Pathological Investigation of the Effect of Bovine Colostrum Against 5-FU-Induced Liver, Kidney, and Heart Toxicity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Histopathologic Examination
2.3. Immunohistochemical Examination
2.4. Statistical Analysis
3. Results
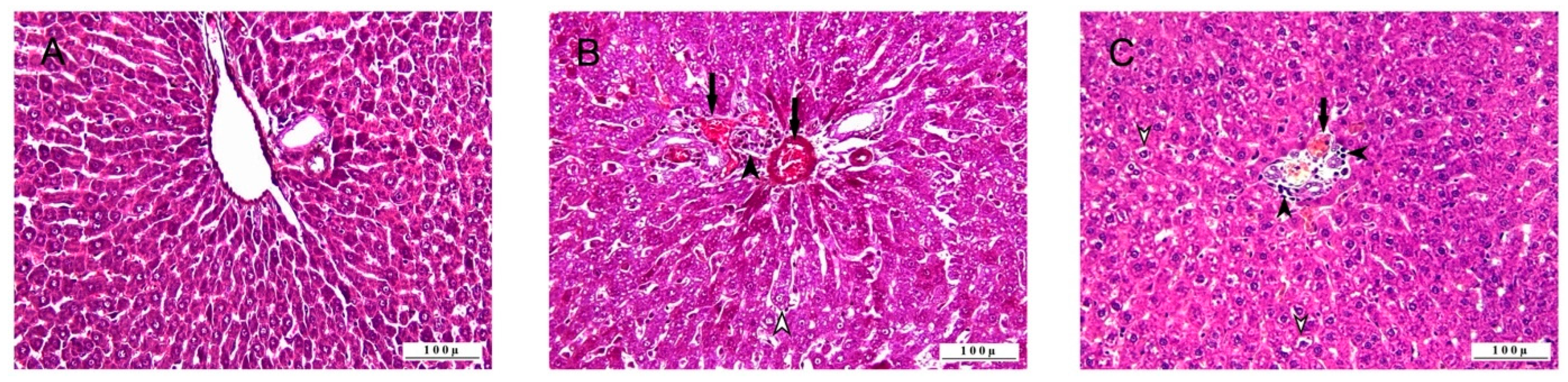
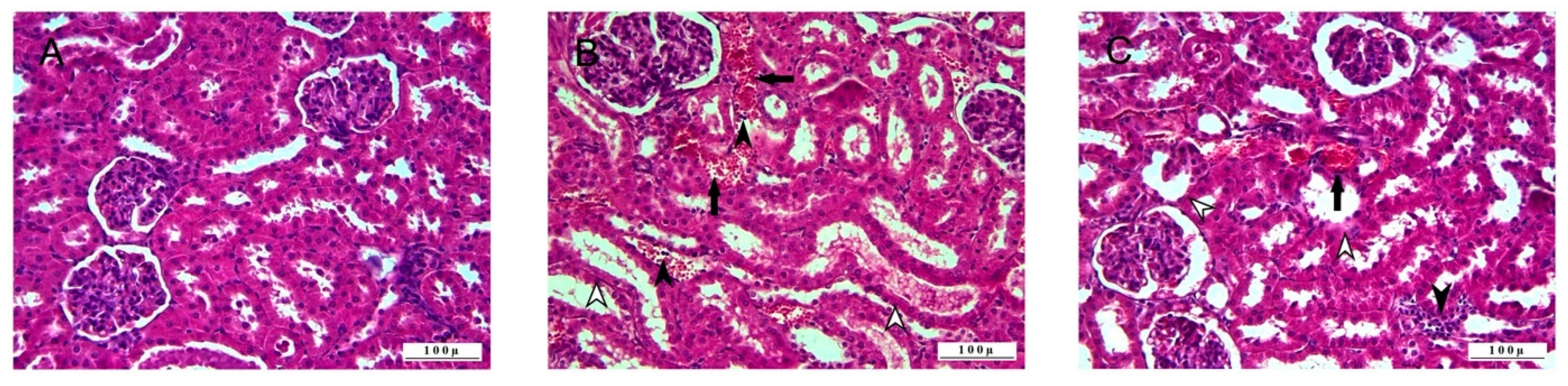
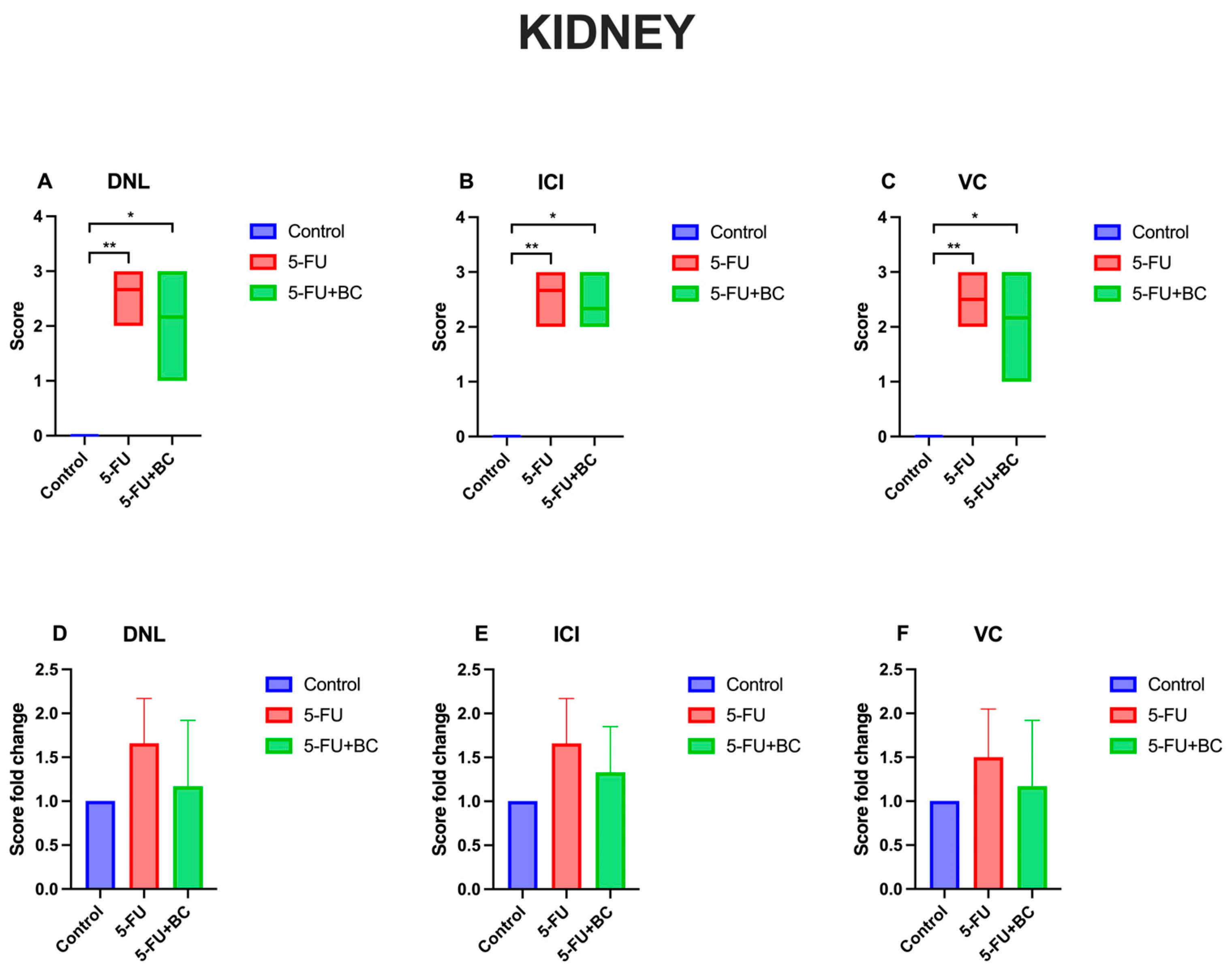
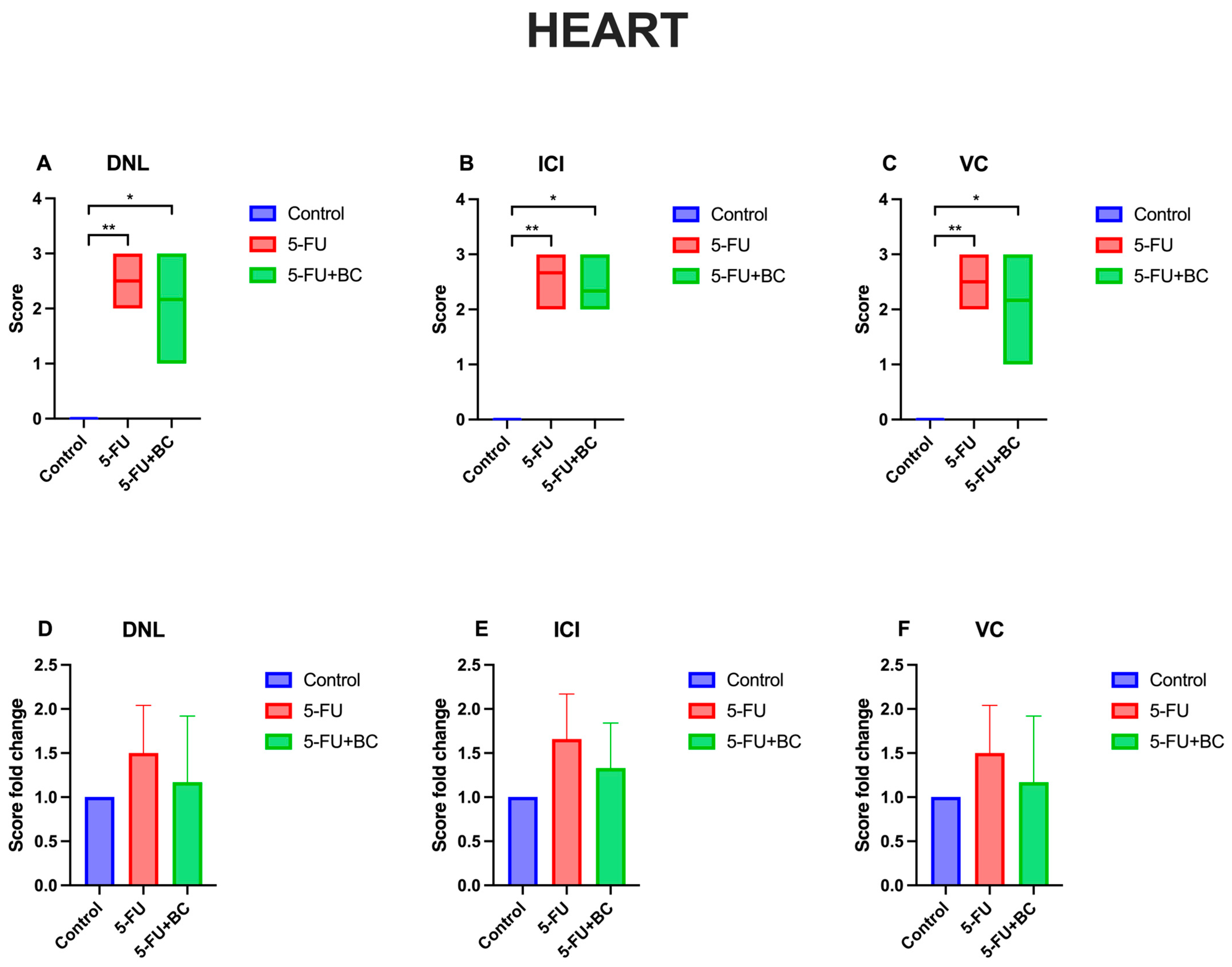
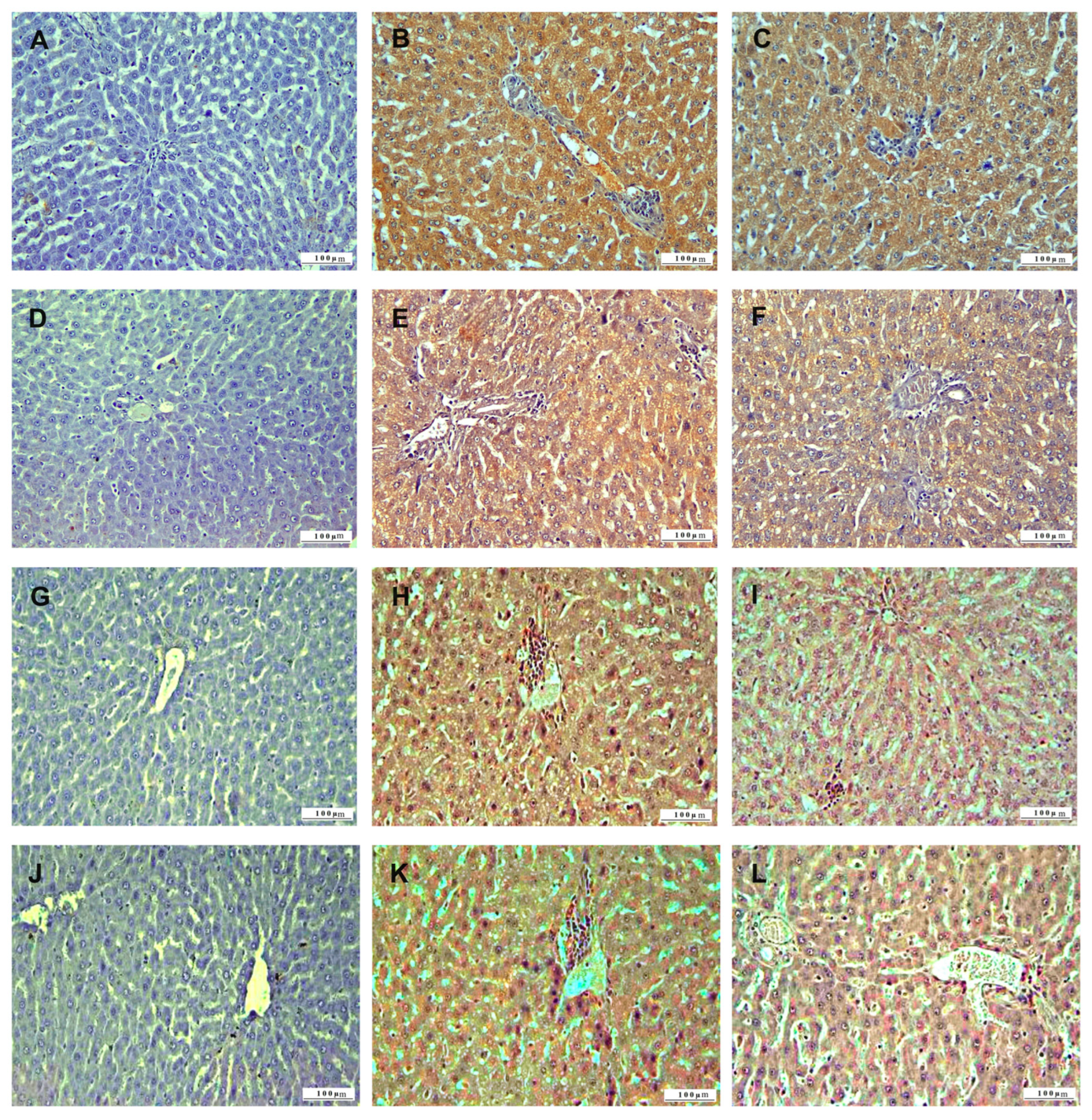
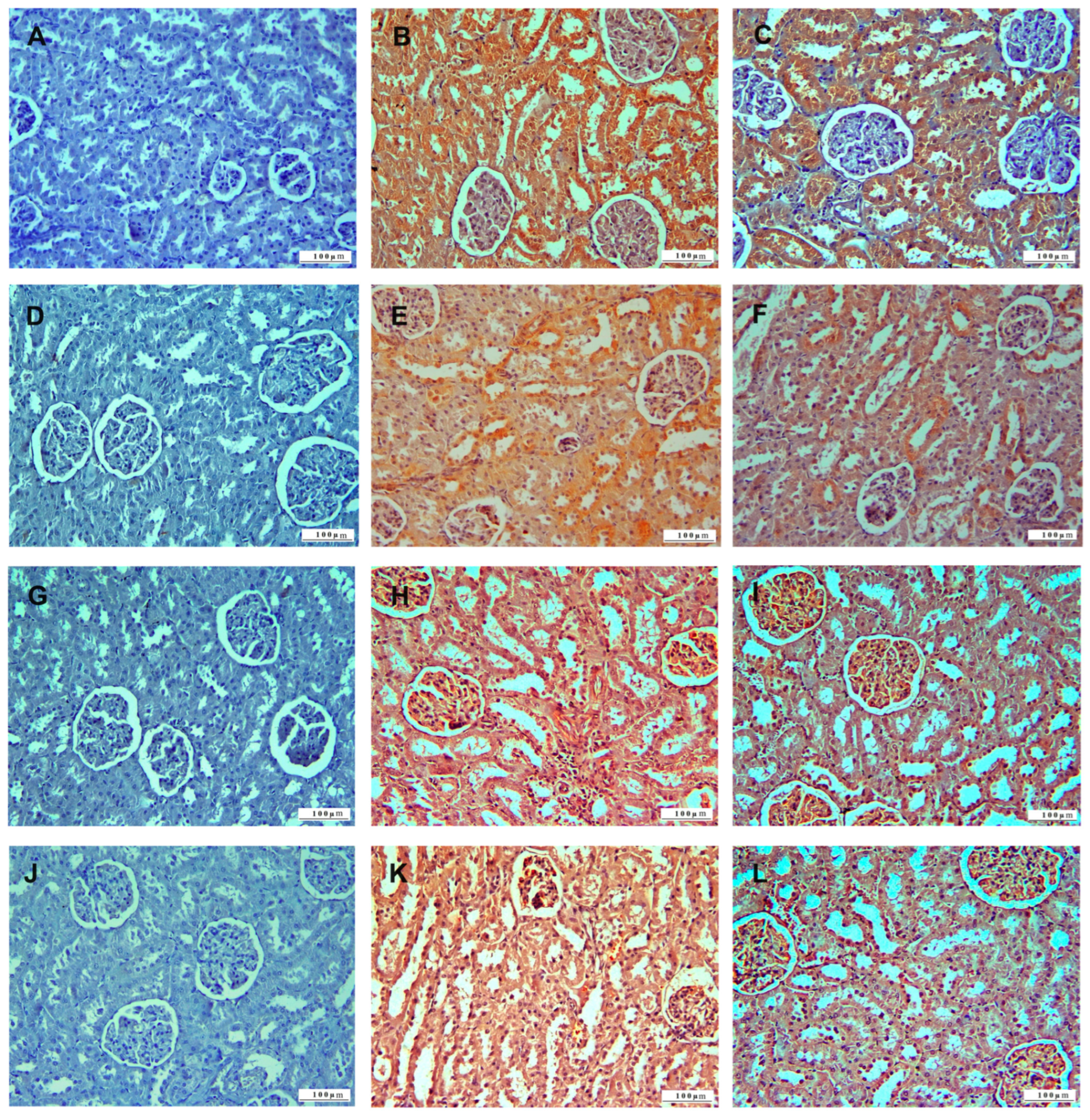
3.1. Histopathologic Analysis
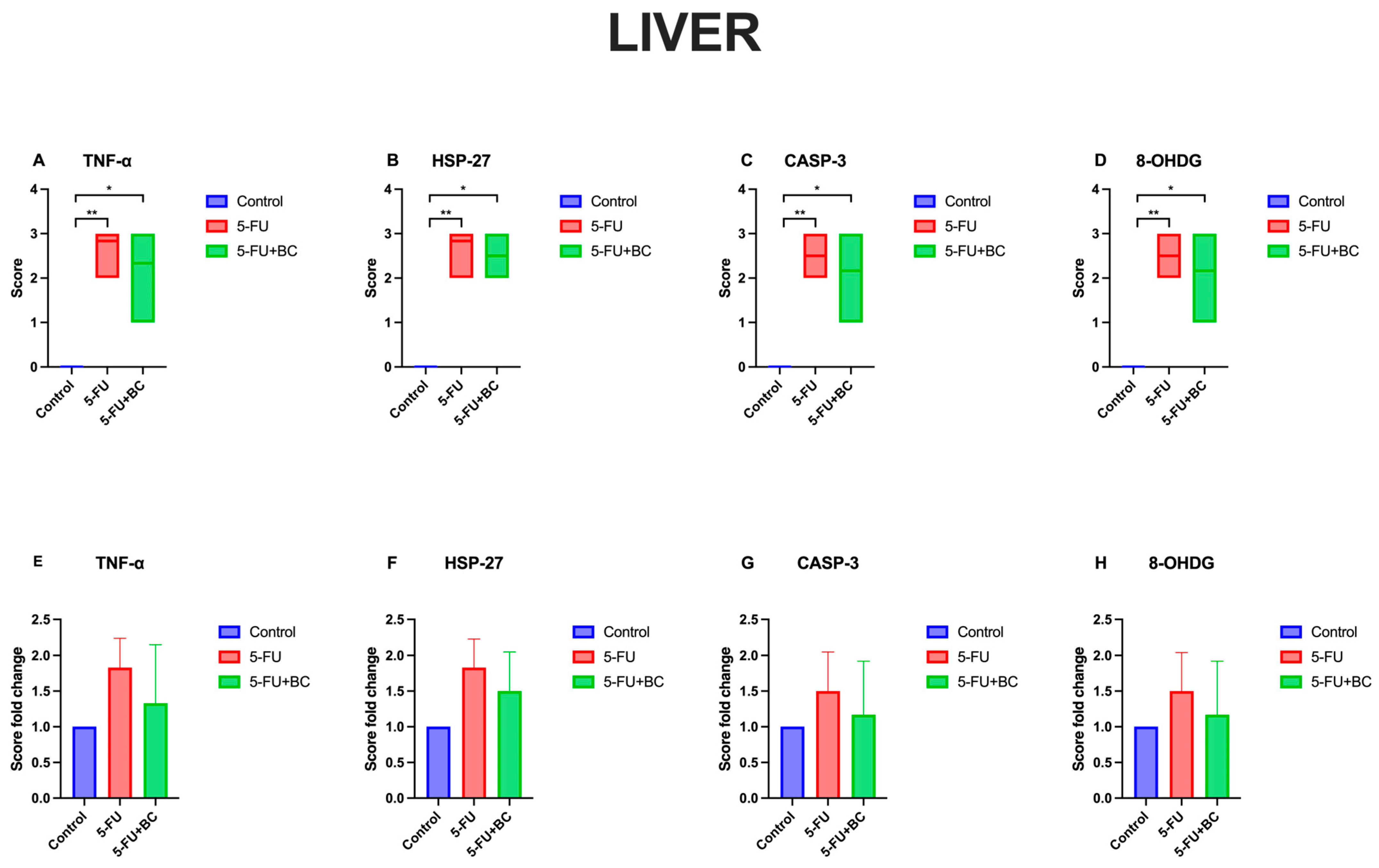
3.2. Immunohistochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| BC | Bovine colostrum |
| dTMP | Deoxythymidine monophosphate |
| NO | Nitric oxide |
| Ig | Immunoglobulin |
| IGF | Insulin-like growth factor |
| EGF | Epidermal growth factor |
| TGF | Transforming growth factor |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet-derived growth factor |
| DNLs | Degenerative-necrotic lesions |
| ICIs | Inflammatory cell infiltrates |
| VCs | Vascular changes |
References
