Effectiveness of Multicomponent Balance Training and Sensorimotor Foot Mobilization on Postural Stability in Patients Following Brain Tumor Surgery
Abstract
1. Introduction
1.1. Acute Rehabilitation Treatment
1.1.1. Multicomponent Balance Training Model (MBE)
1.1.2. Sensorimotor Mobilization and Functional Strengthening of the Feet (SMFE)
2. Materials and Methods
2.1. Assessment Procedures in the Acute Phase of Rehabilitation
2.2. Test Patients
2.3. Implementation
2.4. Statistical Analysis
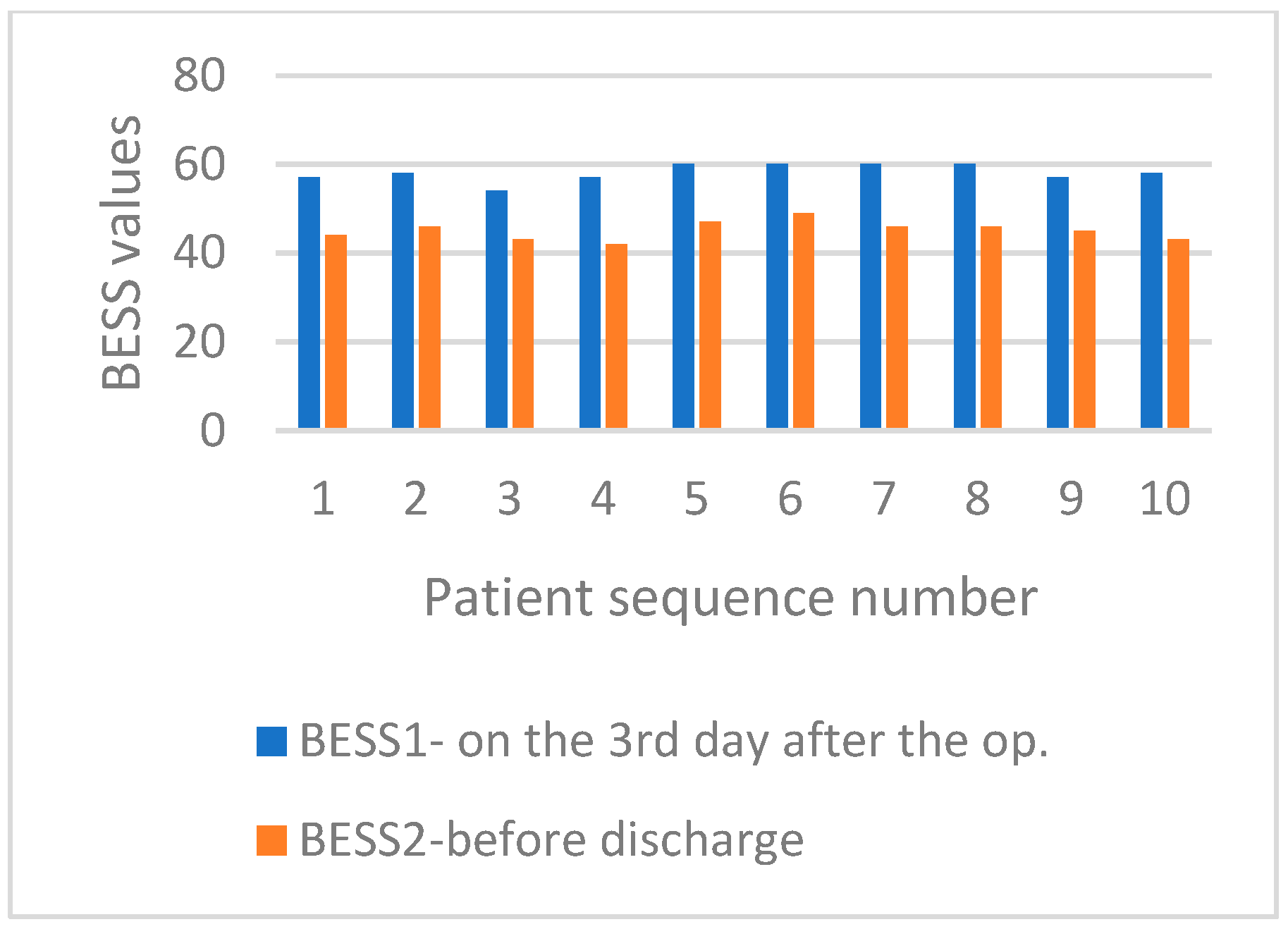
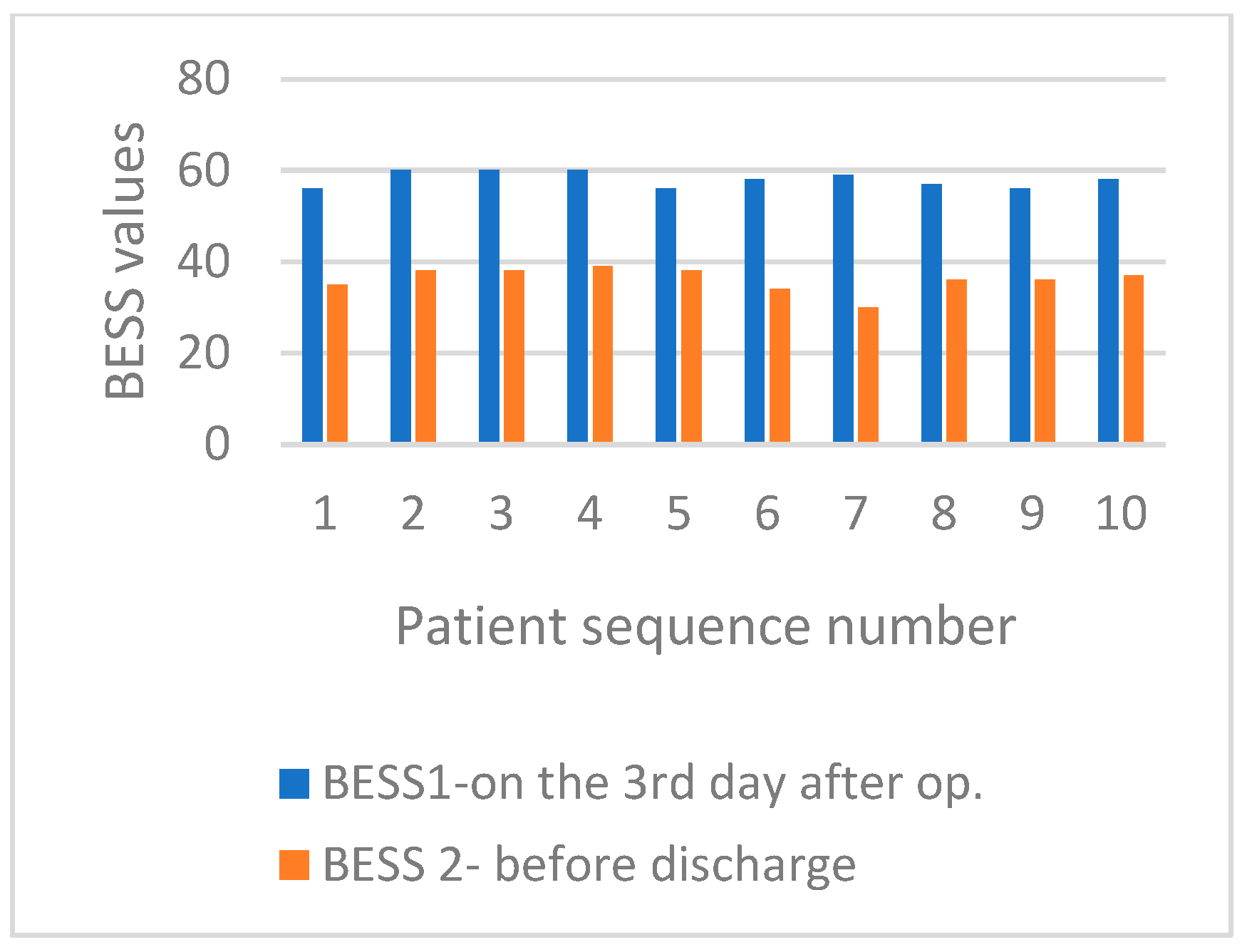
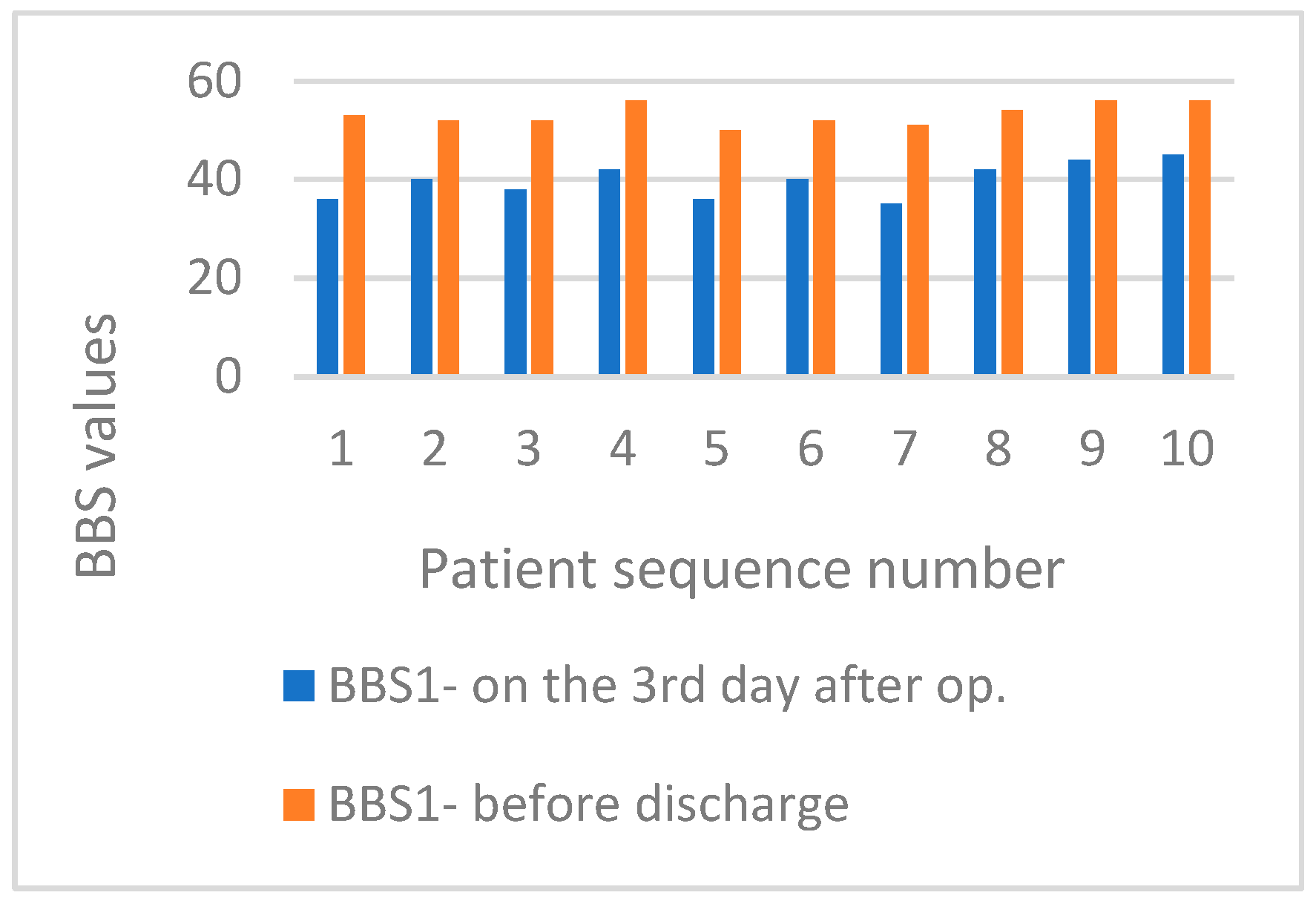
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baldini, A.; Nota, A.; Assi, V.; Ballanti, F.; Cozza, P. Intersession reliability of a posturostabilometric test, using a force platform. J. Electromyogr. Kinesiol. 2013, 23, 1474–1479. [Google Scholar] [PubMed]
- Amiridis, I.G.; Hatzitaki, V.; Arabatzi, F. Age-induced modifications of static postural control in humans. Neurosci. Lett. 2003, 350, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.A.; Black, F.O. The human balance system—A complex coordination of central and peripheral systems. VEDA 2016, 1–5. Available online: https://vestibular.org/sites/default/files/page_files/Documents/Human%20Balance%20System_36.pdf (accessed on 29 March 2025).
- Fransson, P.A.; Gomez, S.; Patel, M.; Johansson, L. Changes in multi-segmented body movements and EMG activity while standing on firm and foam support surfaces. Eur. J. Appl. Physiol. 2007, 101, 81–89. [Google Scholar] [PubMed]
- Mergner, T.; Maurer, C.; Peterka, R.J. A multisensory posture control model of human upright stance. Prog. Brain Res. 2003, 142, 189–201. [Google Scholar]
- Menz, H.B.; Morris, M.E.; Lord, S.R. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1546–1552. [Google Scholar]
- Inglis, J.T.; Kennedy, P.M.; Wells, C.; Chua, R. The role of cutaneous receptors in the foot. Adv. Exp. Med. Biol. 2002, 508, 111–117. [Google Scholar]
- Kelly, L.A.; Kuitunen, S.; Racinais, S.; Cresswell, A.G. Recruitment of the plantar intrinsic foot muscles with increasing postural demand. Clin. Biomech. 2012, 27, 46–51. [Google Scholar]
- Howe, E.E.; Toth, A.J.; Vallis, L.A.; Bent, L.R. Baseline skin information from the foot dorsum controls lower limb kinematics during level walking. Exp. Brain Res. 2015, 233, 2477–2487. [Google Scholar]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24, 1–95. [Google Scholar]
- Westphal, M.; Saladino, A.; Tatagiba, M. Skull base meningiomas. Adv. Exp. Med. Biol. 2023, 1416, 47–68. [Google Scholar] [PubMed]
