Metabolic Profile, Antioxidant, Antimicrobial, Contractile, and Anti-Inflammatory Potential of Moringa oleifera Leaves (India)
Abstract
1. Introduction
- To determine the metabolic profile of Moringa oleifera leaf extracts from two samples from India;
- To evaluate in vitro their antioxidant, antimicrobial, anti-inflammatory, and ex vivo spasmolytic activity.
2. Materials and Methods
2.1. Plant Material
- Sample 1
- Sample 2
2.2. Test Microorganisms
2.3. Culture Media
2.4. Methods
2.4.1. GC-MS Analysis
2.4.2. LC-ESI-QTOF-MS
2.4.3. Quantification of Polyphenols via HPLC-PDA Analysis
2.4.4. Antioxidant Activity Assessment
2.4.5. Total Phenolic Contents (TPC) and Total Flavonoid Contents (TFC)
2.4.6. Antimicrobial Activity Assay
2.4.7. Inhibition of Albumin Denaturation
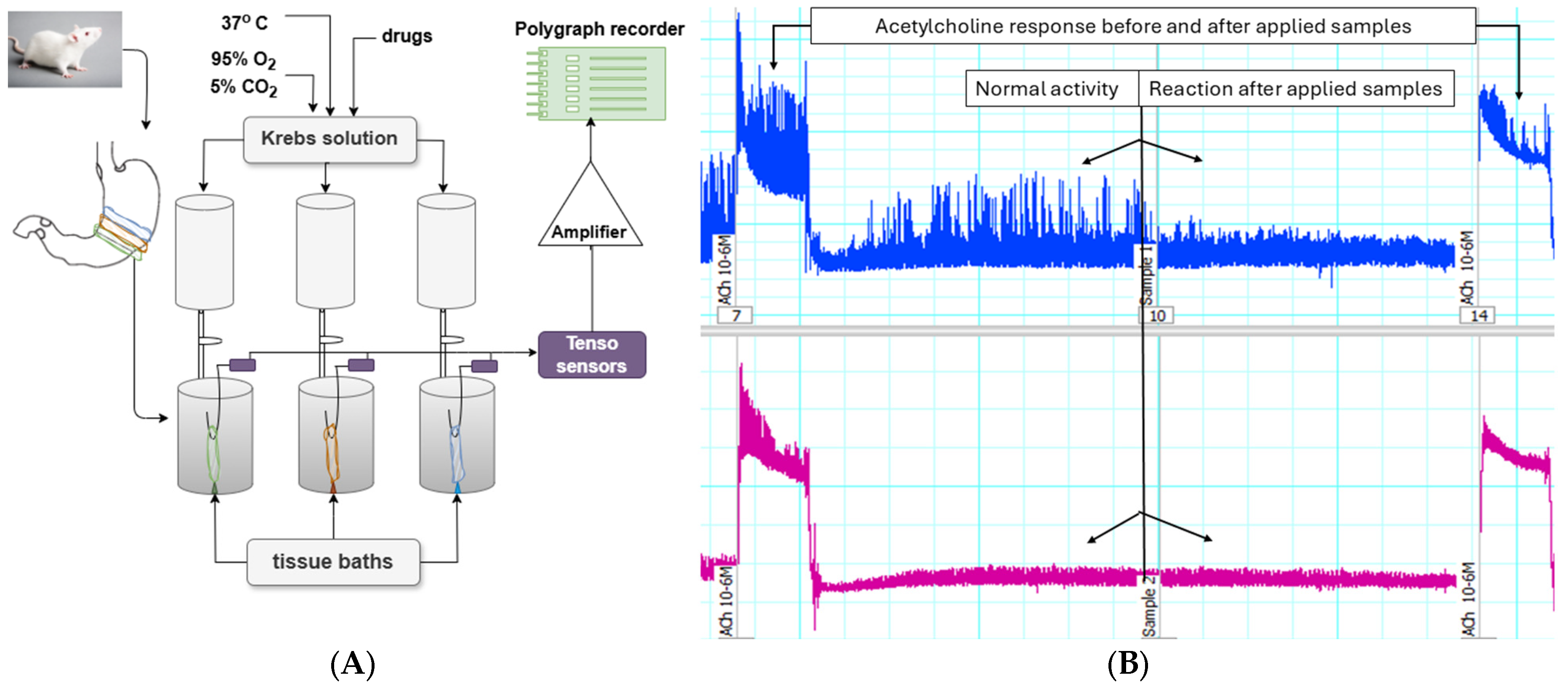
2.5. In Vitro Experiments on Gastric SM Preparations from Rat Wistar
Method of Studying Spontaneous CA of Isolated SM Preparations
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of the Leaves from Moringa oleifera
3.2. Antioxidant Activity
3.3. Antimicrobial Activity Results
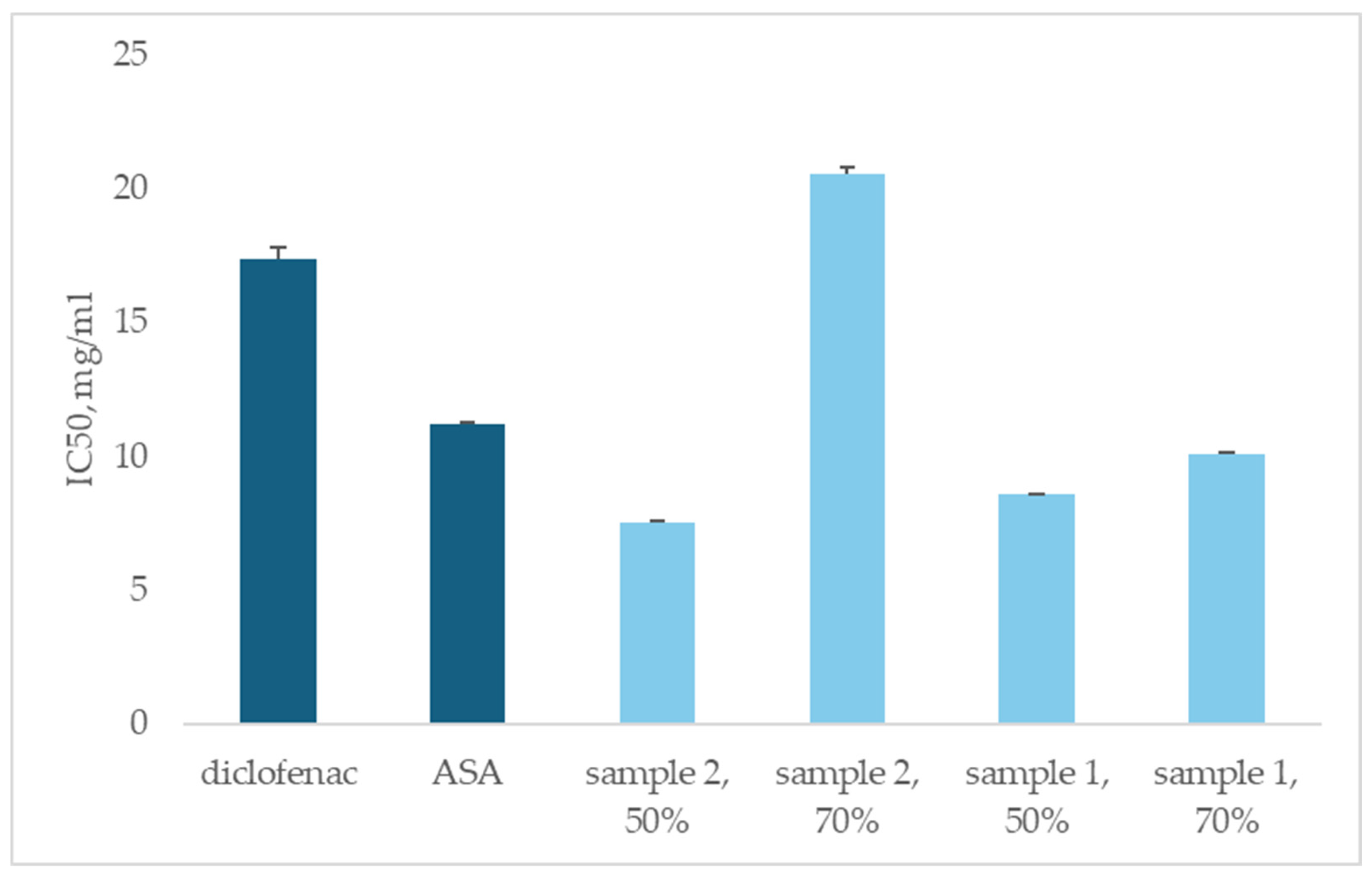
3.4. Anti-Inflammatory Activity
3.5. Ex Vivo Experiments on Gastric SMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teclegeorgish, Z.W.; Aphane, Y.M.; Mokgalaka, N.S.; Steenkamp, P.; Tembu, V.J. Nutrients, secondary metabolites and anti-oxidant activity of Moringa oleifera leaves and Moringa-based commercial products. S. Afr. J. Bot. 2021, 142, 409–421. [Google Scholar] [CrossRef]