- Azwar, S.; Seow, H.F.; Abdullah, M.; Faisal Jabar, M.; Mohtarrudin, N. Recent Updates on Mechanisms of Resistance to 5-Fluorouracil and Reversal Strategies in Colon Cancer Treatment. Biology 2021, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Barathan, M.; Zulpa, A.K.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Innovative Strategies to Combat 5-Fluorouracil Resistance in Colorectal Cancer: The Role of Phytochemicals and Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 7470. [Google Scholar] [CrossRef]
- Souliotis, V.L.; Vlachogiannis, N.I.; Pappa, M.; Argyriou, A.; Ntouros, P.A.; Sfikakis, P.P. DNA Damage Response and Oxidative Stress in Systemic Autoimmunity. Int. J. Mol. Sci. 2019, 21, 55. [Google Scholar] [CrossRef]
- Zoetemelk, M.; Ramzy, G.M.; Rausch, M.; Nowak-Sliwinska, P. Drug-Drug Interactions of Irinotecan, 5-Fluorouracil, Folinic Acid and Oxaliplatin and Its Activity in Colorectal Carcinoma Treatment. Molecules 2020, 25, 2614. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Capra, A.P.; Hasan, A.; Basilotta, R.; Scuderi, S.A.; Campolo, M.; Paterniti, I.; Esposito, E.; Ardizzone, A. Ulva pertusa Modulated Colonic Oxidative Stress Markers and Clinical Parameters: A Potential Adjuvant Therapy to Manage Side Effects During 5-FU Regimen. Int. J. Mol. Sci. 2024, 25, 12988. [Google Scholar] [CrossRef]
