Prognostic Value of Biomarkers in COVID-19: Associations with Disease Severity, Viral Variants, and Comorbidities—A Retrospective Observational Single-Center Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Demographic and Clinical Characteristics
3.2. Association of the Analyzed Biomarkers with COVID-19 Severity and Fatal Outcomes
3.3. Predictive Ability of the Analyzed Biomarkers for COVID-19 Outcomes
3.4. Association of the Analyzed Biomarkers with Viral Variants
3.5. Association of the Analyzed Biomarkers with Vaccination Status and Comorbidities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus Disease 2019 |
| CRP | C-reactive protein |
| LDH | Lactate dehydrogenase |
| RT-PCR | Real-time polymerase chain reaction |
| NT-proBNP | N-terminal pro–brain natriuretic peptide |
| CK | Creatine kinase |
| CKMB | Creatine kinase myocardial band |
| GGT | Gamma-glutamyl transferase |
| ALP | Alkaline phosphatase |
| IQR | Interquartile range |
| ANOIVA | Analysis of variance |
| CI | Confidence interval |
| AUC | Area under the curve |
| ICU | Intensive-care unit |
| RBC | Red blood cell |
| WBC | White blood cell |
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C. COVID-19-lessons for zoonotic disease. Science 2022, 375, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, Y.; Tang, X.; He, D. The Disease Severity and Clinical Outcomes of the SARS-CoV-2 Variants of Concern. Front. Public Health 2021, 9, 775224. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, R.; Samieefar, N.; Akhlaghdoust, M.; Mashhadi, M.; Darzi, P.; Rezaei, N. Delta Variant: The New Challenge of COVID-19 Pandemic, an Overview of Epidemiological, Clinical, and Immune Characteristics. Acta Biomed. 2022, 93, e2022179. [Google Scholar]
- Maryam, K.; Mohamadi, M.H.; Yashmi, I.; Shahabi, A.H.; Shabani, A.H.; Heidary, M.; Khoshnood, S. Clinical Manifestations, Treatment Options, and Comorbidities in COVID-19 Relapse Patients: A Systematic Review. J. Clin. Lab. Anal. 2022, 36, e24402. [Google Scholar]
- Moneim, A.A.; Radwan, M.A.; Yousef, A.I. COVID-19 and Cardiovascular Disease: Manifestations, Pathophysiology, Vaccination, and Long-Term Implication. Curr. Med. Res. Opin. 2022, 38, 1071–1079. [Google Scholar] [CrossRef]
- Moutchia, J.; Pokharel, P.; Kerri, A.; McGaw, K.; Uchai, S.; Nji, M.; Goodman, M. Clinical Laboratory Parameters Associated with Severe or Critical Novel Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0239802. [Google Scholar] [CrossRef]