- Swatia, A.; Virk, A.K.; Kumari, C.; Ali, A.; Garg, P.; Thakur, P.; Attri, C.; Kulshrestha, S. Moringa oleifera—A never die tree: An overview. Asian J. Pharm. Clin. Res. 2018, 11, 57–65. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Fuglie, L.J. Producing Food without Pesticides: Local Solutions to Crop Pest Control in West Africa, 1st ed.; Church World Service: Dakar, Senegal, 1998; pp. 1–158. [Google Scholar]
- Gandji, K.; Chadare, F.J.; Idohou, R.; Salako, V.K.; Assogbadjo, A.E.; Glèlè, R.L.K. Status and utilisation of Moringa oleifera Lam: A review. Afr. Crop Sci. J. 2018, 26, 137–156. [Google Scholar] [CrossRef]
- Guevara, A.P.; Vargas, C.; Sakurai, H.; Fujiwara, Y.; Hashimoto, K.; Maoka, T.; Kozuka, M.; Ito, Y.; Tokuda, H.; Nishino, H. An antitumor promoter from Moringa oleifera Lam. Mutat. Res. 1999, 440, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wael, M.A.E.; Walaa, S.M.A. Effect of Moringa oleifera Seed Oil on Antimi-Crobial Activity of Some Antibiotics Against Some Pathogenic Gram Negative Bacteria. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 140–151. [Google Scholar]
- Sokovi, M.; Glamoèlija, J.; Marin, P.D.; Brki, D.; Van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amin, M.F.; Ariwibowo, T.; Putri, S.A.; Kurnia, D. Moringa oleifera: A Review of the Pharmacology, Chemical Constituents, and Application for Dental Health. Pharmaceuticals 2024, 17, 142. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Wu, A. Mini Review on Antimicrobial Activity and Bioactive Compounds of Moringa oleifera. J. Med. Chem. 2016, 6, 578–582. [Google Scholar] [CrossRef]
- Rebecca, H.S.U.; Sharon, M.; Arbainsyah, A.; Lucienne, D. Moringa oleifera medicinal and socio-economic uses. In International Course on Economic Botany; National Herbarium Leiden: Leiden, The Netherlands, 2006; pp. 2–6. [Google Scholar]