- Fideles, L.S.; de Miranda, J.A.L.; Martins, C.D.S.; Barbosa, M.L.L.; Pimenta, H.B.; Pimentel, P.V.S.; Teixeira, C.S.; Scafuri, M.A.S.; Facanha, S.O.; Barreto, J.E.F.; et al. Role of Rutin in 5-Fluorouracil-Induced Intestinal Mucositis: Prevention of Histological Damage and Reduction of Inflammation and Oxidative Stress. Molecules 2020, 25, 2786. [Google Scholar] [CrossRef] [PubMed]
- Mahran, Y.F.; Badr, A.M.; Al-Kharashi, L.A.; Alajami, H.N.; Aldamry, N.T.; Bayoumy, N.M.; Elmongy, E.I.; Soliman, S. Thymol Protects against 5-Fluorouracil-Induced Hepatotoxicity via the Regulation of the Akt/GSK-3beta Pathway in In Vivo and In Silico Experimental Models. Pharmaceuticals 2024, 17, 1094. [Google Scholar] [PubMed]
- da Silva, M.C.; Fabiano, L.C.; da Costa Salomao, K.C.; de Freitas, P.L.Z.; Neves, C.Q.; Borges, S.C.; de Souza Carvalho, M.D.G.; Breithaupt-Faloppa, A.C.; de Thomaz, A.A.; Dos Santos, A.M.; et al. A Rodent Model of Human-Dose-Equivalent 5-Fluorouracil: Toxicity in the Liver, Kidneys, and Lungs. Antioxidants 2023, 12, 1005. [Google Scholar] [CrossRef]