- Kesmez Can, F.; Alay, H.; Albayrak, A.; Özden, K.; Yilmaz, S.; Öztürk, N.; Özkurt, Z.; Parlak, E.; Tekin, E.; Koçak, A.O. The Effects of Laboratory Parameters on the Prognosis of COVID-19. Eurasian J. Med. 2022, 54, 242–247. [Google Scholar] [CrossRef]
- Dymicka-Piekarska, V.; Dorf, J.; Milewska, A.; Łukaszyk, M.; Kosidło, J.W.; Kamińska, J.; Wolszczak-Biedrzycka, B.; Naumnik, W. Neutrophil/Lymphocyte Ratio (NLR) and Lymphocyte/Monocyte Ratio (LMR)—Risk of Death Inflammatory Biomarkers in Patients with COVID-19. J. Inflamm. Res. 2023, 16, 2209–2222. [Google Scholar] [CrossRef]
- Marin, G.; Ghazal Aghagoli, B.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 Severity: A Literature Review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Vilibić-Čavlek, T.; Stevanović, V.; Brlek-Gorski, D.; Ferenčak, I.; Ferenc, T.; Ujević-Bošnjak, M.; Tabain, I.; Janev-Holcer, N.; Perkovic, I.; Anticevic, M.; et al. Emerging Trends in the Epidemiology of COVID-19: The Croatian ’One Health’ Perspective. Viruses 2021, 13, 2354. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of the Republic of Croatia. Treatment Guidelines for Patients with Coronavirus Disease 2019 (COVID-19). 2022. Available online: https://bfm.hr/wp-content/uploads/2020/08/Smjernice-za-lijecenje-oboljelih-od-koronavirusne-bolesti-2019-COVID-19-verzija-8-od-17.-listopada-2022.-godine.pdf (accessed on 16 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersal, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Al-Hajeri, H.; Baroun, F.; Abutiban, F.; Al-Mutairi, M.; Ali, Y.; Alawadhi, A.; Albasri, A.; Aldei, A.; AlEnizi, A.; Alhadhood, N.; et al. Therapeutic role of immunomodulators during the COVID-19 pandemic—A narrative review. Postgrad. Med. 2022, 134, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Rommasi, F.; Nasiri, M.J.; Mirsaeidi, M. Immunomodulatory agents for COVID-19 treatment: Possible mechanism of action and immunopathology features. Mol. Cell. Biochem. 2022, 477, 711–726. [Google Scholar] [CrossRef]
- Singh, N.; Anchan, R.K.; Besser, S.A.; Belkin, M.N.; Cruz, M.D.; Lee, L.; Yu, D.; Mehta, N.; Nguyen, A.B.; Alenghat, F.J. High sensitivity Troponin-T for prediction of adverse events in patients with COVID-19. Biomarkers 2020, 25, 626–633. [Google Scholar] [CrossRef]