- Winther, T.L.; Torp, S.H. The significance of the extent of resection in modern neurosurgical practice of WHO grade I meningiomas. World Neurosurg. 2017, 99, 104–110. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Kane, A.J.; Shangari, G.; Rutkowski, M.J.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. The relevance of Simpson Grade I and II resection in the modern neurosurgical treatment of World Health Organization Grade I meningiomas. J. Neurosurg. 2010, 113, 1029–1035. [Google Scholar] [CrossRef]
- Akagami, R.; Napolitano, M.; Sekhar, L.N. Patient-evaluated outcome after surgery for basal meningiomas. Neurosurgery 2002, 50, 941–949. [Google Scholar]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of central nervous system tumors: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Rugelj, D.; Tomšič, M.; Sevšek, F. Effectiveness of multi-component balance specific training on active community-dwelling elderly. HealthMed 2012, 6, 3856–3865. [Google Scholar]
- Ansai, J.; Aurichio, T.R.; Gonçalves, R.; Rebelatto, J.R. Effects of two physical performance related to falls in the oldest old: A randomized controlled trial. Geriatr. Gerontol. Int. 2016, 16, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.E.; Carpenter, M.C.; van der Kooij, H.; Bloem, B.R. The clinical utility of posturography. Clin. Neurophysiol. 2008, 119, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J. The foot is half-dome. Br. Med. J. 1955, 1, 1068–1069. [Google Scholar] [CrossRef]
- Soysa, A.; Hiller, C.; Refshauge, K.; Burns, J. Importance and challenges of measuring intrinsic foot muscle strength. J. Foot Ancle Res. 2012, 5, 29. [Google Scholar] [CrossRef]
- Baker, M.K.; Atlantis, E.; Fiatarone Singh, M.A. Multi-modal exercise programs for older adults. Age Ageing 2007, 36, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Guskiewicz, K.M.; Clark, M.A.; Padua, D.A. A systematic review of the Balance Error Scoring System. Sports Health 2011, 3, 287–295. [Google Scholar] [CrossRef]
- Tolar Rešić, A. Najmanjša klinično pomembna razlika testov in lestvic za oceno izida rehabilitacije. Rehabilitacija 2016, 15, 43–62. [Google Scholar]
- Iverson, G.I.; Koehle, M.S. Normative data for the Balance Error Scoring System in Adults. Rehabil. Res. Pract. 2013, 2013, 846418. [Google Scholar] [CrossRef] [PubMed]
- Cameron, P.W.; Soltero, N.C.; Byers, J. Effects of a 60 Minute on Ice Game Stimulation on the Balance Error Scoring System. Int. J. Exerc. Sci. 2018, 11, 462–467. [Google Scholar] [CrossRef]
- Miyashita, T.L.; Diakogeorgiou, E.; Marrie, K. Correlation of Head Impacts to Change in Balance Error Scoring System Scores in Division I Men’s Lacrosse Players. Sports Health 2017, 9, 318–323. [Google Scholar] [PubMed]
- Vikram, M.; Sundaraganesh, K.; Justine, M.; Kurup, M.; Leonard, J.H. Evaluating postural control impairment using balance error scoring system among athletes with ankle injury: An effective tool in daily practice. Clin. Ter. 2012, 163, 293–297. [Google Scholar]
