Optimizing Revascularization in Ischemic Cardiomyopathy: Comparative Evidence on the Benefits and Indications of CABG and PCI
Abstract
1. Introduction
2. Pathophysiology and Rationale for Revascularization
2.1. Mechanisms of Ischemic Left Ventricular Dysfunction
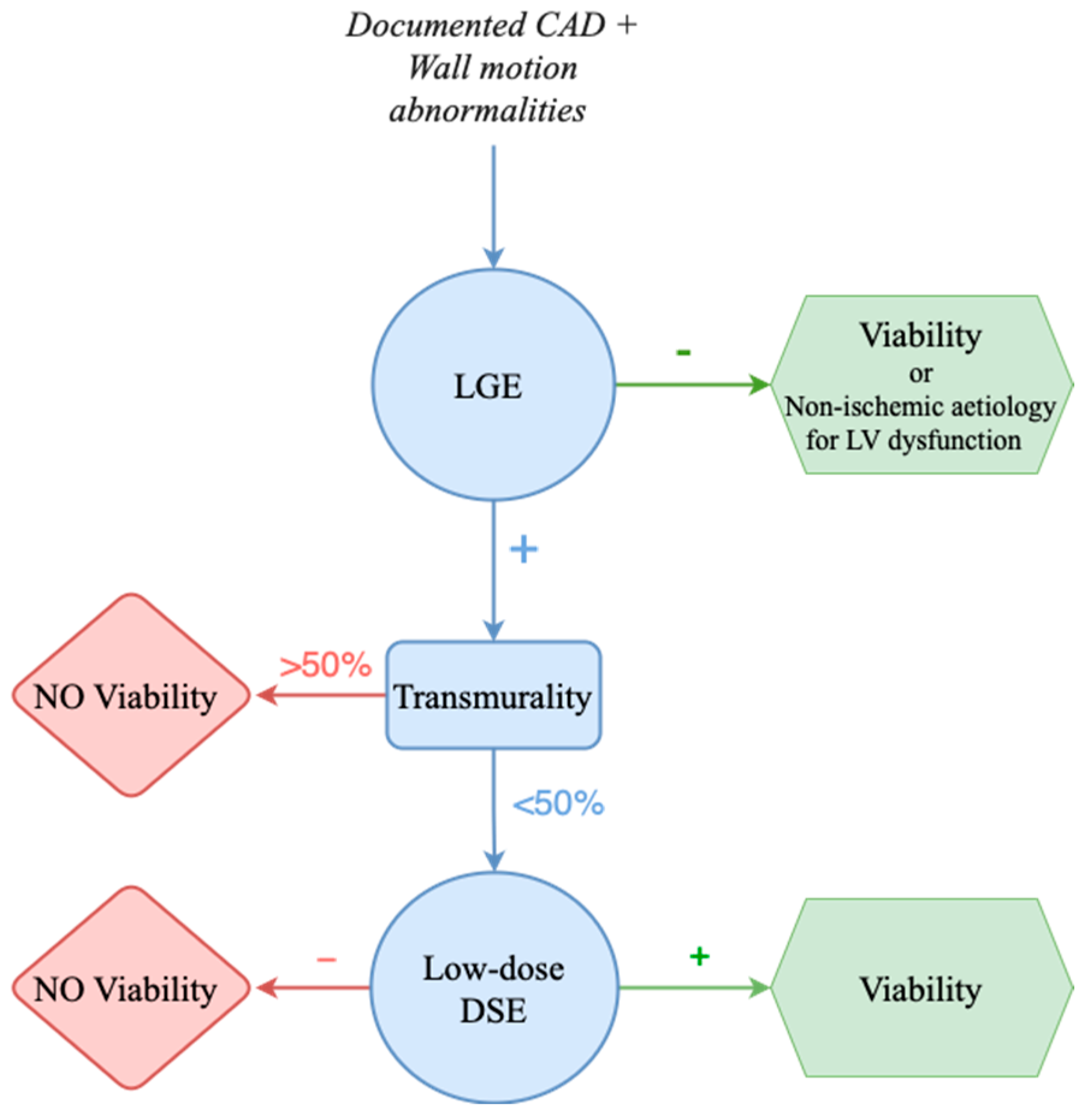
2.2. Myocardial Viability and Its Role in Decision-Making
2.3. Potential Benefits of Revascularization
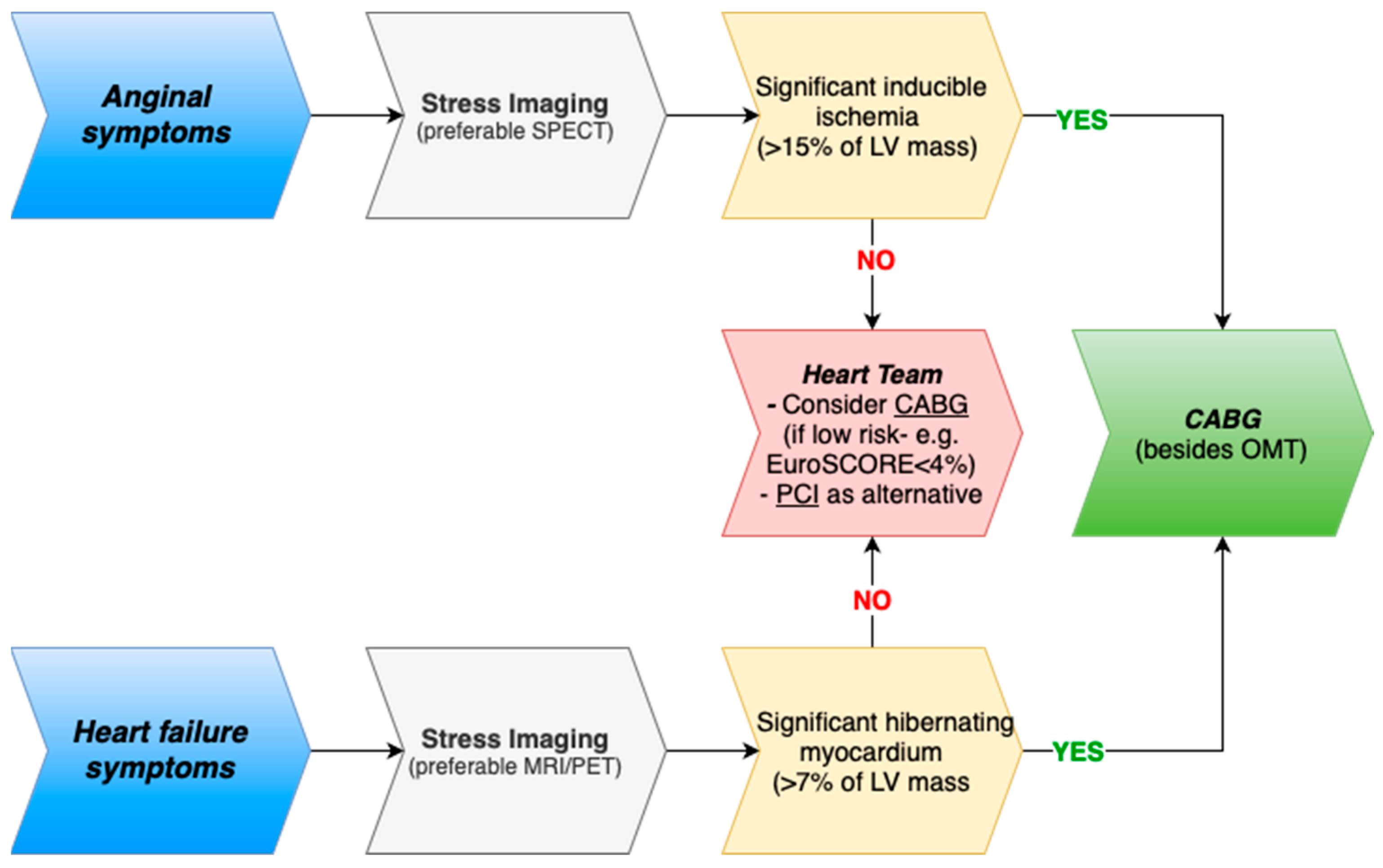
3. Evidence for Revascularization in Ischemic Cardiomyopathy: CABG and PCI
3.1. Evidence for CABG in Left Ventricular Dysfunction
3.1.1. Historical Perspective
3.1.2. How Beneficial Is CABG Really?
3.2. Evidence for PCI in Left Ventricular Dysfunction
3.2.1. Prior Evidence Base for PCI
3.2.2. The REVIVED-BCIS2 Trial
3.2.3. Strengths and Limitations of PCI and of the REVIVED-BCIS2 Trial
4. Comparative Outcomes: PCI Versus CABG
4.1. But What Do the Guidelines Say?
4.2. Current Challenges and Unresolved Questions
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme [inhibitors] |
| ACS | Acute Coronary Syndrome |
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| AI | Artificial Intelligence |
| ARB | Angiotensin II Receptor Blockers |
| ARNI | Angiotensin Receptor-Neprilysin Inhibitors |
| BCIS JS | British Cardiovascular Intervention Society Jeopardy Score |
| CABG | Coronary Artery Bypass Grafting |
| CAD | Coronary Artery Disease |
| CCS | Canadian Cardiovascular Society |
| cMRI | Cardiac Magnetic Resonance Imaging |
| CTO | Chronic Total Occlusion |
| CV | Cardiovascular |
| DSE | Dobutamine Stress Echocardiography |
| EF | Ejection Fraction |
| ESC | European Society of Cardiology |
| EACTS | European Association for Cardio-Thoracic Surgery |
| FDG | Fluorodeoxyglucose |
| FFR | Fractional Flow Reserve |
| HCR | Hybrid Coronary Revascularization |
| HF | Heart Failure |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| ICM | Ischemic Cardiomyopathy |
| IVUS | Intravascular Ultrasound |
| LAD | Left Anterior Descending Artery |
| LGE | Late Gadolinium Enhancement |
| LVD | Left Ventricular Dysfunction |
| LVEF | Left Ventricular Ejection Fraction |
| LV | Left Ventricle |
| MACE | Major Adverse Cardiovascular Events |
| MCS | Mechanical Circulatory Support |
| MI | Myocardial Infarction |
| ML | Machine Learning |
| MVD | Multivessel Disease |
| NYHA | New York Heart Association |
| OCT | Optical Coherence Tomography |
| OMT | Optimal Medical Therapy |
| PET | Positron Emission Tomography |
| PCI | Percutaneous Coronary Intervention |
| REVIVED-BCIS2 | Revascularization for Ischemic Ventricular Dysfunction—Study of Efficacy and Safety of Percutaneous Coronary Intervention to Improve Survival in Heart Failure |
| SCARR | Swedish Coronary Angiography and Angioplasty Registry |
| SCAI | Society for Cardiovascular Angiography and Interventions |
| SPECT | Single Photon Emission Computed Tomography |
| STICH | Surgical Treatment for Ischemic Heart Failure |
| STS | Society of Thoracic Surgeons |
| SGLT2 | Sodium–Glucose Cotransporter 2 |
References
- Gheorghiade, M.; Sopko, G.; De Luca, L.; Velazquez, E.J.; Parker, J.D.; Binkley, P.F.; Sadowski, Z.; Golba, K.S.; Prior, D.L.; Rouleau, J.L.; et al. Navigating the Crossroads of Coronary Artery Disease and Heart Failure. Circulation 2006, 114, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.A.; Zaccardi, F.; Squire, I.; Ling, S.; Davies, M.J.; Lam, C.S.P.; Mamas, M.A.; Khunti, K.; Kadam, U.T. 20-Year Trends in Cause-Specific Heart Failure Outcomes by Sex, Socioeconomic Status, and Place of Diagnosis: A Population-Based Study. Lancet Public Health 2019, 4, e406–e420. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The Global Health and Economic Burden of Hospitalizations for Heart Failure. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Albakri, A. Ischemic Cardiomyopathy: A Review of Literature on Clinical Status and Meta-Analysis of Diagnostic and Clinical Management. Biol. Eng. Med. 2018, 3, 1–13. [Google Scholar] [CrossRef]
