Multivessel Coronary Artery Disease in Cancer Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection of Studies
2.2. Data Extraction and Quality Assessment
2.3. Statistics and Data Analysis
3. Results
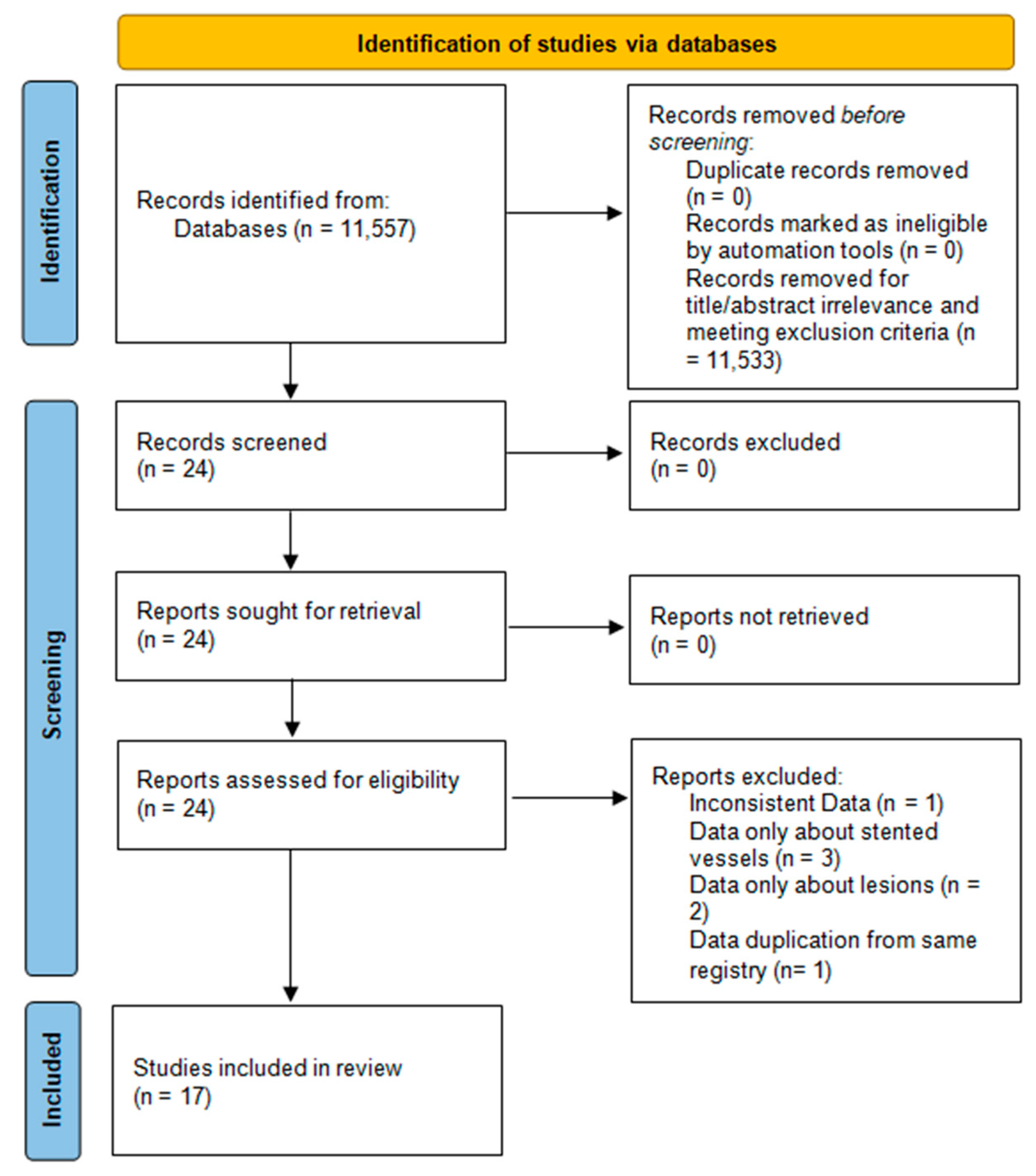
3.1. Literature Search and Study Selection
3.2. Characteristics of Eligible Studies
3.3. Patient Characteristics
3.4. Mortality Data
3.5. Extent and Complexity of CAD
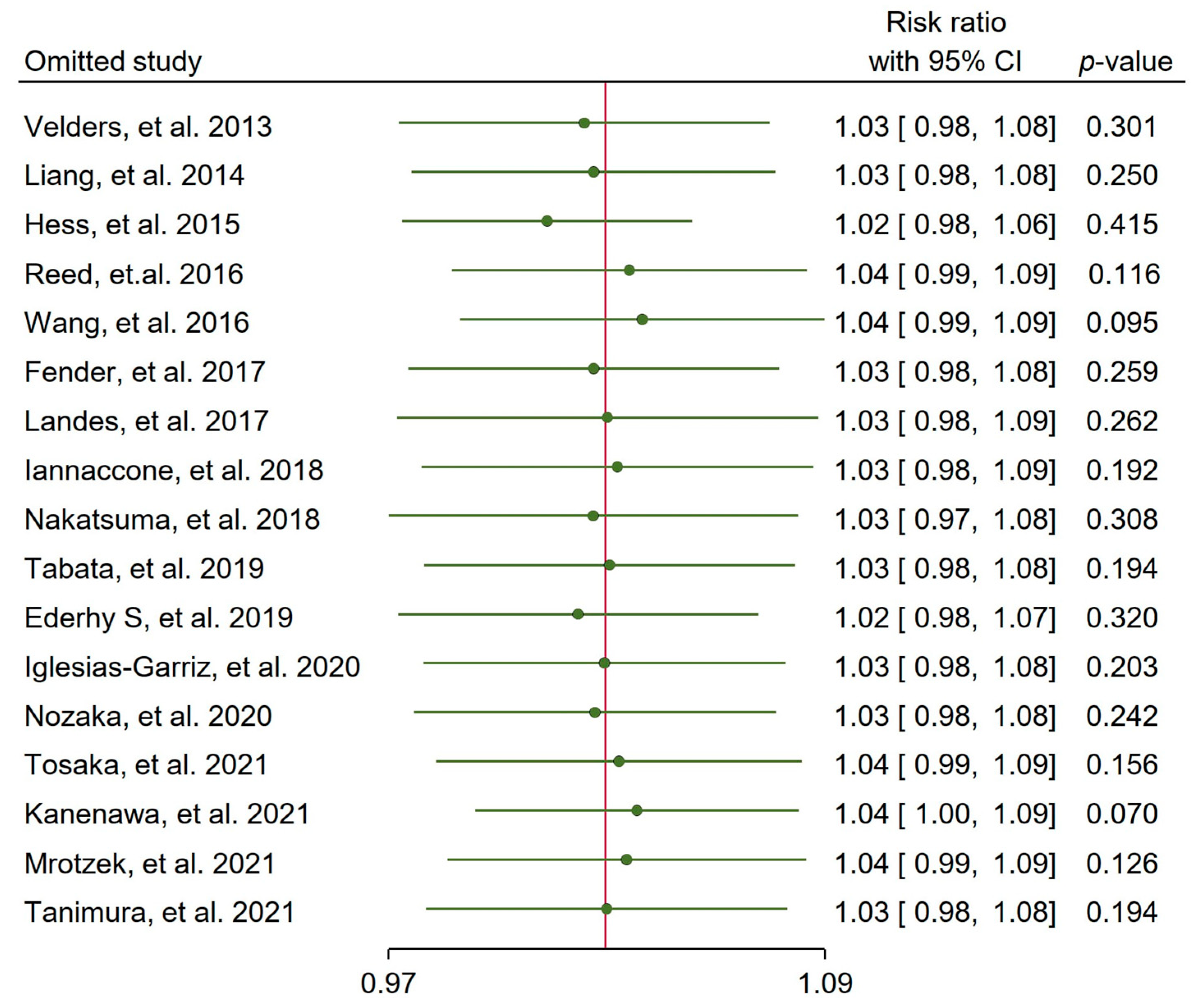
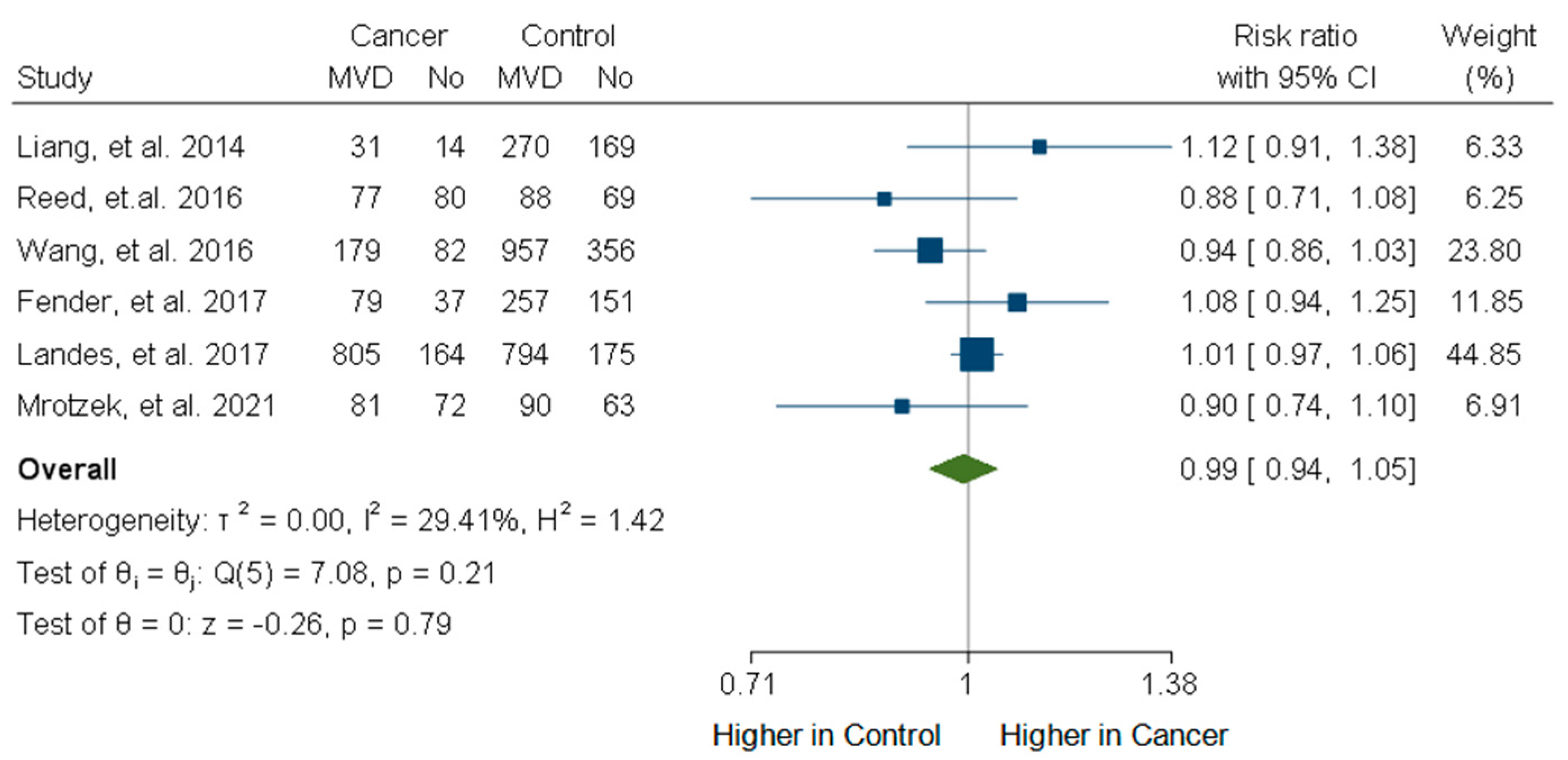
3.6. Incidence of Multivessel CAD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| CABG | Coronary artery bypass grafting |
| CAD | Coronary artery disease |
| CCS | Chronic coronary syndrome |
| CI | Confidence interval |
| MI | Myocardial infarction |
| MVD | Multivessel disease |
| NSTEMI | Non-ST elevation myocardial infarction |
| OCT | Optical coherence tomography |
| PCI | Percutaneous coronary intervention |
| RR | Risk ratio |
| STEMI | ST elevation myocardial infarction |
References
- Blaes, A.; Prizment, A.; Koene, R.; Konety, S. Cardio-oncology Related to Heart Failure: Common Risk Factors Between Cancer and Cardiovascular Disease. Heart Fail. Clin. 2017, 13, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Masoudkabir, F.; Sarrafzadegan, N.; Gotay, C.; Ignaszewski, A.; Krahn, A.D.; Davis, M.K.; Franco, C.; Mani, A. Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017, 263, 343–351. [Google Scholar] [PubMed]
- Herrmann, J.; Yang, E.H.; Iliescu, C.; Marmagkiolis, K. Response by Herrmann et al to Letter Regarding Article, “Vascular Toxicities of Cancer Therapies: The Old and the New-An Evolving Avenue”. Circulation 2016, 134, e466–e467. [Google Scholar] [CrossRef]
- Berardi, R.; Caramanti, M.; Savini, A.; Chiorrini, S.; Pierantoni, C.; Onofri, A.; Ballatore, Z.; De Lisa, M.; Mazzanti, P.; Cascinu, S. State of the art for cardiotoxicity due to chemotherapy and to targeted therapies: A literature review. Crit. Rev. Oncol. Hematol. 2013, 88, 75–86. [Google Scholar]
- Fanous, I.; Dillon, P. Cancer treatment-related cardiac toxicity: Prevention, assessment and management. Med. Oncol. 2016, 33, 84. [Google Scholar] [CrossRef]
- Yeh, E.T.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar]