- Caro-Codón, J.; Rey, J.R.; Buño, A.; Iniesta, A.M.; Rosillo, S.O.; Castrejon-Castrejon, S.; Rodriguez-Sotelo, L.; Martinez, L.A.; Marco, I.; Merino, C.; et al. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Kitbalian, A.; Brovko, M.; Akulkina, L.; Potapov, P.; Moiseev, A.; Schepalina, A.; Yakovleva, A.; Sholomova, V.; Moiseev, S. Association between creatine kinase level and mortality in high-risk patients with COVID-19. Eur. Respir. J. 2021, 58, PA650. [Google Scholar]
- Flaumenhaft, R.; Enjyoji, K.; Schmaier, A.A. Vasculopathy in COVID-19. Blood 2022, 140, 222–235. [Google Scholar] [CrossRef]
- Acharya, Y.; Alameer, A.; Calpin, G.; Alkhattab, M.; Sultan, S. A comprehensive review of vascular complications in COVID-19. J. Thromb. Thrombolysis 2022, 53, 586–593. [Google Scholar] [CrossRef]
- Zhao, S.W.; Lin, Y.M.; Yi, L.L.; Su, C. Liver injury in COVID-19: Clinical features, potential mechanisms, risk factors and clinical treatments. World J. Gastroenterol. 2023, 29, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Hosseiniyan Khatibi, S.M.; Razi Soofiyani, S.; Abediazer, S.; Shoja, M.M.; Ardalan, M.; Zunini Vahed, S. Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2020, 31, e2176. [Google Scholar] [CrossRef] [PubMed]
- Tsanakas, A.T.; Mueller, Y.M.; van de Werken, H.J.G.; Pujol Borrell, R.; Ouzounis, C.A.; Katsikis, P.T. An explainable machine learning model for COVID-19 severity prognosis at hospital admission. Inform. Med. Unlocked 2025, 52, 101602. [Google Scholar] [CrossRef]
- Xiong, Y.; Ma, Y.; Ruan, L.; Li, D.; Lu, C.; Huang, L.; The National Traditional Chinese Medicine Medical Team. Comparing different machine learning techniques for predicting COVID-19 severity. Infect. Dis. Poverty 2022, 11, 19. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, J.Y.; Kim, M.C.; Park, S.Y.; Kim, B.N.; Bae, S.; Cha, H.H.; Jung, J.; Kim, M.J.; Lee, M.J.; et al. Factors of Severity in Patients with COVID-19: Cytokine/Chemokine Concentrations, Viral Load, and Antibody Responses. Am. J. Trop. Med. Hyg. 2020, 103, 2412–2418. [Google Scholar] [CrossRef]
- Çelik, N.; Çelik, O.; Laloğlu, G.; Özkaya, A. The CXCL9/10/11-CXCR3 axis as a predictor of COVID-19 progression: A prospective, case-control study. Rev. Soc. Bras. Med. Trop. 2023, 56, e01282023. [Google Scholar] [CrossRef]