- Bakaeen, F.G.; Gaudino, M.; Whitman, G.; Doenst, T.; Ruel, M.; Taggart, D.P.; Stulak, J.M.; Benedetto, U.; Anyanwu, A.; Chikwe, J.; et al. 2021: The American Association for Thoracic Surgery Expert Consensus Document: Coronary Artery Bypass Grafting in Patients with Ischemic Cardiomyopathy and Heart Failure. J. Thorac. Cardiovasc. Surg. 2021, 162, 829–850.e1. [Google Scholar] [CrossRef]
- Allman, K.C.; Shaw, L.J.; Hachamovitch, R.; Udelson, J.E. Myocardial Viability Testing and Impact of Revascularization on Prognosis in Patients with Coronary Artery Disease and Left Ventricular Dysfunction: A Meta-Analysis. J. Am. Coll. Cardiol. 2002, 39, 1151–1158. [Google Scholar] [CrossRef]
- Sun, L.Y.; Gaudino, M.; Chen, R.J.; Bader Eddeen, A.; Ruel, M. Long-Term Outcomes in Patients With Severely Reduced Left Ventricular Ejection Fraction Undergoing Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting. JAMA Cardiol. 2020, 5, 631–641. [Google Scholar] [CrossRef]
- Spadaccio, C.; Benedetto, U. Coronary Artery Bypass Grafting (CABG) vs. Percutaneous Coronary Intervention (PCI) in the Treatment of Multivessel Coronary Disease: Quo Vadis?—A Review of the Evidences on Coronary Artery Disease. Ann. Cardiothorac. Surg. 2018, 7, 506–515. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Schäfer, A.; Alasnag, M.; Giacoppo, D.; Collet, C.; Rudolph, T.K.; Roguin, A.; Buszman, P.P.; Colleran, R.; Stefanini, G.; Lefevre, T.; et al. High-Risk Percutaneous Coronary Intervention in Patients with Reduced Left Ventricular Ejection Fraction Deemed Not Suitable for Surgical Revascularisation. A Clinical Consensus Statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in Collaboration with the ESC Working Group on Cardiovascular Surgery. EuroIntervention 2025, 21, 22–34. [Google Scholar] [CrossRef]
- Shields, M.C.; Ouellette, M.; Kiefer, N.; Kohan, L.; Taylor, A.M.; Ailawadi, G.; Ragosta, M. Characteristics and Outcomes of Surgically Ineligible Patients with Multivessel Disease Treated with Percutaneous Coronary Intervention. Catheter. Cardiovasc. Interv. 2021, 98, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Ross, J. Myocardial Perfusion-Contraction Matching. Implications for Coronary Heart Disease and Hibernation. Circulation 1991, 83, 1076–1083. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The Pathophysiology of Acute Myocardial Infarction and Strategies of Protection beyond Reperfusion: A Continual Challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Dilsizian, V.; Curiel, R.V.; Unger, E.F.; Laurienzo, J.M.; Kitsiou, A.N. Myocardial Blood Flow at Rest and Contractile Reserve in Patients with Chronic Coronary Artery Disease and Left Ventricular Dysfunction. J. Nucl. Cardiol. 1999, 6, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Ellis, A.M.; Al-Khalidi, H.R.; Holly, T.A.; Berman, D.S.; Oh, J.K.; Pohost, G.M.; Sopko, G.; Chrzanowski, L.; Mark, D.B.; et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N. Engl. J. Med. 2019, 381, 739–748. [Google Scholar] [CrossRef]
- Hoole, S.P.; Heck, P.M.; White, P.A.; Read, P.A.; Khan, S.N.; West, N.E.J.; O’Sullivan, M.; Dutka, D.P. Stunning and Cumulative Left Ventricular Dysfunction Occurs Late After Coronary Balloon Occlusion in Humans. JACC Cardiovasc. Interv. 2010, 3, 412–418. [Google Scholar] [CrossRef]
- Ladwiniec, A.; White, P.A.; Nijjer, S.S.; O’Sullivan, M.; West, N.E.J.; Davies, J.E.; Hoole, S.P. Diastolic Backward-Traveling Decompression (Suction) Wave Correlates With Simultaneously Acquired Indices of Diastolic Function and Is Reduced in Left Ventricular Stunning. Circ. Cardiovasc. Interv. 2016, 9, e003779. [Google Scholar] [CrossRef]