- Vinoth, B.; Manivasagaperumal, R.; Balamurugan, S. Phytochemical analysis and antibacterial activity of Moringa oleifera Lam. Int. J. Res. Biol. Sci. (IJRBS) 2012, 2, 98–102. [Google Scholar]
- Pal, S.K.; Mukherjee, P.K.; Saha, B.P. Studies on the antiulcer activity of Moringa oleifera leaf extract on gastric ulcer models in rats. Phytother. Res. 1995, 9, 463–465. [Google Scholar] [CrossRef]
- Posmontier, B. The medicinal qualities of Moringa oleifera. Holist. Nurs. Pract. 2011, 25, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Aekthammarat, D.; Pannangpetch, P.; Tangsucharit, P. Moringa oleifera leaf extract lowers high blood pressure by alleviating vascular dysfunction and decreasing oxidative stress in L-NAME hypertensive rats. Phytomedicine 2019, 54, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Kang, J.Y.; Mohibbullah, M.; Hong, Y.K.; Lee, H.; Choi, J.S.; Choi, I.S.; Moon, I.S. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J. Ethnopharmacol. 2014, 152, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Ahmed, M.; Marrez, D.A.; Abdelmoeen, N.M.; Mahmoud, E.A.; Abdel-Shakur Ali, M.; Decsi, K.; Tóth, Z. Proximate Analysis of Moringa oleifera Leaves and the Antimicrobial Activities of Successive Leaf Ethanolic and Aqueous Extracts Compared with Green Chemically Synthesized Ag-NPs and Crude Aqueous Extract against Some Pathogens. Int. J. Mol. Sci. 2023, 24, 3529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ph.Eur. Supplement 9.8, 9th Edition of European Pharmacopoeia. 2019. Available online: https://pheur.edqm.eu/home (accessed on 18 February 2025).
