Clinical Significance of Neutralizing Antibodies in COVID-19: Implications for Disease Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Samples
2.3. Serologic Assay
2.3.1. IgM and IgG Measurements
2.3.2. Neutralization Antibody Measurements
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. IgM and IgG Results
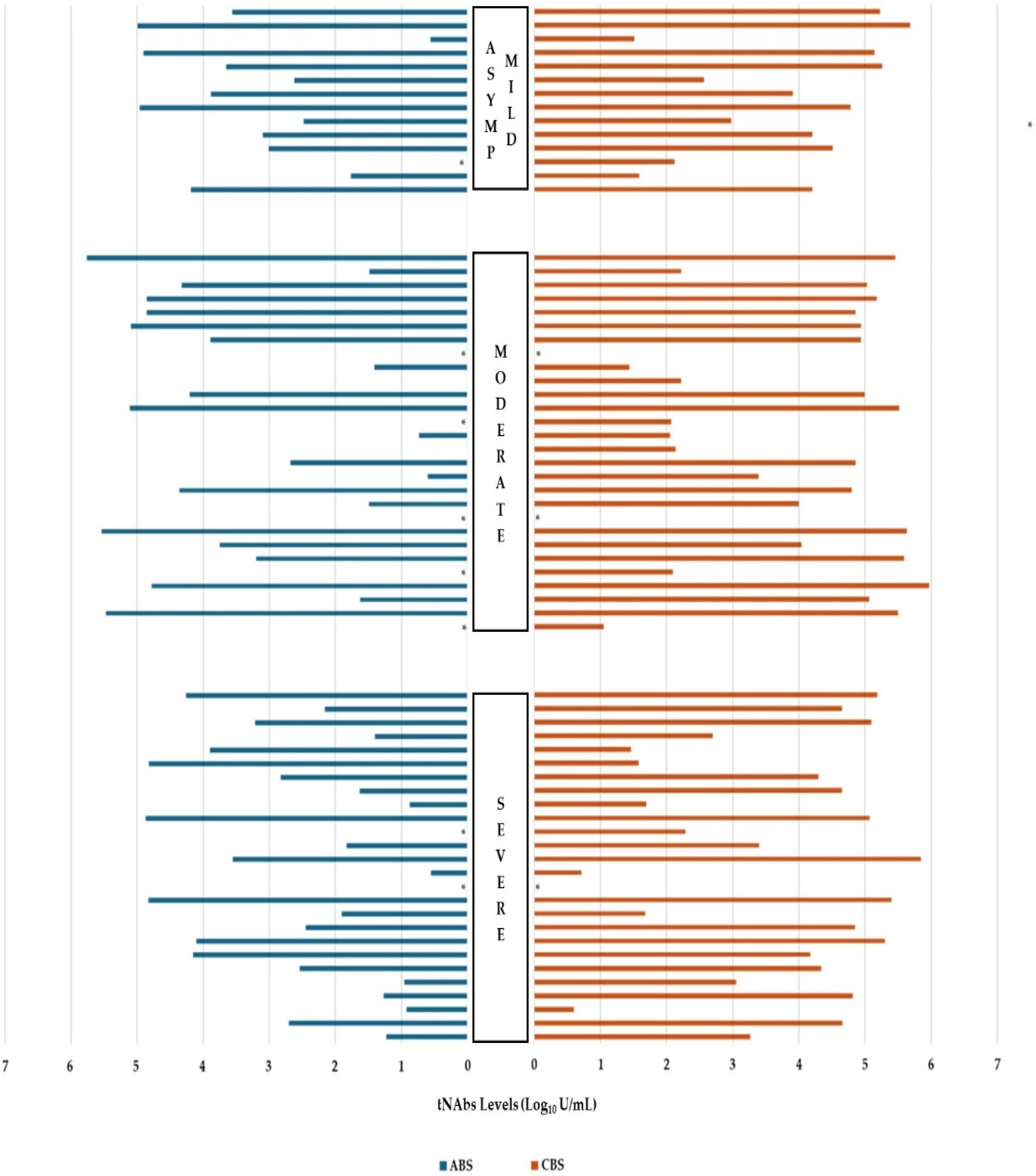
3.3. Neutralization Antibody Results
3.4. Vaccination Results
3.5. Correlation Analysis Results
3.6. Regression Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Röltgen, K.; Boyd, S.D. Antibody and B Cell Responses to SARS-CoV-2 Infection and Vaccination: The End of the Beginning. Annu. Rev. Pathol. Mech. Dis. 2024, 19, 69–97. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. Sars-Cov-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef]
- Yushan, J.; Zihan, H.; Zhuolin, L.; Chenguang, S. Immunology and Immun System Disorders. In Neutralizing Antibodies and Their Role in Health and Disease. Development of Anti-SARS-CoV-2 Neutralizing Antibodies; Nova Publishers: New York, NY, USA, 2024; Chapter 7; pp. 153–165. [Google Scholar]
- Lebedin, M.; Ratswohl, C.; Garg, A.; Schips, M.; García, C.V.; Spatt, L.; Thibeault, C.; Obermayer, B.; Weiner, J.; Velásquez, I.M.; et al. Soluble ACE2 correlates with severe COVID-19 and can impair antibody responses. IScience 2024, 27, 109330. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef] [PubMed]
- Schasfoort, R.B.M.; van Weperen, J.; van Amsterdam, M.; Parisot, J.; Hendriks, J.; Koerselman, M.; Karperien, M.; Mentink, A.; Bennink, M.; Krabbe, H.; et al. Presence and strength of binding of IgM, IgG and IgA antibodies against SARS-CoV-2 during COVİD-19 infection. Biosens. Bioelectron. 2021, 183, 113165. [Google Scholar] [CrossRef]
- Manuylov, V.; Dolzhikova, I.; Kudryashova, A.; Cherepovich, B.; Kovyrshina, A.; Iliukhina, A.; Kharchenko, O.; Semashko, M.; Tkachuk, A.; Gushchin, V.; et al. Simple ELISA Methods to Estimate Neutralizing Antibody Titers to SARS-CoV-2: IgG Quantification; the Avidity Index; and the Surrogate Virus Neutralization Test. Arch. Microbiol. Immunol. 2022, 6, 213. [Google Scholar] [CrossRef]