- Chang, H.M.; Moudgil, R.; Scarabelli, T.; Okwuosa, T.M.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 1. J. Am. Coll. Cardiol. 2017, 70, 2536–2551. [Google Scholar]
- Chang, H.M.; Okwuosa, T.M.; Scarabelli, T.; Moudgil, R.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J. Am. Coll. Cardiol. 2017, 70, 2552–2565. [Google Scholar] [CrossRef]
- Todaro, M.C.; Oreto, L.; Qamar, R.; Paterick, T.E.; Carerj, S.; Khandheria, B.K. Cardioncology: State of the heart. Int. J. Cardiol. 2013, 168, 680–687. [Google Scholar] [CrossRef]
- DeZorzi, C. Radiation-Induced Coronary Artery Disease and Its Treatment: A Quick Review of Current Evidence. Cardiol. Res. Pract. 2018, 2018, 8367268. [Google Scholar] [CrossRef] [PubMed]
- Armanious, M.A.; Mohammadi, H.; Khodor, S.; Oliver, D.E.; Johnstone, P.A.; Fradley, M.G. Cardiovascular effects of radiation therapy. Curr. Probl. Cancer 2018, 42, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Nabialek-Trojanowska, I.; Lewicka, E.; Wrona, A.; Kaleta, A.M.; Lewicka-Potocka, Z.; Raczak, G.; Dziadziuszko, R. Cardiovascular complications after radiotherapy. Cardiol. J. 2020, 27, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Guo, W.; Al-Hijji, M.; El Sabbagh, A.; Begna, K.H.; Habermann, T.M.; Witzig, T.E.; Lewis, B.R.; Lerman, A.; Herrmann, J. Acute coronary syndromes in patients with active hematologic malignancies—Incidence, management, and outcomes. Int. J. Cardiol. 2019, 275, 6–12. [Google Scholar] [CrossRef]
- Mohanty, B.D.; Mohanty, S.; Hussain, Y.; Padmaraju, C.; Aggarwal, S.; Gospin, R.; Yu, A.F. Management of ischemic coronary disease in patients receiving chemotherapy: An uncharted clinical challenge. Future Cardiol. 2017, 13, 247–257. [Google Scholar] [CrossRef]
- Banasiak, W.; Zymlinski, R.; Undas, A. Optimal management of cancer patients with acute coronary syndrome. Pol. Arch. Intern. Med. 2018, 128, 244–253. [Google Scholar] [CrossRef]
- Machanahalli, B.A.; Ismayl, M.; Srinivasamurthy, R.; Gowda, R.M.; Aboeata, A. Early Outcomes of Percutaneous Coronary Intervention in Patients with Cancer: A Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2022, 47, 101305. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Itzhaki Ben Zadok, O.; Hasdai, D.; Gottlieb, S.; Porter, A.; Beigel, R.; Shimony, A.; Cohen, T.; Shlomo, N.; Shohat, T.; Silverman, B.; et al. Characteristics and outcomes of patients with cancer presenting with acute myocardial infarction. Coron. Artery Dis. 2019, 30, 332–338. [Google Scholar] [CrossRef]