- Lee, S.M.; Lee, J.H. The immediate effects of ankle balance taping with kinesiology tape on ankle active range of motion and performance in the Balance Error Scoring System. Phys. Ther. Sport. 2016, 25, 99–105. [Google Scholar]
- Susco, T.M.; Valovich McLeod, T.C.; Gansneder, B.M.; Shultz, S.J. Balance recovers within 20 minutes after exertion as measured by the Balance error scoring system. J. Athl. Train. 2004, 39, 241–246. [Google Scholar]
- Wilkins, J.C.; Valovich McLeod, T.; Perrin, D.H.; Gansneder, B.M. Performance on the Balance Error Scoring System decreases after fatigue. J. Athl. Train. 2004, 39, 156–161. [Google Scholar]
- Ozinga, S.J.; Linder, S.M.; Miller Koop, M.; Dey, T.; Figler, R.; Russman, A.N.; So, R.; Rosenthal, A.H.; Cruickshank, J.; Alberts, J.L. Normative Performance on the Balance Error Scoring System by Youth, High School, and Collegiate Athletes. J. Athl. Train. 2018, 53, 636–645. [Google Scholar] [PubMed]
- Amin, D.J.; Coleman, J.; Herrington, L.C. The Test-Retest Reliability and Minimal Detectable Change of the Balance Error Scoring System. J. Sports Sci. 2014, 2, 200–207. [Google Scholar]
- Lima, C.A.; Ricci, N.A.; Nogueira, E.C.; Perracini, M.R. The Berg Balance Scale as a clinical screening tool to predict fall risk in older adults: A systematic review. Physiotherapy 2018, 104, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.; Clark, M.S.; Shipley, S. The Mini-Mental Status Examination. Am. Fam. Physician 2016, 94, 635–641. [Google Scholar] [PubMed]
- Cheung, J.T.; Zhang, M.; An, K.N. Effect of Achilles tendon loading on plantar fascia tension in the standing foot. Clin. Biomech. 2006, 21, 194–203. [Google Scholar]
- Kuo, A.D. An optimal state estimation model of sensory integration in human postural balance. J. Neural Eng. 2005, 2, 235–249. [Google Scholar]
- Natour, J.; Cazotti Lde, A.; Ribeiro, L.H.; Baptista, A.S.; Jones, A. Pilates improves pain, function and quality of life in patients with chronic low back pain: A randomized controlled trial. Clin. Rehabil. 2015, 29, 59–68. [Google Scholar]
- Shojaa, M.; von Stengel, S.; Kohl, M.; Schoene, D.; Kemmler, W. Effects of dynamic resistance exercise on bone mineral density in postmenopausal women: A systematic review and meta-analysis with special emphasis on exercise parameters. Osteoporos. Int. 2020, 31, 1427–1444. [Google Scholar] [CrossRef]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed. Pharmacother. 2020, 131, 110767. [Google Scholar]
- Massion, J. Postural control system. Curr. Opin. Neurobiol. 1994, 4, 877–887. [Google Scholar]