- Salerno, N.; Scalise, M.; Marino, F.; Filardo, A.; Chiefalo, A.; Panuccio, G.; Torella, M.; De Angelis, A.; De Rosa, S.; Ellison-Hughes, G.M.; et al. A Mouse Model of Dilated Cardiomyopathy Produced by Isoproterenol Acute Exposure Followed by 5-Fluorouracil Administration. J. Cardiovasc. Dev. Dis. 2023, 10, 225. [Google Scholar] [CrossRef]
- Fasse, S.; Alarinta, J.; Frahm, B.; Wirtanen, G. Bovine Colostrum for Human Consumption—Improving Microbial Quality and Maintaining Bioactive Characteristics through Processing. Dairy 2021, 2, 556–575. [Google Scholar] [CrossRef]
- Puppel, K.; Golebiewski, M.; Grodkowski, G.; Slosarz, J.; Kunowska-Slosarz, M.; Solarczyk, P.; Lukasiewicz, M.; Balcerak, M.; Przysucha, T. Composition and Factors Affecting Quality of Bovine Colostrum: A Review. Animals 2019, 9, 1070. [Google Scholar] [CrossRef]
- Kazimierska, K.; Kalinowska-Lis, U. Milk Proteins-Their Biological Activities and Use in Cosmetics and Dermatology. Molecules 2021, 26, 3253. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Sun, Q.; Chen, C.; Wang, R.; Yan, W. A Review: The Effect of Bovine Colostrum on Immunity in People of All Ages. Nutrients 2024, 16, 2007. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M. Colostrum Proteins in Protection against Therapy-Induced Injuries in Cancer Chemo- and Radiotherapy: A Comprehensive Review. Biomedicines 