- Dincheva, I.; Badjakov, I.; Georgiev, V.; Semerdjieva, I.; Vrancheva, R.; Ivanov, I.; Pavlov, A. Comprehensive GC-MS Characterization and Histochemical Assessment of Various Parts of Three Colchicum Species from Bulgarian Flora. Plants 2025, 14, 270. [Google Scholar] [CrossRef]
- The Golm Metabolome Database (GMD). Available online: http://gmd.mpimp-golm.mpg.de/ (accessed on 18 February 2025).
- National Institute of Standards and Technology. NIST Mass Spectral Database, PC-Version 5.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008.
- Ivanov, I.; Vrancheva, R.; Marchev, A.; Petkova, N.; Aneva, I.; Denev, P.; Georgiev, V.; Pavlov, A. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 296–306. [Google Scholar]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, İ.; Duru, M.E.; Öztürk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009, 116, 470–479. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Lincheva, V.; Petkova, N.; Nikolova, R.; Vrancheva, R.; Ivanov, I. Antimicrobial activity of extract from aerial parts of potentilla (Potentilla reptans L.). Acad. J. Ind. Technol. 2017, 4, 37–43. [Google Scholar]
- Milusheva, M.; Todorova, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Pencheva, M.; Nedialkov, P.; Tumbarski, Y.; Yanakieva, V.; Tsoneva, S.; et al. Novel Anthranilic Acid Hybrids—An Alternative Weapon against Inflammatory Diseases. Pharmaceuticals 2023, 16, 1660. [Google Scholar] [CrossRef] [PubMed]
- Milusheva, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Mihaylova, R.; Nedialkov, P.; Cherneva, E.; Tumbarski, Y.; Tsoneva, S.; Todorova, M.; et al. Synthesis, Molecular Docking, and Biological Evaluation of Novel Anthranilic Acid Hybrid and Its Diamides as Antispasmodics. Int. J. Mol. Sci. 2023, 24, 13855. [Google Scholar] [CrossRef] [PubMed]
- Slavchev, V.; Gledacheva, V.; Pencheva, M.; Milusheva, M.; Nikolova, S.; Stefanova, I. Therapeutic Potential of 1-(2-Chlorophenyl)-6,7-dimethoxy-3-methyl-3,4-dihydroisoquinoline. Molecules 2024, 29, 3804. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J. Nat. Prod. 1994, 57, 1256–1261. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A Food Plant with Multiple Medicinal Uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10-Hydroxy-2-Decenoic Acid, a Major Fatty Acid from Royal Jelly, Inhibits VEGF-Induced Angiogenesis in Human Umbilical Vein Endothelial Cells. Evid. Based Complement. Altern. Med. 2009, 6, 489–494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banov, D.; Banov, F.; Bassani, A.S. Case series: The effectiveness of fatty acids from pracaxi oil in a topical silicone base for scar and wound therapy. Dermatol. Ther. 2014, 4, 259–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guevara, A.P. Antioxidant and anti-inflammatory activity of Moringa oleifera leaves from Cuba. Plant Foods Hum. Nutr. 1999, 54, 53–60. [Google Scholar]