- Schelbert, E.B.; Cao, J.J.; Sigurdsson, S.; Aspelund, T.; Kellman, P.; Aletras, A.H.; Dyke, C.K.; Thorgeirsson, G.; Eiriksdottir, G.; Launer, L.J.; et al. Prevalence and Prognosis of Unrecognized Myocardial Infarction Determined by Cardiac Magnetic Resonance in Older Adults. JAMA 2012, 308, 890. [Google Scholar] [CrossRef]
- Sutton, M.G.S.J.; Sharpe, N. Left Ventricular Remodeling After Myocardial Infarction. Circulation 2000, 101, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Briceno, N.; Schuster, A.; Lumley, M.; Perera, D. Ischaemic Cardiomyopathy: Pathophysiology, Assessment and the Role of Revascularisation. Heart 2016, 102, 397–406. [Google Scholar] [CrossRef]
- Schuster, A.; Morton, G.; Chiribiri, A.; Perera, D.; Vanoverschelde, J.-L.; Nagel, E. Imaging in the Management of Ischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2012, 59, 359–370. [Google Scholar] [CrossRef]
- Nagel, E.; Schuster, A. Shortening Without Contraction: New Insights Into Hibernating Myocardium. JACC Cardiovasc. Imaging 2010, 3, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Nihoyannopoulos, P.; Vanoverschelde, J.L. Myocardial Ischaemia and Viability: The Pivotal Role of Echocardiography. Eur. Heart J. 2011, 32, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Prasad, S.K.; Rimoldi, O.E. Stunning, Hibernation, and Assessment of Myocardial Viability. Circulation 2008, 117, 103–114. [Google Scholar] [CrossRef]
- Nagel, E.; Schuster, A. Myocardial Viability. JACC Cardiovasc. Imaging 2012, 5, 509–512. [Google Scholar] [CrossRef]
- Romero, J.; Xue, X.; Gonzalez, W.; Garcia, M.J. CMR Imaging Assessing Viability in Patients With Chronic Ventricular Dysfunction Due to Coronary Artery Disease. JACC Cardiovasc. Imaging 2012, 5, 494–508. [Google Scholar] [CrossRef]
- Chareonthaitawee, P.; Gersh, B.J.; Araoz, P.A.; Gibbons, R.J. Revascularization in Severe Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2005, 46, 567–574. [Google Scholar] [CrossRef]
- Bonow, R.O.; Maurer, G.; Lee, K.L.; Holly, T.A.; Binkley, P.F.; Desvigne-Nickens, P.; Drozdz, J.; Farsky, P.S.; Feldman, A.M.; Doenst, T.; et al. Myocardial Viability and Survival in Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1617–1625. [Google Scholar] [CrossRef]
- Ypenburg, C.; Schalij, M.J.; Bleeker, G.B.; Steendijk, P.; Boersma, E.; Dibbets-Schneider, P.; Stokkel, M.P.M.; van der Wall, E.E.; Bax, J.J. Impact of Viability and Scar Tissue on Response to Cardiac Resynchronization Therapy in Ischaemic Heart Failure Patients. Eur. Heart J. 2006, 28, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, K.; Nührenberg, T.G.; Toma, A.; Gick, M.; Ferenc, M.; Hochholzer, W.; Comberg, T.; Rothe, J.; Valina, C.M.; Löffelhardt, N.; et al. A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion. JACC Cardiovasc. Interv. 2018, 11, 1982–1991. [Google Scholar] [CrossRef]
- Carson, P.; Wertheimer, J.; Miller, A.; O’Connor, C.M.; Pina, I.L.; Selzman, C.; Sueta, C.; She, L.; Greene, D.; Lee, K.L.; et al. The STICH Trial (Surgical Treatment for Ischemic Heart Failure). JACC Heart Fail. 2013, 1, 400–408. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; Petrie, M.C.; Greenwood, J.P.; O’Kane, P.D.; Evans, R.; Sculpher, M.; Mcdonagh, T.; Gershlick, A.; de Belder, M.; et al. Percutaneous Revascularization for Ischemic Ventricular Dysfunction: Rationale and Design of the REVIVED-BCIS2 Trial. JACC Heart Fail. 2018, 6, 517–526. [Google Scholar] [CrossRef]
- Meluzín, J.; Černý, J.; Špinarová, L.; Toman, J.; Groch, L.; Štětka, F.; Frélich, M.; Hude, P.; Krejčí, J.; Rambousková, L.; et al. Prognosis of Patients with Chronic Coronary Artery Disease and Severe Left Ventricular Dysfunction. The Importance of Myocardial Viability. Eur. J. Heart Fail. 2003, 5, 85–93. [Google Scholar] [CrossRef]