2023, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Weiser, M.J.; Marchbank, T. Methods to improve efficacy of orally administered bioactive peptides using bovine colostrum as an exemplar. PLoS ONE 2021, 16, e0253422. [Google Scholar]
- Celebi, A.; Dortbudak, M.B.; Keskinruzgar, A.; Yuksel, H. The therapeutic effect of bovine colostrum on 5-Fluorouracil-Induced oral mucositis in rats. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e682–e686. [Google Scholar]
- Uztimur, M.; Unal, C.N.; Dortbudak, M.B.; Firat, R.; Ekinci, A.I. Assessment of brain injury in cattle with Theileria annulata: Neuron-specific biomarkers, inflammation, oxidative stress and apoptosis. Vet. J. 2024, 309, 106269. [Google Scholar]
- Uztimur, M.; Dortbudak, M.B. Evaluation of brain injury in goats naturally infected with Coenurus cerebralis; brain specific biomarkers, acute inflammation, and DNA oxidation. Res. Vet. Sci. 2023, 165, 105043. [Google Scholar]
- Neal, R.D.; Johnson, P.; Clarke, C.A.; Hamilton, S.A.; Zhang, N.; Kumar, H.; Swanton, C.; Sasieni, P. Cell-Free DNA-Based Multi-Cancer Early Detection Test in an Asymptomatic Screening Population (NHS-Galleri): Design of a Pragmatic, Prospective Randomised Controlled Trial. Cancers 2022, 14, 4818. [Google Scholar] [CrossRef]