- Al-Owaisi, M.; Al-Hadiwi, N.; Khan, S.A. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac. J. Trop. Biomed. 2014, 4, 964–970. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chandrasekar, M.J.N.; Nanjan, M.J.; Suresh, B. Antioxidant Activity of Caesalpinia Digyna Root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, E.I.; Olaleye, A.A.; Idowu, O.T.; Adubiaro, H.O.; Ayeni, K.E. Comparative Amino Acid Composition and Quality Parameters of Moringa oleifera Testa and Cotyledon. Mal. J. Nutr. 2022, 28, 227–238. [Google Scholar] [CrossRef]
- Mahmood, K.T.; Mugal, T.; Haq, I.U. Moringa oleifera: A natural gift—A review. J. Pharm. Sci. Res. 2010, 2, 775–781. [Google Scholar]
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleifera Leaves in Two Stages of Maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Chiș, A.; Noubissi, P.A.; Pop, O.-L.; Mureșan, C.I.; Fokam Tagne, M.A.; Kamgang, R.; Fodor, A.; Sitar-Tăut, A.-V.; Cozma, A.; Orășan, O.H.; et al. Bioactive Compounds in Moringa oleifera: Mechanisms of Action, Focus on Their Anti-Inflammatory Properties. Plants 2023, 13, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohamed, A.; Dayo, M.; Alahmadi, S.; Ali, S. Anti-Inflammatory and Antimicrobial Activity of Silver Nanoparticles Green-Synthesized Using Extracts of Different Plants. Nanomaterials 2024, 14, 1383. [Google Scholar] [CrossRef]
- Anzano, A.; de Falco, B.; Ammar, M.; Ricciardelli, A.; Grauso, L.; Sabbah, M.; Capparelli, R.; Lanzotti, V. Chemical Analysis and Antimicrobial Activity of Moringa oleifera Lam. Leaves and Seeds. Molecules 2022, 27, 8920. [Google Scholar] [CrossRef]
- Venugopal, J.; Bagyalakshmi, S.; Manjula, K.; Richard, D. In Vitro Anti-Inflammatory Activity of 4-Benzylpiperidine. Asian J. Pharm. Clin. Res. 2016, 9, 108–110. [Google Scholar] [CrossRef]
- Caceres, A.; Saravia, A.; Rizzo, S.; Zabala, L.; De Leon, E.; Nave, F. Pharmacologic Proper-ties of Moringa oleifera 2: Screening Antispasmodic, Antiinflammatory and Diuretic Activity. J. Ethnopharmacol. 1992, 36, 233–237. [Google Scholar]
- Sanders, K.M. Spontaneous Electrical Activity and Rhythmicity in Gastrointestinal Smooth Muscles. Adv. Exp. Med. Biol. 2019, 1124, 3–46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurahashi, M.; Kito, Y.; Baker, S.A.; Jennings, L.K.; Dowers, J.G.R.; Koh, S.D.; Sanders, K.M. A novel postsynaptic signal pathway of sympathetic neural regulation of murine colonic motility. FASEB J. 2020, 34, 5563–5577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Bacteria | Fungi | Yeasts | |
|---|---|---|---|
| Gram-Positive | Gram-Negative | ||
| Bacillus subtilis ATCC 6633 | Salmonella enteritidis ATCC 13076 | Aspergillus niger ATCC 1015 | Candida albicans NBIMCC 74 |
| Staphylococcus aureus ATCC 25923 | Klebsiella pneumoniae ATCC 13883 | Aspergillus flavus | Saccharomyces cerevisiae ATCC 9763 |
| Bacillus cereus NCTC 11145 | Escherichia coli ATCC 25922 | Penicillium chrysogenum | |