- Borovac, J.A.; Kwok, C.S.; Iliescu, C.; Lee, H.J.; Kim, P.Y.; Palaskas, N.L.; Zaman, A.; Butler, R.; Lopez-Mattei, J.C.; Mamas, M.A. Percutaneous Coronary Intervention and Outcomes in Patients with Lymphoma in the United States (Nationwide Inpatient Sample [NIS] Analysis. Am. J. Cardiol. 2019, 124, 1190–1197. [Google Scholar] [PubMed]
- Potts, J.; Mohamed, M.O.; Lopez Mattei, J.C.; Iliescu, C.A.; Konopleva, M.; Rashid, M.; Bagur, R.; Mamas, M.A. Percutaneous coronary intervention and in-hospital outcomes in patients with leukemia: A nationwide analysis. Catheter. Cardiovasc. Interv. 2020, 96, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Wong, C.W.; Kontopantelis, E.; Barac, A.; Brown, S.A.; Velagapudi, P.; Hilliard, A.A.; Bharadwaj, A.S.; Chadi Alraies, M.; Mohamed, M.; et al. Percutaneous coronary intervention in patients with cancer and readmissions within 90 days for acute myocardial infarction and bleeding in the USA. Eur. Heart J. 2021, 42, 1019–1034. [Google Scholar] [PubMed]
- Ueki, Y.; Vögeli, B.; Karagiannis, A.; Zanchin, T.; Zanchin, C.; Rhyner, D.; Otsuka, T.; Praz, F.; Siontis, G.C.M.; Moro, C.; et al. Ischemia and Bleeding in Cancer Patients Undergoing Percutaneous Coronary Intervention. JACC Cardio Oncol. 2019, 1, 145–155. [Google Scholar]
- Guo, W.; Fan, X.; Lewis, B.R.; Johnson, M.P.; Rihal, C.S.; Lerman, A.; Herrmann, J. Cancer Patients Have a Higher Risk of Thrombotic and Ischemic Events After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2021, 14, 1094–1105. [Google Scholar]
- Tabata, N.; Sueta, D.; Yamamoto, E.; Takashio, S.; Arima, Y.; Araki, S.; Yamanaga, K.; Ishii, M.; Sakamoto, K.; Kanazawa, H.; et al. Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: A Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 290–300. [Google Scholar]
- Liang, J.J.; Sio, T.T.; Slusser, J.P.; Lennon, R.J.; Miller, R.C.; Sandhu, G.; Prasad, A. Outcomes after percutaneous coronary intervention with stents in patients treated with thoracic external beam radiation for cancer. JACC Cardiovasc. Interv. 2014, 7, 1412–1420. [Google Scholar] [CrossRef]