- Balestrucci, P.; Daprati, E.; Lacquaniti, F.; Maffei, V. Effects of visual motion consistent or inconsistent with gravity on postural sway. Exp. Brain Res. 2017, 235, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Assländer, L.; Peterka, R.J. Sensory reweighting dynamics in human postural control. J. Neurophysiol. 2014, 11, 1852–1864. [Google Scholar]
- Vereeck, L.; Wuyts, F.; Truijen, S.; van de Heyning, P. Clinical assessment of balance: Normative data, and gender and age effects. Int. J. Audiol. 2008, 47, 67–75. [Google Scholar] [CrossRef]
- Taube, W. Neurophysiological adaptations in response to balance training. Dtsch. Z. Sportmed. 2012, 63, 373–377. [Google Scholar]
- Taube, W.; Gruber, M.; Beck, S.; Faist, M.; Gollhofer, A.; Schubert, M. Cortical and spinal adaptations induced by balance training: Correlation between stance stability and corticospinal activation. Acta Physiol. 2007, 189, 347–358. [Google Scholar] [CrossRef]
- Floyer-Lea, A.; Matthews, P.M. Changing brain networks for visuomotor control with increased movement automaticity. J. Neurophysiol. 2004, 92, 2405–2412. [Google Scholar]
- Granacher, U.; Gruber, M.; Golhofer, A. Auswirkungen von sensomotorischen Training auf die posturale Kontrolle älterer Männer. Dtsch. Z. Sportmed. 2009, 60, 387–393. [Google Scholar]
- Shubert, T.E. Evidence-based exercise prescription for balance and falls prevention: A current review of the literature. J. Geriatr. Phys. Ther. 2011, 34, 100–108. [Google Scholar]
- Avelar, B.P.; Costa, J.N.; Safons, M.P.; Dutra, M.T.; Bottaro, M.; Gobbi, S.; Tiedemann, A.; de David, A.C.; Lima, R.M. Balance exercises circuit improves muscle strength, balance, and functional performance in older women. Age 2016, 38, 14. [Google Scholar] [CrossRef]
- Mohapatra, S.; Kukkar, K.K.; Aruin, A.S. Support surface-related changes in feedforward and feedback control of standing posture. J. Electromyogr. Kinesiol. 2014, 24, 144–152. [Google Scholar]
- Tortolero, X.; Masani, K.; Maluly, C.; Popovic, M.R. Body movement induced by electrical stimulation of toe muscles during standing. Artif. Organs 2008, 32, 5–12. [Google Scholar] [PubMed]
- Saeki, J.; Tojima, M.; Torii, S. Clarification of functional differences between the hallux and lesser toes during the single leg stance: Immediate effects of conditioning contraction of the toe plantar flexion muscles. J. Phys. Ther. Sci. 2015, 27, 2701–2704. [Google Scholar]
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 2015, 49, 290. [Google Scholar]
- Mulligan, I.J.; Boland, M.A.; McIlhenny, C.V. The Balance Error Scoring System Learned Response Among Young Adults. Sports Health 2013, 5, 22–26. [Google Scholar]
- DeFord, S.M.; Wilson, M.S.; Rice, A.C.; Clausen, T.; Rice, L.K.; Barabnova, A.; Bullock, R.; Hamm, R.J. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J. Neurotrauma 2002, 19, 427–438. [Google Scholar] [PubMed]