| Listeria monocytogenes NBIMCC 8632 | Proteus vulgaris ATCC 6380 | Fusarium moniliforme ATCC 3893 | |
| Enterococcus faecalis ATCC 29212 | Pseudomonas aeruginosa ATCC 9027 | ||
| Leaves Sample 1 | Leaves Sample 2 | |
|---|---|---|
| Content ± SD, mg g−1 Extract | Content ± SD, mg g−1 Extract | |
| Amino acids | ||
| Non-essential amino acid | ||
| l-Glutamic acid | 4.77 ± 0.11 a | 4.15 ± 0.10 b |
| Essential amino acid | ||
| l-Valine | 1.00 ± 0.23 a | 0.44 ± 0.10 b |
| l-Leucine | 0.37 ± 0.09 b | 0.73 ± 0.17 a |
| l-Isoleucine | 0.93 ± 0.22 b | 1.83 ± 0.43 a |
| Phenylalanine | 2.44 ± 0.57 a | 1.44 ± 0.34 b |
| l-Threonine | 1.20 ± 0.28 a | 1.07 ± 0.25 a,b |
| Conditional non-essential amino acid | ||
| l-Aspartic acid | 6.21 ± 1.45 a | 5.53 ± 1.29 b |
| Proline | 1.24 ± 0.29 a | n.d. |
| Pyroglutamic acid | 1.72 ± 0.40 a | 0.66 ± 0.15 b |
| l-Serine | 0.62 ± 0.15 a | 0.50 ± 0.12 a,b |
| Organic acids | ||
| Malic acid | 3.92 ± 0.92 a | 3.45 ± 0.81 a,b |
| Succinic acid | 0.87 ± 0.20 a,b | 1.19 ± 0.28 a |
| Fumaric acid | 0.50 ± 0.12 b | 0.94 ± 0.22 a |
| Citric acid | 0.33 ± 0.11 b | 0.51 ± 0.14 a |
| Succinic acid | 0.25 ± 0.08 a | 0.33 ± 0.16 a |
| Quinic acid | 6.64 ± 0.77 a | 3.01 ± 0.45 b |
| Phenolic acids | ||
| Gentisic acid | 0.67 ± 0.16 a | 0.40 ± 0.12 b |
| Neochlorogenic acid | 0.85 ± 0.23 a | 0.58 ± 0.17 b |
| Chlorogenic acid | 3.26 ± 0.90 a | 1.80 ± 0.48 b |
| Caffeic acid | 1.09 ± 0.29 a | 0.38 ± 0.19 b |
| 3-p-Coumaroylquinic acid | 0.78 ± 0.30 a | 0.62 ± 0.15 a,b |
| ρ-Coumaric acid | 0.41 ± 0.08 a | 0.28 ± 0.07 a,b |
| Flavonoids | ||
| Hyperoside (Quercetin 3-galactoside) | 6.74 ± 0.83 a | 4.59 ± 0.71 b |
| Isoquercitrin (Quercetin 3-glucoside) | 1.99 ± 0.29 a | 1.05 ± 0.18 b |
| Quercetin 3-(6″-malonylglucoside) | 1.24 ± 0.46 a | 0.64 ± 0.16 b |
| Rutin (Quercetin 3-rutinoside) | 2.85 ± 0.82 a | 2.00 ± 0.67 a,b |
| Quercetin 3-(6″-acetylglucoside) | 0.96 ± 0.15 a | 0.71 ± 0.22 a,b |
| Kaempherol-3-O-α-rhamnoside | 0.57 ± 0.30 a | 0.30 ± 0.11 b |
| Kaempferol 3-galactoside | 1.48 ± 0.21 a | 1.18 ± 0.24 a,b |
| Kaempferol-3-O-glucoside | 1.19 ± 0.37 a | 0.70 ± 0.29 b |
| Kaempferol-3-O-rutinoside | 0.76 ± 0.29 a | 0.63 ± 0.28 a,b |
| Leaves Sample 1 | Leaves Sample 2 | |
|---|---|---|
| Content ± SD, mg g−1 Extract | Content ± SD, mg g−1 Extract | |
| Fatty acids | ||
| Miristic acid (C14:0) | 5.30 ± 0.72 a | 4.36 ± 0.59 b |
| Oleic acid (C18:1) | 0.56 ± 0.07 b | 3.65 ± 0.49 a |
| Palmitic acid (C16:0) | 3.24 ± 0.44 a,b | 3.96 ± 0.53 a |
| Margaric acid (C17:0) | 11.10 ± 1.50 a | 2.37 ± 0.32 b |
| Linoleic acid (C18:2) | 1.74 ± 0.23 a | 1.07 ± 0.14 b |
| Stearic acid (C18:0) | 1.04 ±0.14 b | 2.13 ± 0.29 a |
| Arachidic acid (C20:0) | 1.47 ± 0.20 b | 2.38 ± 0.32 a |
| Behenic acid (C22:0) | 4.67 ± 0.63 b | 6.17 ± 0.83 a |
| Lignoceric acid (C24:0) | 5.20 ± 0.70 a | 1.31 ± 0.18 b |