- Wang, C.; Huo, X.; Gao, L.; Sun, G.; Li, C. Hepatoprotective Effect of Carboxymethyl Pachyman in Fluorouracil-Treated CT26-Bearing Mice. Molecules 2017, 22, 756. [Google Scholar] [CrossRef]
- Yahya, S.A.; Al-Shawi, N.N. Hepatoprotective effect of Nobiletin against 5-fluorouracil induce hepatotoxicity. Curr. Res. Pharmacol. Drug Discov. 2024, 7, 100199. [Google Scholar] [PubMed]
- Mansoori, R.; Kazemi, S.; Almasi, D.; Hosseini, S.M.; Karim, B.; Nabipour, M.; Moghadamnia, A.A. Therapeutic benefit of melatonin in 5-fluorouracil-induced renal and hepatic injury. Basic. Clin. Pharmacol. Toxicol. 2024, 134, 397–411. [Google Scholar] [PubMed]
- Yahya, R.A.M.; Attia, A.M.; Yehia, M.A.; Azab, A.E.; Shkal, K.E.M. Cyclophosphamide Induces Hepatorenal Toxicity and Attenuation by 5-fluorouracil in Male Albino Rats. World J. Clin. Med. Res. 2022, 2, 8–21. [Google Scholar]
- Alharbi, A.M.; Kafl, H.E.; Abdelaziz, R.R.; Suddek, G.M. Saroglitazar ameliorates 5- Fluorouracil-induced hepatorenal damage in rats. Int. Immunopharmacol. 2024, 143, 113407. [Google Scholar]