- Hess, C.N.; Roe, M.T.; Clare, R.M.; Chiswell, K.; Kelly, J.; Tcheng, J.E.; Hagstrom, E.; James, S.K.; Khouri, M.G.; Hirsch, B.R.; et al. Relationship Between Cancer and Cardiovascular Outcomes Following Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2015, 4, e001779. [Google Scholar]
- Reed, G.W.; Masri, A.; Griffin, B.P.; Kapadia, S.R.; Ellis, S.G.; Desai, M.Y. Long-Term Mortality in Patients With Radiation-Associated Coronary Artery Disease Treated with Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2016, 9, e003483. [Google Scholar] [CrossRef]
- Fender, E.A.; Liang, J.J.; Sio, T.T.; Stulak, J.M.; Lennon, R.J.; Slusser, J.P.; Ashman, J.B.; Miller, R.C.; Herrmann, J.; Prasad, A.; et al. Percutaneous revascularization in patients treated with thoracic radiation for cancer. Am. Heart J. 2017, 187, 98–103. [Google Scholar]
- Landes, U.; Kornowski, R.; Bental, T.; Assali, A.; Vaknin-Assa, H.; Lev, E.; Iakobishvili, Z. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron. Artery Dis. 2017, 28, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuma, K.; Shiomi, H.; Morimoto, T.; Watanabe, H.; Nakagawa, Y.; Furukawa, Y.; Kadota, K.; Ando, K.; Ono, K.; Shizuta, S.; et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Sueta, D.; Yamamoto, E.; Takashio, S.; Arima, Y.; Araki, S.; Yamanaga, K.; Ishii, M.; Sakamoto, K.; Kanazawa, H.; et al. A retrospective study of arterial stiffness and subsequent clinical outcomes in cancer patients undergoing percutaneous coronary intervention. J. Hypertens. 2019, 37, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.A.; Boden, H.; Hofma, S.H.; Osanto, S.; van der Hoeven, B.L.; Heestermans, A.A.; Cannegieter, S.C.; Jukema, J.W.; Umans, V.A.; Schalij, M.J.; et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am. J. Cardiol. 2013, 112, 1867–1872. [Google Scholar] [CrossRef]
- Ederhy, S.; Cohen, A.; Boccara, F.; Puymirat, E.; Aissaoui, N.; Elbaz, M.; Bonnefoy-Cudraz, E.; Druelles, P.; Andrieu, S.; Angoulvant, D.; et al. In-hospital outcomes and 5-year mortality following an acute myocardial infarction in patients with a history of cancer: Results from the French registry on Acute ST-elevation or non-ST-elevation myocardial infarction (FAST-MI) 2005 cohort. Arch. Cardiovasc. Dis. 2019, 112, 657–669. [Google Scholar] [CrossRef]