| Cerotic acid (C26:0) | 8.32 ± 1.12 a | 2.26 ± 0.30 b |
| Sterols | ||
| β-Amyrin | 1.48 ± 0.20 a | 0.80 ± 0.11 b |
| α-Amyrin | 0.70 ± 0.09 b | 1.15 ± 0.16 a |
| Betulin | 0.82 ± 0.11 b | 2.26 ± 0.31 a |
| β-Sitosterol | 4.99 ± 0.67 b | 7.10 ± 0.96 a |
| Stigmasterol | 3.29 ± 0.44 a | 2.48 ± 0.33 b |
| Leaves Sample 1 | Leaves Sample 2 | |
|---|---|---|
| Content ± SD, mg g−1 Extract | Content ± SD, mg g−1 Extract | |
| Saccharides (mono-, di-) | ||
| Fructose | 15.72 ± 3.68 b | 19.95 ± 4.67 a |
| Glucose | 12.09 ± 2.83 b | 16.54 ± 3.87 a |
| Sucrose | 2.44 ± 0.46 b | 3.13 ± 0.73 a |
| Sugar alcohols | ||
| Myo-Inositol | 30.00 ± 7.02 a | 26.25 ± 6.14 b |
| Sorbitol | 12.90 ± 3.02 a | 10.88 ± 2.55 b |
| Sample | TPC, mg GAE 100 g−1 | TFC, μg QE 100 g−1 | Antioxidant Activities, mM TE 100 g−1 | |||
|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | CuPRAC | |||
| Moringa oleifera leaves (CH3OH), sample 1 | 11.49 ± 0.15 e | 26.83 ± 1.60 f | 142.91 ± 5.14 d | 52.55 ± 0.58 c | 29.46 ± 2.00 b | 80.49 ± 7.36 c |
| Moringa oleifera leaves (50% C2H5OH), sample 1 | 23.06 ± 0.11 a | 87.73 ± 0.50 a | 208.90 ± 9.10 a | 105.46 ± 3.60 a | 44.67 ± 2.50 a | 164.37 ± 8.28 b |
| Moringa oleifera leaves (70% C2H5OH), sample 1 | 22.10 ± 0.14 b | 83.76 ± 1.10 b | 209.46 ± 5.14 a | 92.03 ± 0.29 b | 46.26 ± 1.75 a | 170.22 ± 7.36 a |
| Moringa oleifera leaves (CH3OH), sample 2 | 14.08 ± 0.08 d | 44.79 ± 2.50 e | 108.51 ± 2.37 e | 18.93 ± 0.60 f | 7.56 ± 0.03 d | 50.36 ± 0.10 e |
| Moringa oleifera leaves (50% C2H5OH), sample 2 | 19.61 ± 0.12 c | 77.83 ± 4.03 c | 193.52 ± 3.07 b | 27.76 ± 0.80 d | 14.34 ± 0.06 c | 83.35 ± 0.09 c |
| Moringa oleifera leaves (70% C2H5OH), sample 2 | 19.59 ± 0.11 c | 63.78 ± 1.40 d | 155.49 ± 0.79 c | 23.97 ± 0.57 e | 12.62 ± 0.04 c | 63.58 ± 0.06 d |
| Test Microorganism | Inhibition Zones, mm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf Extracts (10 mg mL−1) Sample 1 | Leaf Extracts (10 mg mL−1) Sample 2 | Controls * (10 mg mL−1) | ||||||||
| CH3OH | 50% C2H5OH | 70% C2H5OH | CH3OH | 50% C2H5OH | 70% C2H5OH | A | P | N | F | |
| Bacillus subtilis ATCC 6633 | - | 16.0 ± 0.0 | 17.0 ± 0.0 | 8.0 ± 0.0 | 12.5 ± 0.7 | 16.0 ± 0.0 | 16.0 ± 0.0 | - | n.a. | n.a. |
| Bacillus cereus NCTC 11145 | - | 14.5 ± 0.7 | 17.0 ± 0.0 | 9.0 ± 0.0 | 13.0 ± 0.0 | 16.0 ± 0.0 | 20.0 ± 0.0 | - | n.a. | n.a. |
| Staphylococcus aureus ATCC 25923 | - | 11.0 ± 0.0 | 13.0 ± 0.0 | - | - | 13.0 ± 0.0 | 35.0 ± 0.0 | 30.0 ± 0.0 | n.a. | n.a. |
| Listeria monocytogenes NBIMCC 8632 | - | 11.0 ± 0.0 | 12.5 ± 0.7 | - | 9.5 ± 0.7 | 12.0 ± 0.0 | 40.0 ± 0.0 | 12.0 ± 0. | n.a. | n.a. |
| Enterococcus faecalis ATCC 29212 | - | 12.0 ± 0.0 | 13.0 ± 0.0 | - | 10.0 ± 0.0 | 12.5 ± 0.7 | 38.0 ± 0.0 | - | n.a. | n.a. |
| Salmonella enteritidis ATCC 13076 | - | 12.0 ± 0.0 | 13.0 ± 0.0 | - | 10.0 ± 0.0 | 12.5 ± 0.7 | 40.0 ± 0.0 | - | n.a. | n.a. |