- Lapuente, D.; Winkler, T.H.; Tenbusch, M. B-cell and antibody responses to SARS-CoV-2: Infection; vaccination; and hybrid immunity. Cell Mol. Immunol. 2024, 21, 144–158. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2 spike protein protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Hajilooi, M.; Keramat, F.; Moazenian, A.; Rastegari-Pouyani, M.; Solgi, G. The quantity and quality of anti-SARS-CoV-2 antibodies show contrariwise association with COVID-19 severity: Lessons learned from IgG avidity. Med. Microbiol. Immunol. 2023, 212, 203–220. [Google Scholar] [CrossRef]
- Dopico, X.C.; Ols, S.; Lore, K.; Hedestam, G.B.K. Immunity to SARS-CoV-2 induced by infection or vaccination. J. Intern. Med. 2022, 291, 32–50. [Google Scholar] [CrossRef]
- Theel, E.S.; Kirby, J.E.; Pollock, N.R. Testing for SARS-CoV-2: Lessons learned and current use cases. Clin. Microbiol. Rev. 2024, 37, e00072-23. [Google Scholar] [CrossRef]
- Li, C.J.; Chao, T.L.; Chang, T.Y.; Hsiao, C.C.; Lu, D.C.; Chiang, Y.W.; Lai, G.C.; Tsai, Y.M.; Fang, J.T.; Ieong, S.; et al. Neutralizing Monoclonal Antibodies Inhibit SARS-CoV-2 Infection through Blocking Membrane Fusion. Microbiol. Spectr. 2022, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, M.; Nishina, N.; Moriyama, S.; Takahashi, Y.; Uwamino, Y.; Nagata, M.; Aoki, W.; Masaki, K.; Ishii, M.; Saya, H.; et al. Incomplete humoral response including neutralizing antibodies in asymptomatic to mild COVID-19 patients in Japan. Virology 2021, 555, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Palkar, S.; Shah, J.; Rane, P.; Lalwani, S.; Mishra, A.C.; Arankalle, V.A. Early and high SARS-CoV-2 neutralizing antibodies are associated with severity in COVID-19 patients from India. Am. J. Trop. Med. Hyg. 2021, 105, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Bang, M.S.; Kim, C.M.; Cho, N.H.; Seo, J.W.; Kim, D.Y.; Yun, N.R.; Kim, D.M. Evaluation of humoral immune response in relation to COVID-19 severity over 1 year post-infection: Critical cases higher humoral immune response than mild cases. Front. Immunol. 2023, 14, 1203803. [Google Scholar] [CrossRef]
- Franchini, M.; Focosi, D. Hyperimmune Plasma and Immunoglobulins against COVID-19: A Narrative Review. Life 2024, 14, 214. [Google Scholar] [CrossRef]
- Misra, A.; Theel, E.S. Immunity to SARS-CoV-2: What do we know and should we be testing for it? J. Clin. Microbiol. 2022, 60, e00482-21. [Google Scholar] [CrossRef]
- Du, Z.; Liu, C.; Bai, Y.; Wang, L.; Lim, W.W.; Lau, E.H.; Cowling, B.J. Predicting Efficacies of Fractional Doses of Vaccines by Using Neutralizing Antibody Levels: Systematic Review and Meta-Analysis. JMIR Public Health Surveill. 2024, 10, e49812. [Google Scholar] [CrossRef]
