relationship metrics are limited. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifu, D.-M.; Leucuța, D.-C.; Pintea-Trifu, M.-L.; Elec, F.; Crișan, N.; Eniu, D.; Coman, I. Comparative Effects of Tadalafil Cream Versus Oral Tadalafil on Males with Erectile Disfunction Regarding Relationship Dynamics: A Secondary Analysis of Dyadic Adjustment Outcomes in a Randomized Crossover Trial. Life 2025, 15, 668. https://doi.org/10.3390/life15040668
Trifu D-M, Leucuța D-C, Pintea-Trifu M-L, Elec F, Crișan N, Eniu D, Coman I. Comparative Effects of Tadalafil Cream Versus Oral Tadalafil on Males with Erectile Disfunction Regarding Relationship Dynamics: A Secondary Analysis of Dyadic Adjustment Outcomes in a Randomized Crossover Trial. Life. 2025; 15(4):668. https://doi.org/10.3390/life15040668
Chicago/Turabian StyleTrifu, Dragoș-Mihail, Daniel-Corneliu Leucuța, Martina-Luciana Pintea-Trifu, Florin Elec, Nicolae Crișan, Dan Eniu, and Ioan Coman. 2025. "Comparative Effects of Tadalafil Cream Versus Oral Tadalafil on Males with Erectile Disfunction Regarding Relationship Dynamics: A Secondary Analysis of Dyadic Adjustment Outcomes in a Randomized Crossover Trial" Life 15, no. 4: 668. https://doi.org/10.3390/life15040668
APA StyleTrifu, D.-M., Leucuța, D.-C., Pintea-Trifu, M.-L., Elec, F., Crișan, N., Eniu, D., & Coman, I. (2025). Comparative Effects of Tadalafil Cream Versus Oral Tadalafil on Males with Erectile Disfunction Regarding Relationship Dynamics: A Secondary Analysis of Dyadic Adjustment Outcomes in a Randomized Crossover Trial. Life, 15(4), 668. https://doi.org/10.3390/life15040668