- Horak, F.B.; Hlavacka, F. Somatosensory loss increases vestibulospinal sensitivity. J. Neurophysiol. 2001, 86, 575–585. [Google Scholar] [PubMed]
- Linder, S.M.; Ozinga, S.J.; Koop, M.M.; Dey, T.; Figler, R.; Cruickshank, J.; Alberts, J.L. Cleveland Clinic Postural Stability Index Norms for the Balance Error Scoring System. Med. Sci. Sports Exerc. 2018, 50, 1998–2006. [Google Scholar]
- Hunt, T.N.; Ferrara, M.S.; Bornstein, R.; Baumgartner, T.A. The reliability of the modified Balance Error Scoring System. Clin. J. Sport. Med. 2009, 19, 471–475. [Google Scholar]
| Assessed for Eligibility (n = 45) | ||
| Enrollment | Excluded (n = 25): Not meeting inclusion criteria (n = 11) Declined to participate (n = 9) Other reasons (n = 5) | |
| Randomized (n = 20) | ||
| Allocation | Allocated to MBE intervention (n = 10) Received allocated MBE intervention (n = 10) Did not receive allocated MBE intervention (give reasons) (n = 0) | Allocated to SMFE intervention (n = 10) Received allocated SMFE intervention (n = 10) Did not receive allocated SMFE intervention (give reasons) (n = 0) |
| Follow-up | Lost to follow-up MBE (give reasons) (n = 0) Discontinued MBE intervention (give reasons) (n = 0) | Lost to follow-up SMFE (give reasons) (n = 0) Discontinued SMFE intervention (give reasons) (n = 0) |
| Analysis | Analyzed MBE (n = 10) Excluded from analysis MBE (give reasons) (n = 0) | Analyzed SMFE (n = 10) Excluded from analysis SMFE (give reasons) (n = 0) |
| Multicomponent Balance Exercises | Various Balance Components with an Impact on Different Sensorimotor Systems |
|---|---|
| Sit down and stand up five times with open and closed eyes. | Stability during movement in the vertical and horizontal planes increases the proprioceptive response of the body. |
| Stand with feet together on a hard surface. Close and open your eyes five times for 10 s each. | A change in the size of the support surface maintains the body’s center of gravity and increases the body’s proprioceptive response. |
| Stand on a soft surface with feet together. Close and open your eyes five times for 10 s each. | Stabilization of posture in case of altered quantity and quality of proprioceptive stimuli, as well as proactive and reactive responsiveness of the body. |
| Stand with feet together on a hard surface. Turn your head, eyes, and torso in each direction five times. Repeat with closed eyes. | Changing direction, vestibulocochlear component, reactive response of the body, attention to the task, and increased conscious training of the body. |
| Stand with your feet together on a soft surface with your eyes open. Turn your eyes, head, and torso in each direction five times. You can repeat with closed eyes. | Changing direction, vestibulocochlear component, reactive responsiveness of the body, focus on the task, and the proprioceptive response of the body. |