- Kanenawa, K.; Yamaji, K.; Morinaga, T.; Hiromasa, T.; Hayashi, M.; Hiramori, S.; Tomoi, Y.; Kuramitsu, S.; Domei, T.; Hyodo, M.; et al. Clinical Outcomes After Percutaneous Coronary Intervention in Patients With Cancer. Circ. J. 2021, 85, 837–846. [Google Scholar] [CrossRef]
- Nozaka, M.; Yokoyama, H.; Kitayama, K.; Nagawa, D.; Hamadate, M.; Miura, N.; Kawamura, Y.; Nakata, M.; Nishizaki, F.; Hanada, K.; et al. Clinical Outcomes of Acute Myocardial Infarction Patients With a History of Malignant Tumor. In Vivo 2020, 34, 3589–3595. [Google Scholar] [CrossRef]
- Iglesias-Garriz, I.; Delgado, I.; Prieto-Salvador, I.; Garrote, C.; Garcia-Palomo, A.; Fernandez-Vazquez, F. Previously diagnosed cancer and mortality after ST-segment elevation acute myocardial infarction treated with primary angioplasty. Catheter. Cardiovasc. Interv. 2020, 95, 1269–1274. [Google Scholar] [CrossRef]
- Tosaka, K.; Ishida, M.; Tsuji, K.; Kanehama, N.; Koeda, Y.; Niiyama, M.; Ishikawa, Y.; Shimoda, Y.; Kimura, T.; Fusazaki, T.; et al. Prevalence, clinical characteristics, and impact of active cancer in patients with acute myocardial infarction: Data from an all-comer registry. J. Cardiol. 2021, 78, 193–200. [Google Scholar] [CrossRef]
- Iannaccone, M.; D’Ascenzo, F.; Vadala, P.; Wilton, S.B.; Noussan, P.; Colombo, F.; Raposeiras Roubín, S.; Abu Assi, E.; González-Juanatey, J.R.; Simao Henriques, J.P.; et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: A BleeMACS substudy. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 631–638. [Google Scholar] [CrossRef]
- Wang, F.; Gulati, R.; Lennon, R.J.; Lewis, B.R.; Park, J.; Sandhu, G.S.; Wright, R.S.; Lerman, A.; Herrmann, J. Cancer History Portends Worse Acute and Long-term Noncardiac (but Not Cardiac) Mortality After Primary Percutaneous Coronary Intervention for Acute ST-Segment Elevation Myocardial Infarction. Mayo Clin. Proc. 2016, 91, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, K.; Otake, H.; Kawamori, H.; Toba, T.; Nagasawa, A.; Nakano, S.; Takahashi, Y.; Fukuyama, Y.; Kozuki, A.; Shite, J.; et al. Morphological Plaque Characteristics and Clinical Outcomes in Patients With Acute Coronary Syndrome and a Cancer History. J. Am. Heart Assoc. 2021, 10, e020243. [Google Scholar] [CrossRef] [PubMed]