- Alonso, R.; Gil-Manso, S.; Catalán, P.; Sánchez-Arcilla, I.; Marzola, M.; Correa-Rocha, R.; Pion, M.; Marañón, G. Neutralizing antibody levels detected early after mRNA-based vaccination do not predict by themselves subsequent breakthrough infections of SARS-CoV-2. Front. Immunol. 2024, 15, 1341313. [Google Scholar] [CrossRef]
- Higgins, V.; Fabous, A.; Kulasingam, V. Quantitative Measurement of Anti-SARS-CoV-2 Antibodies: Analytical and Clinical Evaluation. J. Clin. Microbiol. 2021, 59, 1–7. [Google Scholar] [CrossRef]
- Mink, S.; Reimann, P.; Fraunberger, P. Prognostic value of anti-SARS-CoV-2 antibodies: A systematic review. Clin. Chem. Lab. Med. 2024, 62, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.M.; Haralambieva, I.H.; Warner, N.D.; Grill, D.E.; Quach, H.Q.; Kennedy, R.B. Longitudinal antibody titer; avidity; and neutralizing responses after SARS-CoV-2 infection. Heliyon 2022, 8, e11676. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Stevanovic, V.; Ilic, M.; Barbic, L.; Capak, K.; Tabain, I.; Krleza, J.L.; Ferenc, T.; Hruskar, Z.; Topic, R.Z.; et al. SARS-CoV-2 Seroprevalence and Neutralizing Antibody Response after the First and Second COVID-19 Pandemic W ave in Croatia. Pathogens 2021, 10, 774. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Qin, X.; Wang, W.; Xu, M.; Wang, L.F.; Xu, C.; Tang, S.; Liu, P.; Zhang, L.; et al. SARS-CoV-2 neutralizing antibody levels are correlated with severity of COVID-19 pneumonia. Biomed. Pharmacother. 2020, 130, 110629. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, J.; Wen, F.; Liu, K.S.; Chen, Y. Detection methods and dynamic characteristics of specific antibodies in patients with COVID-19: A review of the early literature. Heliyon 2024, 10, e24580. [Google Scholar] [CrossRef]
- Choe, P.G.; Kang, C.K.; Suh, H.J.; Jung, J.; Song, K.H.; Bang, J.H.; Kim, E.S.; Kim, H.B.; Park, S.W.; Kim, N.J.; et al. Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Emerg. Infect. Dis. 2021, 27, 327. [Google Scholar] [CrossRef] [PubMed]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi., G.; et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef]
- Mink, S.; Saely, C.H.; Leiherer, A.; Reimann, P.; Frick, M.; Cadamuro, J.; Hitzl, W.; Drexel, H.; Fraunberger, P. Antibody levels versus vaccination status in the outcome of older adults with COVID-19. JCI Insight 2024, 9, e183913. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Largescale study of antibody titer decay following BNT162b2 mRNA vaccine or SARSCoV-2 infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef]