| Stand on a hard surface and walk in place while alternating between having your eyes open and closed. | Dynamic body stability, body position control, and reactive/proactive responsiveness. |
| Walk in place on a soft surface with open and closed eyes. | Maintenance and dynamic stabilization of the body’s center of gravity, proprioceptive proactive/reactive responsiveness. |
| Walk up and down the stairs with eyes open and closed. | Muscle performance, depth and distance perception, standing leg stabilization, proprioceptive response. |
| Stand on one leg while balancing on hard and soft ground with your eyes open. | Focus, dynamic stabilization of the body and standing legs, proprioceptive response, muscle strength and endurance, proactive/reactive responsiveness. |
| Tandem stands on hard and soft ground with open and closed eyes. | Attention to task, static and dynamic stabilization, muscle endurance, and proprioceptive response. |
| Tandem stands with eyes, head, and torso turning in both directions on hard and soft ground with eyes open and closed. | Proprioceptive responsiveness, divided attention, proactive/reactive responsiveness, vestibuloocular component, dynamic stabilization. |
| Walk straight while turning your head left and right with your eyes open/closed. | Vestibuloocular component, proactive/reactive component, dynamic body stabilization. |
| Walk on your toes on hard and soft surfaces with open and closed eyes. | Muscle length, passive mobility, muscle endurance, dynamic body control, and proprioceptive body response. |
| Walk on heels on hard and soft ground with eyes open and closed. | Muscle length, passive range of motion, muscle endurance, dynamic body control, and proprioceptive response. |
| Polygon for practicing balance. | Avoid obstacles, change direction, sense height, depth, and ground characteristics, and be proactive or reactive. |
| Balance Exercises with an Emphasis on Foot Muscle Activity | Balance Components with an Impact on Different Body Systems |
|---|---|
| While in a split stance, lift and spread your toes. Perform on hard or soft surfaces with eyes open or closed. | Proprioceptive response, passive mobility, muscle length, strength and endurance, and dynamic body stabilization. |
| While in a split position, curl your toes down and hold on hard and soft surfaces with eyes open and closed. | Proprioceptive response, muscle mobility and length, muscle performance, and body control. |