- Mrotzek, S.M.; Lena, A.; Hadzibegovic, S.; Ludwig, R.; Al-Rashid, F.; Mahabadi, A.A.; Mincu, R.I.; Michel, L.; Johannsen, L.; Hinrichs, L.; et al. Assessment of coronary artery disease during hospitalization for cancer treatment. Clin. Res. Cardiol. 2021, 110, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Quintana, R.A.; Monlezun, D.J.; Davogustto, G.; Saenz, H.R.; Lozano-Ruiz, F.; Sueta, D.; Tsujita, K.; Landes, U.; Denktas, A.E.; Alam, M.; et al. Outcomes following percutaneous coronary intervention in patients with cancer. Int. J. Cardiol. 2020, 300, 106–112. [Google Scholar] [CrossRef]
- Roule, V.; Verdier, L.; Blanchart, K.; Ardouin, P.; Lemaitre, A.; Bignon, M.; Sabatier, R.; Alexandre, J.; Beygui, F. Systematic review and meta-analysis of the prognostic impact of cancer among patients with acute coronary syndrome and/or percutaneous coronary intervention. BMC Cardiovasc. Disord. 2020, 20, 38. [Google Scholar] [CrossRef]
- Carrillo-Estrada, M.; Bobrowski, D.; Carrasco, R.; Nadler, M.B.; Kalra, S.; Thavendiranathan, P.; Abdel-Qadir, H. Coronary artery disease in patients with cancer: Challenges and opportunities for improvement. Curr. Opin. Cardiol. 2021, 36, 597–608. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Tsokkou, S.; Grigoriadis, S.; Chrysavgi, L.; Gavriilaki, E. Cardiotoxicity in Acute Myeloid Leukemia in Adults: A Scoping Study. Cancers 2024, 16, 2474. [Google Scholar] [CrossRef]
- Cadeddu, D.C.; Deidda, M.; Giorgi, M.; Colonna, P. Vascular Damage—Coronary Artery Disease. J. Cardiovasc. Echogr. 2020, 30 (Suppl. S1), S11–S16. [Google Scholar] [CrossRef]
- Siaravas, K.C.; Katsouras, C.S.; Sioka, C. Radiation Treatment Mechanisms of Cardiotoxicity: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6272. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Nobre, M.M.; Tavares, S.M.; Magalhães, A.; Melica, B.; Toste, J.C.; Calé, R.; Almeida, M.; Fiuza, M.; Infante de Oliveira, E. Interventional cardiology in cancer patients: A position paper from the Portuguese Cardiovascular Intervention Association and the Portuguese Cardio-Oncology Study Group of the Portuguese Society of Cardiology. Rev. Port. Cardiol. 2024, 43, 35–48. [Google Scholar] [CrossRef]