- Fagyas, M.; Fejes, Z.; Sütő, R.; Nagy, Z.; Székely, B.; Pócsi, M.; Ivády, G.; Bíró, E.; Bekő, G.; Nagy, A.; et al. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int. J. Infect. Dis. 2022, 115, 8–16. [Google Scholar] [CrossRef]
- Baker, T.B.; Bolt, D.M.; Smith, S.S.; Piasecki, T.M.; Conner, K.L.; Bernstein, S.L.; Hayes-Birchler, T.; Theobald, W.E.; Fiore, M.C. The Relationship of COVID-19 Vaccination with Mortality Among 86,732 Hospitalized Patients: Subpopulations, Patient Factors, and Changes over Time. J. Gen. Intern. Med. 2023, 38, 1248–1255. [Google Scholar] [CrossRef]
- Hall, A.J.; Clement, N.D.; MacLullich, A.M.J.; White, T.O.; Duckworth, A.D. Vaccination against COVID-19 reduced the mortality risk of COVID-positive hip fracture patients to baseline levels: The nationwide data-linked IMPACT Protect study. Osteoporos. Int. 2024, 35, 353–363. [Google Scholar] [CrossRef]
- Beraud, G.; Bouetard, L.; Civljak, R.; Michon, J.; Tulek, N.; Lejeune, S.; Millot, R.; Garchet-Beaudron, A.; Lefebvre, M.; Velikov, P.; et al. Impact of vaccination on the presence and severity of symptoms in hospitalized patients with an infection of the Omicron variant (B.1.1.529) of the SARS-CoV-2 (subvariant BA.1). Clin. Microbiol. Infect. 2023, 29, 642–650. [Google Scholar] [CrossRef]
- Đaković Rode, O.; Bodulić, K.; Zember, S.; Cetinić Balent, N.; Novokmet, A.; Čulo, M.; Rašić, Ž.; Mikulić, R.; Markotić, A. Decline of Anti-SARS-CoV-2 IgG Antibody Levels 6 Months after Complete BNT162b2 Vaccination in Healthcare Workers to Levels Observed Following the First Vaccine Dose. Vaccines 2022, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Zember, S.; Bodulić, K.; Cetinić Balent, N.; Mikulić, R.; Markotić, A.; Đaković Rode, O. Slower Waning of Anti-SARS-CoV-2 IgG Levels Six Months after the Booster Dose Compared to Primary Vaccination. Vaccines 2022, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ge, E.; Li, Y.; Wu, S.; Candido, E.; Wei, X. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLoS ONE 2021, 16, e0258154. [Google Scholar] [CrossRef]
- Turtle, L.; Thorpe, M.; Drake, T.M.; Swets, M.; Palmieri, C.; Russell, C.D.; Ho, A.; Aston, S.; Wootton, D.G.; Richter, A.; et al. Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study. PLoS Med. 2023, 20, e1004086. [Google Scholar] [CrossRef]
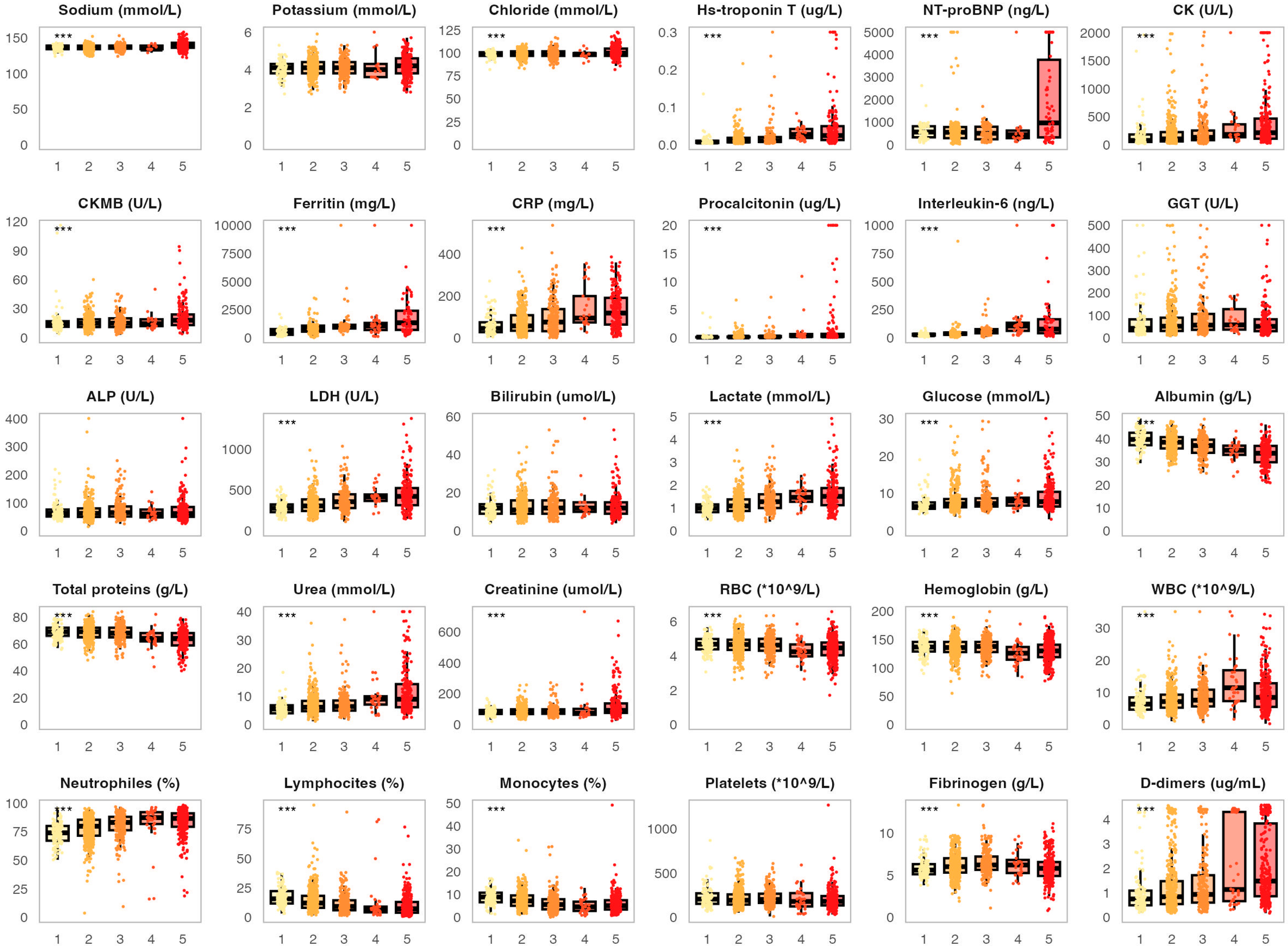
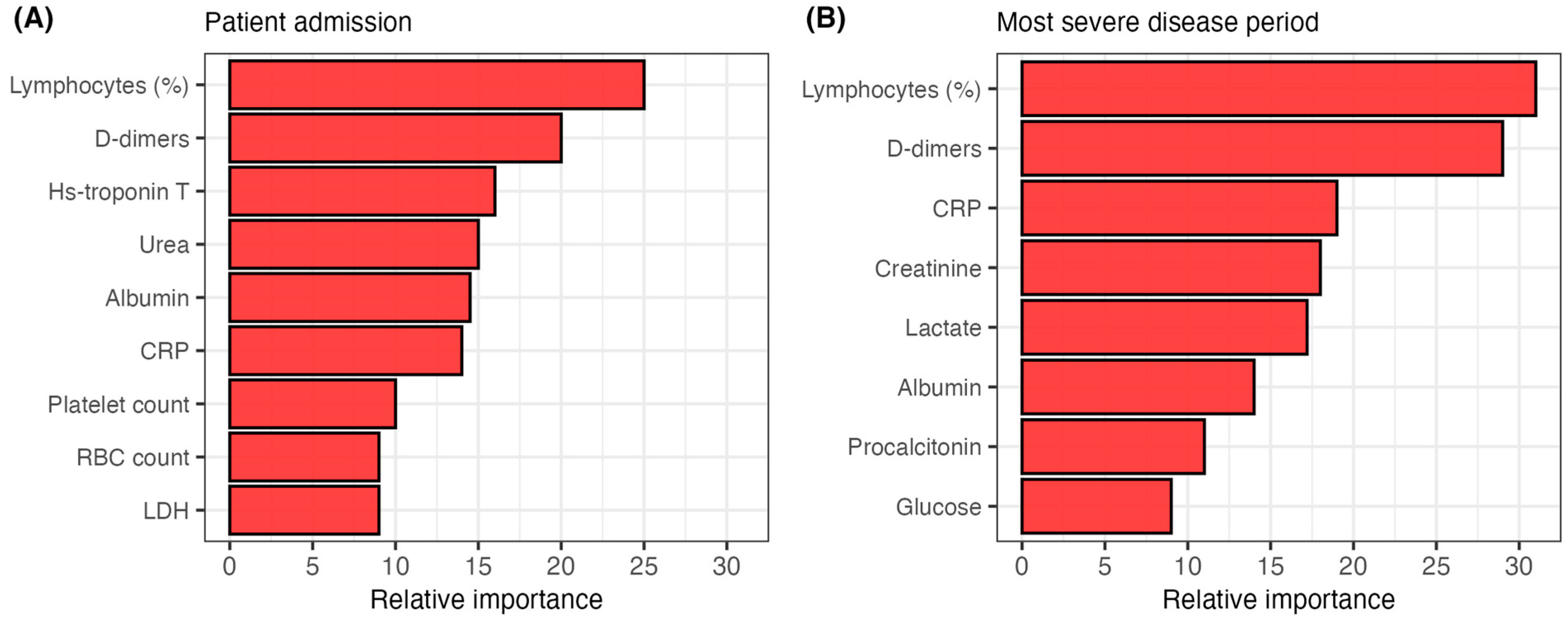