- Sawada, S.G.; Dasgupta, S.; Nguyen, J.; Lane, K.A.; Gradus-Pizlo, I.; Mahenthiran, J.; Feigenbaum, H. Effect of Revascularization on Long-Term Survival in Patients With Ischemic Left Ventricular Dysfunction and a Wide Range of Viability. Am. J. Cardiol. 2010, 106, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Alderman, E.L.; Fisher, L.D.; Litwin, P.; Kaiser, G.C.; Myers, W.O.; Maynard, C.; Levine, F.; Schloss, M. Results of Coronary Artery Surgery in Patients with Poor Left Ventricular Function (CASS). Circulation 1983, 68, 785–795. [Google Scholar] [CrossRef]
- Beanlands, R.S.B.; Nichol, G.; Huszti, E.; Humen, D.; Racine, N.; Freeman, M.; Gulenchyn, K.Y.; Garrard, L.; deKemp, R.; Guo, A.; et al. F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients With Severe Left Ventricular Dysfunction and Suspected Coronary Disease. J. Am. Coll. Cardiol. 2007, 50, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Calvert, M.; Freemantle, N.; Arrow, Y.; Ball, S.G.; Bonser, R.S.; Chattopadhyay, S.; Norell, M.S.; Pennell, D.J.; Senior, R. The Heart Failure Revascularisation Trial (HEART). Eur. J. Heart Fail. 2011, 13, 227–233. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; O’Connor, C.M.; Oh, J.K.; Bonow, R.O.; Pohost, G.M.; Feldman, A.M.; Mark, D.B.; Panza, J.A.; Sopko, G.; et al. The Rationale and Design of the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. J. Thorac. Cardiovasc. Surg. 2007, 134, 1540–1547.e4. [Google Scholar] [CrossRef]
- Wrobel, K.; Stevens, S.R.; Jones, R.H.; Selzman, C.H.; Lamy, A.; Beaver, T.M.; Djokovic, L.T.; Wang, N.; Velazquez, E.J.; Sopko, G.; et al. Influence of Baseline Characteristics, Operative Conduct, and Postoperative Course on 30-Day Outcomes of Coronary Artery Bypass Grafting Among Patients With Left Ventricular Dysfunction. Circulation 2015, 132, 720–730. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Velazquez, E.J.; She, L.; Smith, P.K.; Nicolau, J.C.; Favaloro, R.R.; Gradinac, S.; Chrzanowski, L.; Prabhakaran, D.; Howlett, J.G.; et al. Extent of Coronary and Myocardial Disease and Benefit From Surgical Revascularization in LV Dysfunction. J. Am. Coll. Cardiol. 2014, 64, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Holly, T.A.; Asch, F.M.; She, L.; Pellikka, P.A.; Velazquez, E.J.; Lee, K.L.; Borges-Neto, S.; Farsky, P.S.; Jones, R.H.; et al. Inducible Myocardial Ischemia and Outcomes in Patients With Coronary Artery Disease and Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2013, 61, 1860–1870. [Google Scholar] [CrossRef]
- Jolicœur, E.M.; Dunning, A.; Castelvecchio, S.; Dabrowski, R.; Waclawiw, M.A.; Petrie, M.C.; Stewart, R.; Jhund, P.S.; Desvigne-Nickens, P.; Panza, J.A.; et al. Importance of Angina in Patients With Coronary Disease, Heart Failure, and Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2015, 66, 2092–2100. [Google Scholar] [CrossRef]
- MacDonald, M.R.; She, L.; Doenst, T.; Binkley, P.F.; Rouleau, J.L.; Tan, R.; Lee, K.L.; Miller, A.B.; Sopko, G.; Szalewska, D.; et al. Clinical Characteristics and Outcomes of Patients with and without Diabetes in the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. Eur. J. Heart Fail. 2015, 17, 725–734. [Google Scholar] [CrossRef]
- Li Kam Wa, M.E.; Assar, S.Z.; Kirtane, A.J.; Perera, D. Revascularisation for Ischaemic Cardiomyopathy. Interv. Cardiol. 2023, 18, e24. [Google Scholar] [PubMed]
- Panza, J.A.; Chrzanowski, L.; Bonow, R.O. Myocardial Viability Assessment Before Surgical Revascularization in Ischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 1068–1077. [Google Scholar] [CrossRef]