- Sravathi, V.; Mylaram, J.; Yadala, R.; Kumar, A.; Aasritha, S.; Mounika, K.; Hanuman, D. Ameliorative Effect of Naringenin in 5-Fluorouracil-Induced Hepatotoxicity: An Experimental Study on Albino Wistar Rats. Indian J. Anim. Res. 2024, 58, 1294–1302. [Google Scholar]
- El-Sherbiny, M.; Fahmy, E.K.; Eisa, N.H.; Said, E.; Elkattawy, H.A.; Ebrahim, H.A.; Elsherbiny, N.M.; Ghoneim, F.M. Nanogold Particles Suppresses 5-Flurouracil-Induced Renal Injury: An Insight into the Modulation of Nrf-2 and Its Downstream Targets, HO-1 and gamma-GCS. Molecules 2021, 26, 7684. [Google Scholar]
- Elbanan, M.E.; Amer, M.E.; El-Missiry, M.A.; Othman, A.I.; Shabana, S.M. Melatonin protected against kidney impairment induced by 5-fluorouracil in mice. J. Exp. Zool. A Ecol. Integr. Physiol. 2023, 339, 777–787. [Google Scholar]
- Rashid, S.; Ali, N.; Nafees, S.; Hasan, S.K.; Sultana, S. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem. Toxicol. 2014, 66, 185–193. [Google Scholar]
- Gelen, V.; Sengul, E.; Yildirim, S.; Senturk, E.; Tekin, S.; Kukurt, A. The protective effects of hesperidin and curcumin on 5-fluorouracil-induced nephrotoxicity in mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 47046–47055. [Google Scholar]