| Variable | N (%)/Median (IQR) |
|---|---|
| Sex (male/female) | 797/425 (65.2%/34.8%) |
| Age (years) | 66 (55–76) |
| Age groups (years) | |
| 18–50 | 200 (16.4%) |
| 51–65 | 396 (32.4%) |
| 66–80 | 435 (35.6%) |
| >80 | 191 (15.6%) |
| Viral variant | |
| B.1 (Original) | 397 (32.5%) |
| B.1.1.7 (Alpha) | 293 (24.0%) |
| B.1.617.2 (Delta) | 330 (27.0%) |
| Unknown * | 202 (16.5%) |
| Fully vaccinated against COVID-19 | 178 (14.6%) |
| Number of comorbidities | 2 (1–3) |
| Most common comorbidities | |
| Hypertension | 738 (60.4%) |
| Cardiovascular diseases | 320 (26.2%) |
| Diabetes | 266 (21.8%) |
| Chronic renal disease | 86 (7.0%) |
| Immunodeficiency | 71 (6.3%) |
| Duration of disease on admission (days) | 9 (7–12) |
| Duration of hospitalization (days) | 10 (7–16) |
| ICU admission | 297 (24.3%) |
| Mechanical ventilation | 229 (18.7%) |
| Disease severity | |
| Surviving patients, mild COVID-19 | 123 (10.1%) |
| Surviving patients, moderate COVID-19 | 498 (40.8%) |
| Surviving patients. severe COVID-19 | 210 (17.2%) |
| Surviving patients, critical COVID-19 | 70 (5.7%) |
| Deceased patients | 314 (25.7%) |
| Fold Change (95% CI), p-Value | |||
|---|---|---|---|
| Biomarker | B.1.617.2 vs. B.1 | B.1.617.2 vs. B.1.1.7 | B.1. vs. B.1.1.7 |
| Hs-troponin T | 2.90 (2.67–3.13), <0.001 | 2.97 (2.71–3.24), <0.001 | 1.32 (1.09–1.55), 0.043 |
| CK | 1.67 (1.42–1.92), <0.001 | 1.75 (1.48–2.01), <0.001 | / |
| Procalcitonin | 1.51 (1.25–1.76), 0.004 | 1.50 (1.26–1.74), 0.003 | / |
| Interleukin-6 | 1.60 (1.36–1.85), <0.001 | 1.58 (1.37–1.79), <0.001 | |
| Urea | 1.38 (1.15–1.61), 0.018 | 1.42 (1.13–1.70), 0.020 | / |
| D-dimers | 1.35 (1.08–1.62), 0.034 | 1.48 (1.20–1.76), 0.006 | 1.28 (1.05–1.52), 0.048 |
| Albumin | 0.68 (0.48–0.88), 0.012 | 0.67 (0.46–0.89), 0.014 | 0.71 (0.52–0.91), 0.021 |
| Fold Change (95% CI), p-Value | |||||
|---|---|---|---|---|---|
| Biomarker | Hypertension N = 738 | Cardiovascular Disease N = 320 | Diabetes N = 266 | Chronic Renal Disease N = 86 | Immunodeficiency N = 71 |
| Hs-troponin T | 1.38 (1.31–1.45), <0.001 | 1.45 (1.22–1.68), 0.012 | / | 1.50 (1.06–1.96), 0.049 | / |
| NT-proBNP | 1.40 (1.23–1.57), <0.001 | 1.60 (1.30–1.90), 0.001 | / | 1.70 (1.15–2.25), 0.043 | / |
| CK | 1.32 (1.24–1.40), <0.001 | 1.38 (1.16–1.60), 0.034 | / | / | / |
| CRP | / | / | / | / | 1.48 (1.08–1.89), 0.040 |