- Cancarevic, I.; Malik, B.H. SARS-CoV-2 (COVID 19) Infection in Hypertensive Patients and in Patients With Cardiac Disease. Cureus 2020, 12, e8557. [Google Scholar]
- Kounis, N.G.; Gogos, C.; de Gregorio, C.; Hung, M.Y.; Kounis, S.N.; Tsounis, E.P.; Assimakopoulos, S.; Pourmasumi, S.; Blani, V.; Servos; et al. “When;” “Where;” and “How” of SARS-CoV-2 Infection Affects the Human Cardiovascular System: A Narrative Review. Balkan Med. J. 2024, 41, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Savedchuk, S.; Raslan, R.; Nystrom, S.; Sparks, M.A. Emerging viral infections and the potential impact on hypertension; cardiovascular disease; and kidney disease. Circ. Res. 2022, 130, 1618–1641. [Google Scholar] [CrossRef] [PubMed]
| Parameters | ABS * (n = 68) Median (Min–Max) | p Value | CBS * (n = 68) Median (Min–Max) | p Value | |||
|---|---|---|---|---|---|---|---|
| Gender | Female (n = 35) | 477.7 (0.4–343,370) | 0.854 | 11,080 (0.4–927,920) | 0.902 | ||
| Male (n = 33) | 498.7 (0.4–571,870) | 32,050 (0.4–474,800) | |||||
| Disease severity | Asymptomatic—Mild | 2441 (0.4–98,660) | 0.496 | 16,260 (32.49–474,800) | 0.699 | ||
| Moderate | 1027.35 (0.4–571,870) | 67,360 (0.4–927,920) | |||||
| Severe | 211.75 (0.4–73,640) | 17,330 (0.51–706,590) | |||||
| COVID-19 Vaccination | No | 5.33 (0.4–1619) | <0.001 | 126.8 (0.4–126,360) | <0.001 | ||
| Yes | 5610 (0.4–571,870) | 66,480 (0.4–927,920) | |||||
| Full-dose COVID-19 vaccination (n = 49) 1 | No | 4451 (0.4–571,870) | 0.585 | 72,440 (0.4–927,920) | 0.157 | ||
| Yes | 7758.5 (16.8–90,260) | 24,170 (29–170,410) | |||||
| Time since LV 2 (n = 49) | <6 months | 6682.5 (0.4–343,370) | 0.758 | 71,985 (0.4–927,920) | 0.743 | ||
| >6 months | 1577 (4–571,870) | 46,110 (37.7–393,440) | |||||
| COVID-19 IgM ABS 3 | Negative | 211.75 (0.4–66,090) | <0.001 | ||||
| Positive | 69,600 (5.33–571,870) | ||||||
| COVID-19 IgM CBS 4 | Negative | 15,450 (5.07–313,170) | 0.488 | ||||
| Positive | 38,145 (0.4–927,920) | ||||||
| COVID-19 IgG ABS 3 | Negative | 58.1 (0.4–66,090) | <0.001 | ||||
| Positive | 14,210 (0.4–571,870 | ||||||
| COVID-19 IgG CBS 4 | Negative | 208.09 (5.07–16,290) | 0.090 | ||||
| Positive | 27,070 (0.4–927,920) | ||||||