| Stand in a split position and squeeze your big toe and your other toes together. Make sure not to contract them inward and downward. You can perform the exercise on a hard or soft surface with open or closed eyes. | Dynamic stabilization of the position of the body and lower limbs, muscle performance, and proprioceptive response. |
| While standing in a split, lift the big toe and hold it up while pressing the other toes against the base. Do the exercise on hard and soft surfaces, with eyes open and closed. | Motorically demanding components include flexibility, muscle length and strength, dynamic body stabilization, and proprioceptive response. |
| Press the big toe to the floor while lifting the other toes. Hold the position on hard and soft surfaces with eyes open and closed. | Motor skill, flexibility, endurance, muscle capacity, proprioceptive response, dynamic body stabilization. |
| Lift your toes off the ground while standing in a split. Hold them and lower them from the little toe to the big toe. Perform the exercise on hard and soft surfaces with eyes both closed and open. | Motor skills, muscle length, attention to task, proprioceptive response, and dynamic body stabilization. |
| While standing in a split, rise onto your toes and hold. Perform the exercise on hard and soft surfaces with open and closed eyes. | Dynamic stabilization of the body, proprioceptive response, muscle strength and endurance, flexibility, and muscle length. |
| While standing in a split position, rise on your heels and hold. Perform the exercise on hard and soft surfaces with open and closed eyes. | Dynamic stabilization of the body and limbs, proprioceptive response, mobility, and muscle length. |
| Practice walking on hard and soft ground, forwards, backward, and sideways. Do this with your eyes open and closed while walking on your toes. | Dynamic stabilization of body and limbs, proprioceptive response, proactive/reactive component. |
| On hard and soft ground, with eyes open and closed, practice walking forward, backward, and sideways on your heels. | Dynamic balance, proprioceptive component, proactive/reactive component. |
| While walking, try to alternate between hard and soft ground, and practice it with open and closed eyes. | Increased responsiveness to external stimuli, proprioceptive response, and focus on the task. |
| Practice lunges on varying surfaces with open and closed eyes while walking. | Attention to the task, muscle strength and endurance, dynamic stabilization of the body, and proprioceptive response of the body. |
| When walking on hard and soft ground, try to walk on the outer edge of your foot with your eyes open and closed. | Strengthening the foot’s arch, flexibility, dynamic stabilization of the trunk and limbs, and proprioceptive response. |