| Interleukin-6 | / | / | / | / | 1.50 (1.08–1.91), 0.043 |
| ALP | / | / | 1.60 (1.34–1.86), <0.001 | / | / |
| LDH | / | / | 1.55 (1.21–1.79), 0.017 | / | / |
| Lactate | / | / | 1.40 (1.10–1.70), 0.034 | / | / |
| Glucose | / | / | 1.50 (1.25–1.74), 0.001 | / | / |
| Albumin | / | / | / | 1.88 (1.50–2.26), <0.001 | / |
| Total proteins | / | / | / | 1.60 (1.20–2.01), 0.023 | / |
| Urea | / | / | / | 1.64 (1.20–2.08), 0.021 | / |
| Creatinine | / | / | / | 1.70 (1.35–2.05), <0.001 | |
| Neutrophils (%) | / | / | / | / | 1.45 (1.05–1.86), 0.048 |
| Lymphocytes (%) | / | / | / | / | 0.60 (0.25–0.95), 0.046 |
| Fibrinogen | / | 1.33 (1.10–1.56), 0.038 | / | / | / |
| D-dimers | / | 1.40 (1.15–1.64), 0.027 | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barušić, Z.; Bodulić, K.; Zember, S.; Laškaj, R.; Čivljak, R.; Puljiz, I.; Kurolt, I.-C.; Šafranko, Ž.M.; Krajinović, L.C.; Karić, P.S.; et al. Prognostic Value of Biomarkers in COVID-19: Associations with Disease Severity, Viral Variants, and Comorbidities—A Retrospective Observational Single-Center Cohort Study. Life 2025, 15, 634. https://doi.org/10.3390/life15040634
Barušić Z, Bodulić K, Zember S, Laškaj R, Čivljak R, Puljiz I, Kurolt I-C, Šafranko ŽM, Krajinović LC, Karić PS, et al. Prognostic Value of Biomarkers in COVID-19: Associations with Disease Severity, Viral Variants, and Comorbidities—A Retrospective Observational Single-Center Cohort Study. Life. 2025; 15(4):634. https://doi.org/10.3390/life15040634
Chicago/Turabian StyleBarušić, Zoran, Kristian Bodulić, Sanja Zember, Renata Laškaj, Rok Čivljak, Ivan Puljiz, Ivan-Christian Kurolt, Željka Mačak Šafranko, Lidija Cvetko Krajinović, Petra Svoboda Karić, and et al. 2025. "Prognostic Value of Biomarkers in COVID-19: Associations with Disease Severity, Viral Variants, and Comorbidities—A Retrospective Observational Single-Center Cohort Study" Life 15, no. 4: 634. https://doi.org/10.3390/life15040634
APA StyleBarušić, Z., Bodulić, K., Zember, S., Laškaj, R., Čivljak, R., Puljiz, I., Kurolt, I.-C., Šafranko, Ž. M., Krajinović, L. C., Karić, P. S., & Markotić, A. (2025). Prognostic Value of Biomarkers in COVID-19: Associations with Disease Severity, Viral Variants, and Comorbidities—A Retrospective Observational Single-Center Cohort Study. Life, 15(4), 634. https://doi.org/10.3390/life15040634