| 28 day mortality | No | 677.9 (0.4–571,870) | 0.874 | 16,230 (0.4–927,920) | 0.659 | ||
| Yes | 278.8 (16.8–73,640) | 44,240 (29-706,590) | |||||
| pNAbs ABS 3 | No | 42.9 (17–343,370) | 0.148 | ||||
| Yes | 5610 (4–571,870) | ||||||
| pNAbs CBS 4 | No | 117,230 (22,090–706,590) | 0.167 | ||||
| Yes | 62,600 (4–927,920) | ||||||
| Parameters | Admission (n = 52) | p | Control (n = 52) | p | ||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||||
| Gender (n [%]) | Female | 8 (30.8) | 18 (69.2) | 0.174 | 3 (11.5) | 23 (88.5) | 1.000 | |
| Male | 3 (11.5) | 23 (88.5) | 2 (7.7) | 24 (92.3) | ||||
| Age (Year) (median [min-max]) | 67 (62–85) | 67 (28–89) | 0.419 | 77 (65–89) | 67 (28–88) | 0.232 | ||
| Hospital stay (median [min-max]) | 21 (8–83) | 15 (1–85) | 0.158 | 21 (14–23) | 18 (1–85) | 0.664 | ||
| Disease severity (n [%]) | Asymptomatic—Mild | 0 (0) | 11 (100) | 0.089 | 1 (9.1) | 10 (90.9) | 0.600 | |
| Moderate | 4 (20) | 16 (80) | 1 (5) | 19 (95) | ||||
| Severe | 7 (33.3) | 14 (66.7) | 3 (14.3) | 18 (85.7) | ||||
| COVID-19 Vaccination (n [%]) | No | 1 (14.3) | 6 (85.7) | 1.000 | 0 (0) | 7 (100) | 1.000 | |
| Yes | 10 (22.2) | 35 (77.8) | 5 (11.1) | 40 (88.9) | ||||
| Full-dose COVID-19 vaccination 1 (n = 45) [%]) | No | 8 (22.9) | 27 (77.1) | 1.000 | 4 (11.4) | 31 (88.6) | 1.000 | |
| Yes | 2 (20) | 8 (80) | 1 (10) | 9 (90) | ||||
| Time since LV 2 (n = 45) | <6 months | 7 (25.9) | 20 (74.1) | 0.716 | 4 (14.8) | 23 (85.2) | 0.634 | |
| >6 months | 3 (16.7) | 15 (83.3) | 1 (5.6) | 17 (94.4) | ||||
| COVID-19 IgM ABS 3 (n [%]) | Negative | 8 (25.8) | 23 (74.2) | 0.491 | ||||
| Positive | 3 (14.3) | 18 (85.7) | ||||||
| COVID-19 IgM CBS 4 (n [%]) | Negative | 0 (0) | 18 (100) | 0.150 | ||||
| Positive | 5 (14.7) | 29 (85.3) | ||||||
| COVID-19 IgG ABS 3 (n [%]) | Negative | 7 (36.8) | 12 (63.2) | 0.074 | ||||
| Positive | 4 (12.1) | 29 (87.9) | ||||||
| COVID-19 IgG CBS 4 (n [%]) | 0 (0) | 2 (100) | 1.000 | |||||
| 5 (10) | 45 (90) | |||||||
| 28 day mortality (n [%]) | No | 6 (14.3) | 36 (85.7) | 0.025 | 2 (4.8) | 40 (95.2) | 0.043 | |
| Yes | 5 (50) | 5 (50) | 3 (30) | 7 (70) | ||||
| Risk Factors | Multinomial Analyse | |||||
|---|---|---|---|---|---|---|
| 95% Confidence Interval for Exp(B) | ||||||
| B | Odd’s Ratio | Lower Bound | Upper Bound | p Value | ||
| Moderate symptomatic | IgM admission | −3.521 | 0.03 | 0.001 | 1.352 | 0.071 |
| IgG admission | 5.213 | 183.6 | 0.546 | 61,699.735 | 0.079 | |