| When walking on hard and soft ground, walk on the inner edge of your foot with both your eyes open and closed. | Strengthening the foot arch, dynamic control of the body and limbs, and conscious perception. |
| Stand on the edge of the stairs, rise slowly, hold, then lower and hold again. | Length and strength of muscles, mobility, agility, and dynamic control of the body and limbs in the vertical direction of movement. |
| Interventions | Age (years) | Height (cm) | Body Mass (kg) | BMI | |
|---|---|---|---|---|---|
| MBE group | M ± SD | 30.20 ± 9.841 | 175.00 ± 10.541 | 70.50 ± 11.721 | 22.70 ± 3.020 |
| Min–Max | 20–46 | 162–198 | 55–89 | 19–27 | |
| SMFE group | M ± SD | 34.50 ± 8.606 | 173.50 ± 10.680 | 65.90 ± 11.770 | 21.70 ± 1.829 |
| Min–Max | 23–47 | 162–192 | 53–85 | 18–24 | |
| Intervention | M-W | p | |
|---|---|---|---|
| BESS1 | MBE group | 45.50 | 0.726 |
| SMFE group | |||
| BESS2 | MBE group | 12.50 | 0.000 |
| SMFE group | |||
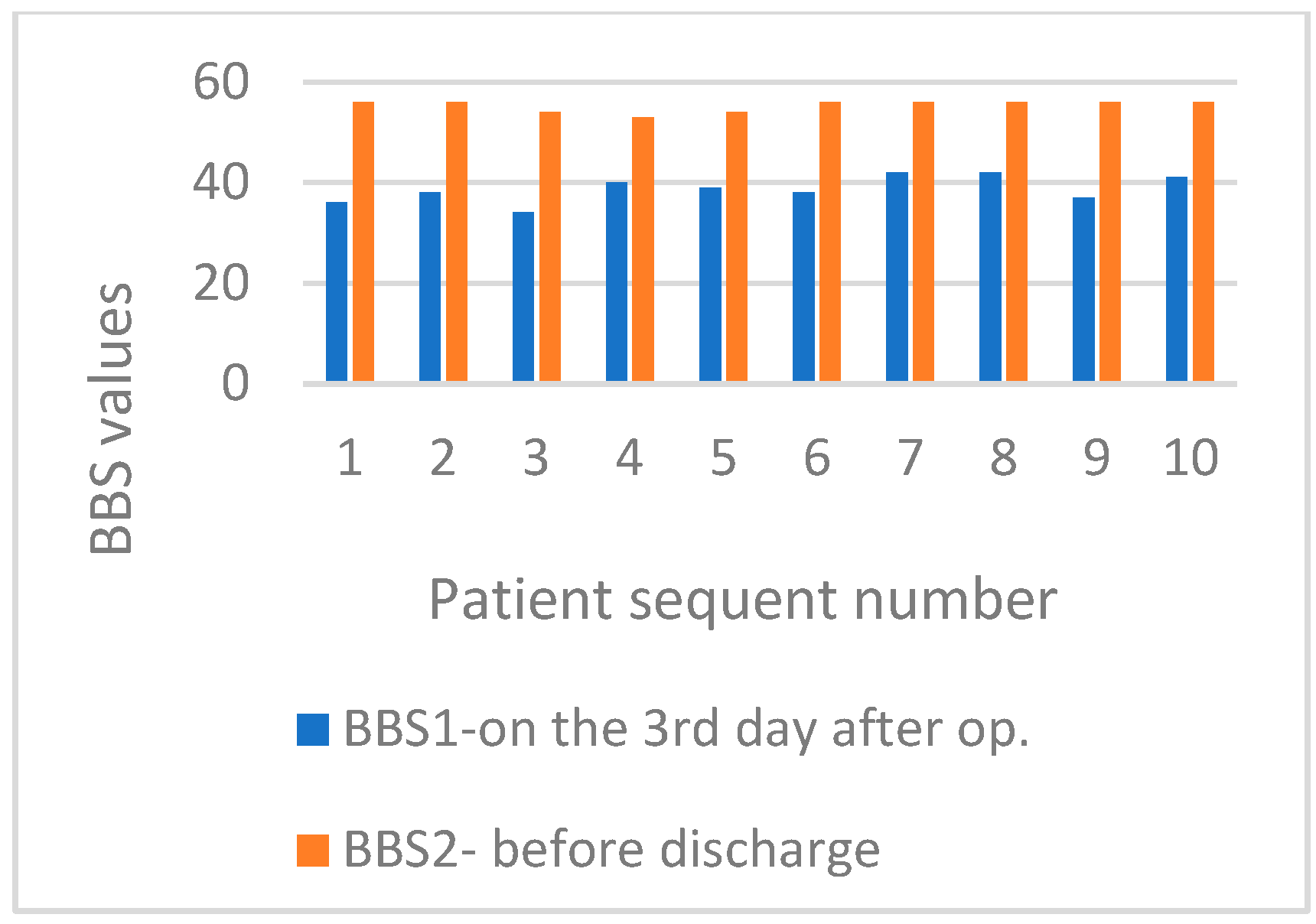
| BBS1 | MBE group | 41.00 | 0.493 |
| SMFE group | |||
| BBS2 | MBE group | 17.50 | 0.012 |
| SMFE group |
| BESS1 | BESS2 | ||
|---|---|---|---|
| BBS1 | rs | −0.075 | 0.006 |
| p | 0.753 | 0.981 | |
| BBS2 | rs | −0.273 | −0.769 |
| p | 0.245 | 0.000 | |
| Intervention Group | M-W | p | ||
|---|---|---|---|---|
| A hard support | Feet together | MBE group | 7.50 | 0.000 |
| SMFE group | ||||
| One leg stand | MBE group | 18.00 | 0.006 | |
| SMFE group | ||||
| Tandem stand | MBE group | 24.00 | 0.035 | |
| SMFE group | ||||
| A soft support | Feet together | MBE group | 1.50 | 0.000 |
| SMFE group | ||||
| One leg stand | MBE group | 35.00 | 0.068 | |
| SMFE group | ||||
| Tandem stand | MBE group | 25.00 | 0.013 | |
| SMFE group | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, N.; Brcar, M.; Brcar, M.; Velnar, T. Effectiveness of Multicomponent Balance Training and Sensorimotor Foot Mobilization on Postural Stability in Patients Following Brain Tumor Surgery. Life 2025, 15, 579. https://doi.org/10.3390/life15040579
Kos N, Brcar M, Brcar M, Velnar T. Effectiveness of Multicomponent Balance Training and Sensorimotor Foot Mobilization on Postural Stability in Patients Following Brain Tumor Surgery. Life. 2025; 15(4):579. https://doi.org/10.3390/life15040579
Chicago/Turabian StyleKos, Natasa, Marusa Brcar, Marko Brcar, and Tomaz Velnar. 2025. "Effectiveness of Multicomponent Balance Training and Sensorimotor Foot Mobilization on Postural Stability in Patients Following Brain Tumor Surgery" Life 15, no. 4: 579. https://doi.org/10.3390/life15040579
APA StyleKos, N., Brcar, M., Brcar, M., & Velnar, T. (2025). Effectiveness of Multicomponent Balance Training and Sensorimotor Foot Mobilization on Postural Stability in Patients Following Brain Tumor Surgery. Life, 15(4), 579. https://doi.org/10.3390/life15040579