- Flaherty, J.D.; Rossi, J.S.; Fonarow, G.C.; Nunez, E.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Greenberg, B.H.; O’Connor, C.M.; Yancy, C.W.; et al. Influence of Coronary Angiography on the Utilization of Therapies in Patients with Acute Heart Failure Syndromes: Findings from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am. Heart J. 2009, 157, 1018–1025. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.-C.; Lembo, N.; Brown, W.M.; et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Morgan, H.; Petrie, M.C.; Perera, D. Coronary Revascularisation in Patients with Ischaemic Cardiomyopathy. Heart 2021, 107, 612–618. [Google Scholar] [CrossRef]
- Sedlis, S.P.; Ramanathan, K.B.; Morrison, D.A.; Sethi, G.; Sacks, J.; Henderson, W. Outcome of Percutaneous Coronary Intervention versus Coronary Bypass Grafting for Patients with Low Left Ventricular Ejection Fractions, Unstable Angina Pectoris, and Risk Factors for Adverse Outcomes with Bypass (the AWESOME Randomized Trial and Registry). Am. J. Cardiol. 2004, 94, 118–120. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Ryan, M.; Perera, D. Revascularization Strategies in Patients with Low Left Ventricular Function. 2023. Available online: https://textbooks.pcronline.com/the-pcr-eapci-textbook/revascularization-strategies-in-patients-with-low-left-ventricular-function (accessed on 29 January 2025).
- Gori, M.; Januzzi, J.L.; D’Elia, E.; Lorini, F.L.; Senni, M. Rationale for and Practical Use of Sacubitril/Valsartan in the Patient’s Journey with Heart Failure and Reduced Ejection Fraction. Card. Fail. Rev. 2021, 7, e06. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Samad, Z.; Shaw, L.K.; Phelan, M.; Ersboll, M.; Risum, N.; Al-Khalidi, H.R.; Glower, D.D.; Milano, C.A.; Alexander, J.H.; O’Connor, C.M.; et al. Management and Outcomes in Patients with Moderate or Severe Functional Mitral Regurgitation and Severe Left Ventricular Dysfunction. Eur. Heart J. 2015, 36, 2733–2741. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Liga, R.; Colli, A.; Taggart, D.P.; Boden, W.E.; De Caterina, R. Myocardial Revascularization in Patients With Ischemic Cardiomyopathy: For Whom and How. J. Am. Heart Assoc. 2023, 12, e026943. [Google Scholar]
- Lee Chuy, K.; Velazquez, E.J.; Lansky, A.J.; Jamil, Y.; Ahmad, Y. Current Landscape and Future Directions of Coronary Revascularization in Ischemic Systolic Heart Failure: A Review. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101197. [Google Scholar] [PubMed]
- Giacoppo, D.; Colleran, R.; Cassese, S.; Frangieh, A.H.; Wiebe, J.; Joner, M.; Schunkert, H.; Kastrati, A.; Byrne, R.A. Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting in Patients With Left Main Coronary Artery Stenosis. JAMA Cardiol. 2017, 2, 1079. [Google Scholar] [CrossRef]
- Ozturk, S. Relationship Between SYNTAX Score and Myocardial Viability in Ischemic Cardiomyopathy. Turk. Kardiyol. Dern. Ars. Arch. Turk. Soc. Cardiol. 2019, 47, 350–356. [Google Scholar] [CrossRef]
- Völz, S.; Redfors, B.; Angerås, O.; Ioanes, D.; Odenstedt, J.; Koul, S.; Valeljung, I.; Dworeck, C.; Hofmann, R.; Hansson, E.; et al. Long-Term Mortality in Patients with Ischaemic Heart Failure Revascularized with Coronary Artery Bypass Grafting or Percutaneous Coronary Intervention: Insights from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur. Heart J. 2021, 42, 2657–2664. [Google Scholar] [CrossRef]
- Pei, J.; Wang, X.; Xing, Z.; Zheng, K.; Hu, X. Short-term and Long-term Outcomes of Revascularization Interventions for Patients with Severely Reduced Left Ventricular Ejection Fraction: A Meta-analysis. ESC Heart Fail. 2021, 8, 634–643. [Google Scholar] [CrossRef]
- Marui, A.; Nishiwaki, N.; Komiya, T.; Hanyu, M.; Tanaka, S.; Kimura, T.; Sakata, R. Comparison of 5-Year Outcomes After Coronary Artery Bypass Grafting in Heart Failure Patients With Versus Without Preserved Left Ventricular Ejection Fraction (from the CREDO-Kyoto CABG Registry Cohort-2). Am. J. Cardiol. 2015, 116, 580–586. [Google Scholar] [CrossRef]