| Klebsiella pneumoniae ATCC 13883 | - | - | - | - | - | - | 25.0 ± 0.0 | - | n.a. | n.a. |
| Escherichia coli ATCC 25922 | - | 13.0 ± 0.0 | 14.0 ± 0.0 | 9.5 ± 0.7 | 11.0 ± 0.0 | 14.0 ± 0.0 | 16.0 ± 0.0 | - | n.a. | n.a. |
| Proteus vulgaris ATCC 6380 | - | - | - | - | - | - | 30.0 ± 0.0 | - | n.a. | n.a. |
| Pseudomonas aeruginosa ATCC 9027 | 8.0 ± 0.0 | 11.0 ± 0.0 | 13.0 ± 0.0 | 10.0 ± 0.0 | 11.0 ± 0.0 | 14.0 ± 0.0 | 16.0 ± 0.0 | - | n.a. | n.a. |
| Candida albicans NBIMCC 74 | - | - | - | - | - | - | n.a. | n.a. | 22.0 ± 0.0 | - |
| Saccharomyces cerevisiae ATCC 9763 | - | - | - | - | - | - | n.a. | n.a. | 31.0 ± 0.0 | - |
| Aspergillus niger ATCC 1015 | - | - | - | - | - | - | n.a. | n.a. | 32.0 ± 0.0 | 25.0 ± 0.0 |
| Aspergillus flavus | - | - | - | - | - | - | n.a. | n.a. | 26.0 ± 0.0 | 20.0 ± 0.0 |
| Penicillium chrysogenum | - | - | - | - | - | - | n.a. | n.a. | 26.0 ± 0.0 | 13.0 ± 0.0 |
| Fusarium moniliforme ATCC 38932 | - | - | - | - | - | - | n.a. | n.a. | 25.0 ± 0.0 | - |
| Applied Substance | Tonus, mN | Amplitude, mN | Frequency, Number/min | Time for Reaction, min | ACh-Induced SMresponse, % | n |
|---|---|---|---|---|---|---|
| Normal activity | 2.00 ± 0.10 | 2.40 ± 0.18 | 5.00 ± 0.05 | 20.00 ± 0.20 | 100% | 10 |
| Moringa oleifera Sample 1 | 1.88 ± 0.13 | 1.02 ± 0.11 a | 5.10 ± 0.15 | 13.50 ± 0.30 | 134% | 9 |
| Moringa oleifera Sample 2 | 1.90 ± 0.12 | 1.67 ± 0.15 a | 5.0. ± 0.12 | 15.00 ± 0.20 | 111% | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panova, N.; Gerasimova, A.; Tumbarski, Y.; Ivanov, I.; Todorova, M.; Dincheva, I.; Gentscheva, G.; Gledacheva, V.; Slavchev, V.; Stefanova, I.; et al. Metabolic Profile, Antioxidant, Antimicrobial, Contractile, and Anti-Inflammatory Potential of Moringa oleifera Leaves (India). Life 2025, 15, 583. https://doi.org/10.3390/life15040583
Panova N, Gerasimova A, Tumbarski Y, Ivanov I, Todorova M, Dincheva I, Gentscheva G, Gledacheva V, Slavchev V, Stefanova I, et al. Metabolic Profile, Antioxidant, Antimicrobial, Contractile, and Anti-Inflammatory Potential of Moringa oleifera Leaves (India). Life. 2025; 15(4):583. https://doi.org/10.3390/life15040583
Chicago/Turabian StylePanova, Natalina, Anelia Gerasimova, Yulian Tumbarski, Ivan Ivanov, Mina Todorova, Ivayla Dincheva, Galia Gentscheva, Vera Gledacheva, Valeri Slavchev, Iliyana Stefanova, and et al. 2025. "Metabolic Profile, Antioxidant, Antimicrobial, Contractile, and Anti-Inflammatory Potential of Moringa oleifera Leaves (India)" Life 15, no. 4: 583. https://doi.org/10.3390/life15040583
APA StylePanova, N., Gerasimova, A., Tumbarski, Y., Ivanov, I., Todorova, M., Dincheva, I., Gentscheva, G., Gledacheva, V., Slavchev, V., Stefanova, I., Petkova, N., Nikolova, S., & Nikolova, K. (2025). Metabolic Profile, Antioxidant, Antimicrobial, Contractile, and Anti-Inflammatory Potential of Moringa oleifera Leaves (India). Life, 15(4), 583. https://doi.org/10.3390/life15040583