| Study | PCI Cause | Exposed Group | Control Group | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|
| Velders M. et al. [34] | STEMI | Active and history of cancer | Non-cancer | 1-year all-cause mortality, cardiac mortality | 1-year survival |
| Liang J. et al. [27] | ACS and CCS | EBRT before or after PCI | No EBRT history | TLR | MI, cardiac mortality, overall mortality |
| Hess C. et al. [28] | ACS and CCS | Recent and non-recent cancer | No pre-PCI cancer | Cardiovascular mortality | Composite cardiovascular mortality, MI, repeat revascularization, all-cause mortality |
| Reed G. et al. [29] | ACS and CCS | EBRT before PCI | Non-cancer | All-cause mortality | Cardiovascular mortality |
| Wang F. et al. [35] | STEMI | Cancer history | Non-cancer | In-hospital and long-term mortality | |
| Fender E. et al. [30] | ACS and CCS | EBRT | No EBRT history | All-cause mortality | Cardiac, non-cardiac mortality, procedural complications, angiographic characteristics, number of diseased vessels |
| Landes U. et al. [31] | ACS and CCS | Cancer history | Non-cancer | All-cause mortality, nonfatal MI, composite of death, TVR, CABG | Cardiac, malignant, infectious cause of death and other cause of death |
| Iannaccone M. et al. [36] | ACS | Active or <2 years cancer history | Non-cancer | 1-year death or MI, bleedings | Death, re-infraction, bleeding after 1-year |
| Nakatsuma K. et al. [32] | ACS and CCS | Cancer history | Non-cancer | All-cause death, cardiac or non-cardiac death, HF hospitalization, stent thrombosis, TLR, stroke | |
| Tabata N. et al. [33] | ACS and CCS | Current or cancer history | Non-malignancy | 1-year cardiovascular death, non-fatal MI, stroke, revascularizations | |
| Ederhy S. et al. [37] | STEMI and NSTEMI | Any cancer history | Non-cancer | 5-year all-cause, cardiovascular, non-cardiovascular mortality | In-hospital complications (recurrent MI, VF, bleeding, transfusion, stroke) |
| Iglesias-Garriz I. et al. [38] | STEMI | History of cancer, no active cancer | Non-cancer | Totalmortality during follow-up | |