- Nagendran, J.; Norris, C.M.; Graham, M.M.; Ross, D.B.; MacArthur, R.G.; Kieser, T.M.; Maitland, A.M.; Southern, D.; Meyer, S.R. Coronary Revascularization for Patients With Severe Left Ventricular Dysfunction. Ann. Thorac. Surg. 2013, 96, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.E.; Vogrin, S.; Reid, C.M.; Ajani, A.E.; Clark, D.J.; Freeman, M.; Hiew, C.; Brennan, A.; Dinh, D.; Williams-Spence, J.; et al. Coronary Artery Bypass Grafting vs. Percutaneous Coronary Intervention in Severe Ischaemic Cardiomyopathy: Long-Term Survival. Eur. Heart J. 2025, 46, 72–80. [Google Scholar] [CrossRef]
- Doenst, T.; Haverich, A.; Serruys, P.; Bonow, R.O.; Kappetein, P.; Falk, V.; Velazquez, E.; Diegeler, A.; Sigusch, H. PCI and CABG for Treating Stable Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 73, 964–976. [Google Scholar] [CrossRef]
- Parikh, P.B.; Bhatt, D.L.; Bhasin, V.; Anker, S.D.; Skopicki, H.A.; Claessen, B.E.; Fonarow, G.C.; Hernandez, A.F.; Mehran, R.; Petrie, M.C.; et al. Impact of Percutaneous Coronary Intervention on Outcomes in Patients With Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2432–2447. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Anderson, M.; Burkhoff, D.; Grines, C.L.; Kapur, N.K.; Lansky, A.J.; Mannino, S.; McCabe, J.M.; Alaswad, K.; Daggubati, R.; et al. Improved Outcomes in Patients with Severely Depressed LVEF Undergoing Percutaneous Coronary Intervention with Contemporary Practices. Am. Heart J. 2022, 248, 139–149. [Google Scholar] [CrossRef]
- van Nunen, L.X.; Zimmermann, F.M.; Tonino, P.A.L.; Barbato, E.; Baumbach, A.; Engstrøm, T.; Klauss, V.; MacCarthy, P.A.; Manoharan, G.; Oldroyd, K.G.; et al. Fractional Flow Reserve versus Angiography for Guidance of PCI in Patients with Multivessel Coronary Artery Disease (FAME): 5-Year Follow-up of a Randomised Controlled Trial. Lancet 2015, 386, 1853–1860. [Google Scholar] [CrossRef]
- Tonino, P.A.L.; De Bruyne, B.; Pijls, N.H.J.; Siebert, U.; Ikeno, F.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef]
- Fearon, W.F.; Zimmermann, F.M.; De Bruyne, B.; Piroth, Z.; van Straten, A.H.M.; Szekely, L.; Davidavičius, G.; Kalinauskas, G.; Mansour, S.; Kharbanda, R.; et al. Fractional Flow Reserve–Guided PCI as Compared with Coronary Bypass Surgery. N. Engl. J. Med. 2022, 386, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, D.; Laudani, C.; Occhipinti, G.; Spagnolo, M.; Greco, A.; Rochira, C.; Agnello, F.; Landolina, D.; Mauro, M.S.; Finocchiaro, S.; et al. Coronary Angiography, Intravascular Ultrasound, and Optical Coherence Tomography for Guiding of Percutaneous Coronary Intervention: A Systematic Review and Network Meta-Analysis. Circulation 2024, 149, 1065–1086. [Google Scholar] [CrossRef] [PubMed]
- Moscardelli, S.; Masoomi, R.; Villablanca, P.; Jabri, A.; Patel, A.K.; Moroni, F.; Azzalini, L. Mechanical Circulatory Support for High-Risk Percutaneous Coronary Intervention. Curr. Cardiol. Rep. 2024, 26, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Chinnakondepalli, K.; Magnuson, E.A.; Kandzari, D.E.; Puskas, J.D.; Ben-Yehuda, O.; van Es, G.-A.; Taggart, D.P.; Morice, M.-C.; Lembo, N.J.; et al. Quality-of-Life After Everolimus-Eluting Stents or Bypass Surgery for Left-Main Disease. J. Am. Coll. Cardiol. 2017, 70, 3113–3122. [Google Scholar] [CrossRef]
- Hinojosa-Gonzalez, D.E.; Bueno-Gutierrez, L.C.; Salan-Gomez, M.; Tellez-Garcia, E.; Ramirez-Mulhern, I.; Sepulveda-Gonzalez, D.; Ramonfaur, D.; Roblesgil-Medrano, A.; Flores-Villalba, E. Hybrid Revascularization vs. Coronary Bypass for Coronary Artery Disease: A Systematic Review and Meta-Analysis. J. Cardiovasc. Surg. 2022, 63, 353–368. [Google Scholar] [CrossRef]