| Days since LV 1 | 0.006 | 1.006 | 0.992 | 1.019 | 0.419 | |
| tNAbs admission | 0.000 | 1.000 | 1.000 | 1.000 | 0.184 | |
| tNAbs control | 0.000 | 1.000 | 1.000 | 1.000 | 0.240 | |
| pNAbs admission | −0.098 | 0.907 | 0.806 | 1.021 | 0.107 | |
| pNAbs control | 0.040 | 1.041 | 0.983 | 1.102 | 0.174 | |
| Severe symptomatic | IgM admission | −0.485 | 0.616 | 0.018 | 30.948 | 0.787 |
| IgG admission | 6.289 | 538.7 | 1.175 | 247,056.786 | 0.044 | |
| Days since LV 1 | 0.007 | 1.007 | 0.993 | 1.021 | 0.350 | |
| tNAbs admission | 0.000 | 1.000 | 1.000 | 1.000 | 0.708 | |
| tNAbs control | 0.000 | 1.000 | 1.000 | 1.000 | 0.310 | |
| pNAbs admission | −0.137 | 0.872 | 0.772 | 0.984 | 0.026 | |
| pNAbs control | 0.021 | 1.021 | 0.974 | 1.071 | 0.388 | |
| Risk Factors | Logistic Analysis | ||
|---|---|---|---|
| B | Odd’s Ratio | p Value | |
| IgM admission | −2.501 | 0.082 | 0.402 |
| IgM control | −0.175 | 0.840 | 0.915 |
| IgG admission | −3.491 | 0.03 | 0.341 |
| IgG control | −15.590 | 0.000 | 1.000 |
| Days since LV 1 | −0.013 | 0.987 | 0.161 |
| tNAbs admission | 0.000 | 1.000 | 0.141 |
| tNAbs control | 0.000 | 1.000 | 0.301 |
| pNAbs admission | −0.089 | 0.914 | 0.041 |
| pNAbs control | −0.004 | 0.006 | 0.792 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çolak, S.M.; İlgar, T.; Bahçeci, İ.; Özkaya, E.; Hüner Yiğit, M.; Durmuş, H.; Atiş, F.; Ertürk, A.; Yazıcı, Z.A. Clinical Significance of Neutralizing Antibodies in COVID-19: Implications for Disease Prognosis. Life 2025, 15, 429. https://doi.org/10.3390/life15030429
Çolak SM, İlgar T, Bahçeci İ, Özkaya E, Hüner Yiğit M, Durmuş H, Atiş F, Ertürk A, Yazıcı ZA. Clinical Significance of Neutralizing Antibodies in COVID-19: Implications for Disease Prognosis. Life. 2025; 15(3):429. https://doi.org/10.3390/life15030429
Chicago/Turabian StyleÇolak, Sudem Mahmutoğlu, Tuba İlgar, İlkay Bahçeci, Esra Özkaya, Merve Hüner Yiğit, Hilal Durmuş, Feyza Atiş, Ayşe Ertürk, and Zihni Acar Yazıcı. 2025. "Clinical Significance of Neutralizing Antibodies in COVID-19: Implications for Disease Prognosis" Life 15, no. 3: 429. https://doi.org/10.3390/life15030429
APA StyleÇolak, S. M., İlgar, T., Bahçeci, İ., Özkaya, E., Hüner Yiğit, M., Durmuş, H., Atiş, F., Ertürk, A., & Yazıcı, Z. A. (2025). Clinical Significance of Neutralizing Antibodies in COVID-19: Implications for Disease Prognosis. Life, 15(3), 429. https://doi.org/10.3390/life15030429