| Nozaka M. et al. [39] | AMI | Current and history of cancer | Non-malignancy | All-cause mortality, readmission for DHF | |
| Tosaka K. [40] | AMI | Active or history of cancer | Non-cancer | Cardiac death | Bleedings, non-cardiac death, MI, stroke |
| Kanenawa K. et al. [41] | CAD | Active or history of cancer | Non-cancer | 1-year NACCE (all-cause death, MI, stroke, major bleeding) | Bleeding, thrombotic composite of MI, stent thrombosis |
| Mrotzek S. et al. [42] | ACS and CCS | Cancer history | Non-cancer | 1-year all-cause mortality | 5-year all-cause mortality |
| Tanimura K. et al. [43] | ACS | Active cancer and history of cancer | Non-cancer | Cardiac death (due to MI, arrhythmia, heart failure, sudden) | Non-fatal MI, revascularization of coronary vessel, stroke TIA, heart failure admission |
| CANCER (N = 5261) | NO CANCER (N = 54,879) | p Value | |
|---|---|---|---|
| Diabetes | 35.9% | 33.6% | 0.068 |
| Hypertension | 72.3% | 70.1% | 0.045 |
| Hyperlipidemia | 58.8% | 63% | 0.007 |
| Smoking | 41.2% | 45.1% | 0.026 |
| Previous MI | 25.2% | 23.8% | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siaravas, K.C.; Papafaklis, M.I.; Moula, A.I.; Michalis, L.K.; Sioka, C.; Katsouras, C.S. Multivessel Coronary Artery Disease in Cancer Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Life 2025, 15, 571. https://doi.org/10.3390/life15040571
Siaravas KC, Papafaklis MI, Moula AI, Michalis LK, Sioka C, Katsouras CS. Multivessel Coronary Artery Disease in Cancer Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Life. 2025; 15(4):571. https://doi.org/10.3390/life15040571
Chicago/Turabian StyleSiaravas, Konstantinos C., Michail I. Papafaklis, Amalia I. Moula, Lampros K. Michalis, Chrissa Sioka, and Christos S. Katsouras. 2025. "Multivessel Coronary Artery Disease in Cancer Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis" Life 15, no. 4: 571. https://doi.org/10.3390/life15040571
APA StyleSiaravas, K. C., Papafaklis, M. I., Moula, A. I., Michalis, L. K., Sioka, C., & Katsouras, C. S. (2025). Multivessel Coronary Artery Disease in Cancer Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Life, 15(4), 571. https://doi.org/10.3390/life15040571