- McKiernan, M.; Halkos, M.E. Hybrid Coronary Revascularization: Are We There Yet? Curr. Opin. Cardiol. 2020, 35, 673–678. [Google Scholar] [CrossRef]
- Saha, T.; Naqvi, S.Y.; Goldberg, S. Hybrid Revascularization: A Review. Cardiology 2018, 140, 35–44. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; Dimaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E18–E114. [Google Scholar] [PubMed]
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction. JACC Cardiovasc. Imaging 2016, 9, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Vetrovec, G.W.; Kaki, A.; Dahle, T.G. A Review of Bleeding Risk with Impella-Supported High-Risk Percutaneous Coronary Intervention. Heart Int. 2020, 14, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Wirtz, H.S.; Sheer, R.; Honarpour, N.; Casebeer, A.W.; Simmons, J.D.; Kurtz, C.E.; Pasquale, M.K.; Globe, G. Real-World Analysis of Guideline-Based Therapy After Hospitalization for Heart Failure. J. Am. Heart Assoc. 2020, 9, e015042. [Google Scholar] [CrossRef]
- El Bèze, N.; Steg, P.G. Heart Failure and Revascularization: Which Method to Choose and Should We Even Do It? Eur. Heart J. 2025, 46, 81–83. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Stevens, S.R.; Doenst, T.; Petrie, M.C.; Al-Attar, N.; Ali, I.S.; Ambrosy, A.P.; Barton, A.K.; Cartier, R.; Cherniavsky, A.; et al. Society of Thoracic Surgeons Risk Score and EuroSCORE-2 Appropriately Assess 30-Day Postoperative Mortality in the STICH Trial and a Contemporary Cohort of Patients With Left Ventricular Dysfunction Undergoing Surgical Revascularization. Circ. Heart Fail. 2018, 11, e005531. [Google Scholar] [CrossRef]
- Fremes, S.E.; Marquis-Gravel, G.; Gaudino, M.F.L.; Jolicoeur, E.M.; Bédard, S.; Masterson Creber, R.; Ruel, M.; Vervoort, D.; Wijeysundera, H.C.; Farkouh, M.E.; et al. STICH3C: Rationale and Study Protocol. Circ. Cardiovasc. Interv. 2023, 16, e012527. [Google Scholar] [CrossRef]
- Rezende, P.C.; Hueb, W.; Bocchi, E.A.; Farkouh, M.; Junior, C.V.S.; Lima, E.G.; Silva, E.E.R.; Dallan, L.A.O.; Gaiotto, F.A.; Garzillo, C.L.; et al. Hypotheses, Rationale, Design, and Methods for Prognostic Evaluation of a Randomized Comparison between Patients with Coronary Artery Disease Associated with Ischemic Cardiomyopathy Who Undergo Medical or Surgical Treatment: MASS-VI (HF). Trials 2020, 21, 337. [Google Scholar] [CrossRef]
- Bani Hani, S.H.; Ahmad, M.M. Machine-Learning Algorithms for Ischemic Heart Disease Prediction: A Systematic Review. Curr. Cardiol. Rev. 2023, 19, e090622205797. [Google Scholar] [CrossRef] [PubMed]
- Leiner, T.; Rueckert, D.; Suinesiaputra, A.; Baeßler, B.; Nezafat, R.; Išgum, I.; Young, A.A. Machine Learning in Cardiovascular Magnetic Resonance: Basic Concepts and Applications. J. Cardiovasc. Magn. Reson. 2019, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Kageyama, S.; Shiomi, H.; Kotoku, N.; Masuda, S.; Revaiah, P.C.; Garg, S.; O’Leary, N.; van Klaveren, D.; Kimura, T.; et al. Can Machine Learning Aid the Selection of Percutaneous vs Surgical Revascularization? J. Am. Coll. Cardiol. 2023, 82, 2113–2124. [Google Scholar] [CrossRef] [PubMed]

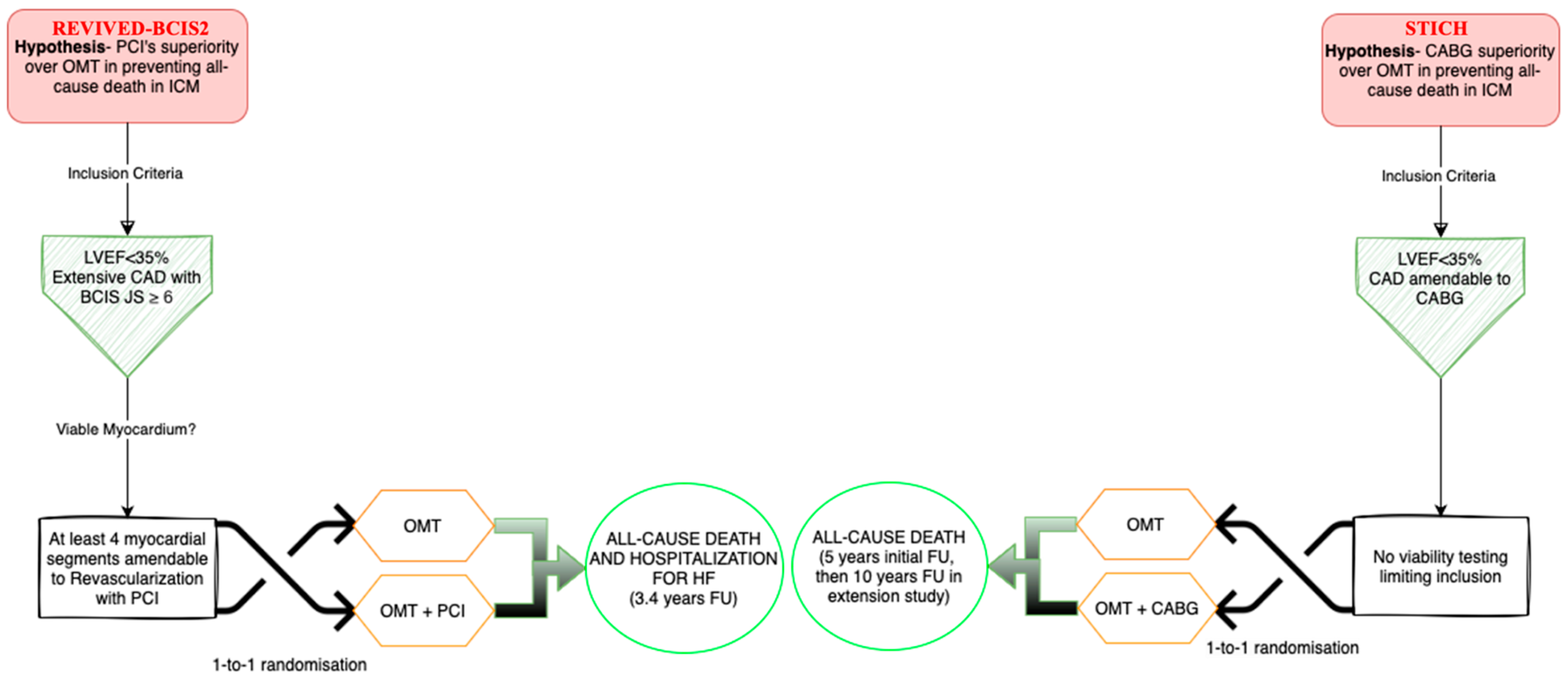
| Trial Acronym | STICH | REVIVED-BCIS2 | HEART | PARR-2 |
|---|---|---|---|---|
| Participants | 1212 | 700 | 138 | 430 |
| Primary outcome | All-cause death | All-cause death and hospitalization for HF | All-cause mortality | Cardiovascular death, MI, rehospitalization due to cardiac cause within 1 year |
| Secondary outcome | Cardiovascular death or death by any cause, hospitalization for cardiovascular cause | LVEF at 6 and 12 months, QoL scores, NYHA and CCS class, cardiovascular death, major bleeding, and NT-proBNP levels | Terminated early due to slow recruitment | Time to primary outcome and cardiovascular death |
| Inclusion criteria | LVEF ≤ 35%, CAD amendable for CABG | LVEF ≤ 35%, extensive coronary artery disease with [BCIS JS Score ≥ 6], viability in at least 4 dysfunctional myocardial segments amenable for PCI | LVEF ≤ 35%, HF ≥ 6 weeks, receiving diuretics, CAD or history of MI, ≥5 viable segments with reduced contractility [assessed by any method] | LVEF ≤ 35%, high suspicion of CAD [from coronary angiogram, previous MI, revascularization, or perfusion imaging] |
| Exclusion criteria | Recent MI responsible for LV dysfunction, cardiogenic shock within 72 h of randomization, history of CABG, important LM disease, life expectancy < 3 years for noncardiac causes | Acute MI within 4 weeks before randomization, decompensated HF, or malignant ventricular arrhythmias within 72 h prior to randomization | Recent ACS, stroke, valve surgery, angina requiring revascularization, malignant ventricular arrhythmias | Predetermined revascularization or transplantation strategy already planned, prior FDG viability imaging, recent MI [<4 weeks], severe comorbidities, or severe valvular disease requiring surgery |
| Median F/U | Initial: 4.7 years Extension study: 9.8 years | 41 months | 59 months | 5 years |
| Imaging technique for myocardial viability | DSE, SPECT | DSE, cMRI, SPECT/PET | DSE, PET, SPECT | PET |
| Revascularization technique | CABG+OMT [n = 610] vs. OMT [n = 602] | PCI+OMT [n = 347] vs. OMT [n = 353] | CABG [n = 30] and PCI [n = 15] vs. conservative [n = 69] | PET-guided revascularization_CABG [n = 71] and PCI [n = 33] vs. standard care |
| Outcomes | Initial study: no difference and benefit in all-cause mortality Extension study: Reduction in all-cause mortality [HR 0.84], 95% CI [0.73 to 0.97] and in combined CV hospitalization and death [HR 0.72], 95% CI [0.64 to 0.82] in the CABG group | No significant benefit of revascularization over OMT; improved QoL scores at 6 and 12 months diminished after 24 months when groups outcomes converged | No significant benefit of revascularization regarding mortality and QoL over OMT | No significant difference in composite primary endpoint [cardiac death/ MI/CV hospitalization] between PET-guided and standard strategies at 1- and 5-year follow-up |
| Limitations | Exclusion of LM disease in the OMT group, crossover between groups, deaths assessed as ‘unknown’ | Open-label design bias, largely pauci-symptomatic patients with limited applicability to patients with significant angina or ACS | Small enrollment, underpowered due to early termination | Low protocol adherence that biased the primary outcome analysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prunea, D.M.; Homorodean, C.; Olinic, M.; Achim, A.; Olinic, D.-M. Optimizing Revascularization in Ischemic Cardiomyopathy: Comparative Evidence on the Benefits and Indications of CABG and PCI. Life 2025, 15, 575. https://doi.org/10.3390/life15040575
Prunea DM, Homorodean C, Olinic M, Achim A, Olinic D-M. Optimizing Revascularization in Ischemic Cardiomyopathy: Comparative Evidence on the Benefits and Indications of CABG and PCI. Life. 2025; 15(4):575. https://doi.org/10.3390/life15040575
Chicago/Turabian StylePrunea, Dan M., Calin Homorodean, Maria Olinic, Alexandru Achim, and Dan-Mircea Olinic. 2025. "Optimizing Revascularization in Ischemic Cardiomyopathy: Comparative Evidence on the Benefits and Indications of CABG and PCI" Life 15, no. 4: 575. https://doi.org/10.3390/life15040575
APA StylePrunea, D. M., Homorodean, C., Olinic, M., Achim, A., & Olinic, D.-M. (2025). Optimizing Revascularization in Ischemic Cardiomyopathy: Comparative Evidence on the Benefits and Indications of CABG and PCI. Life, 15(4), 575. https://doi.org/10.3390/life15040575