- Ahmad Ansari, M.; Shahid, M.; Ahmad, S.F.; Ahmad, A.; Alanazi, A.; Malik, A.; Bin Jardan, Y.A.; Attia, S.M.; Bakheet, S.A.; Raish, M. Sinapic acid alleviates 5-fluorouracil-induced nephrotoxicity in rats via Nrf2/HO-1 signalling. Saudi Pharm. J. 2023, 31, 1351–1359. [Google Scholar]
- Rupert, C.E.; Coulombe, K.L. The roles of neuregulin-1 in cardiac development, homeostasis, and disease. Biomark. Insights 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lasica, R.; Spasic, J.; Djukanovic, L.; Trifunovic-Zamaklar, D.; Orlic, D.; Nedeljkovic-Arsenovic, O.; Asanin, M. Case report: Acute toxic myocardial damage caused by 5-fluorouracil-from enigma to success. Front. Cardiovasc. Med. 2022, 9, 991886. [Google Scholar] [CrossRef]
- Kashyap, M.K.; Mangrulkar, S.V.; Kushwaha, S.; Ved, A.; Kale, M.B.; Wankhede, N.L.; Taksande, B.G.; Upaganlawar, A.B.; Umekar, M.J.; Koppula, S.; et al. Recent Perspectives on Cardiovascular Toxicity Associated with Colorectal Cancer Drug Therapy. Pharmaceuticals 2023, 16, 1441. [Google Scholar] [CrossRef]
- Safarpour, S.; Safarpour, S.; Pirzadeh, M.; Moghadamnia, A.A.; Ebrahimpour, A.; Shirafkan, F.; Mansoori, R.; Kazemi, S.; Hosseini, M. Colchicine Ameliorates 5-Fluorouracil-Induced Cardiotoxicity in Rats. Oxid. Med. Cell. Longev. 2022, 2022, 6194532. [Google Scholar] [CrossRef] [PubMed]
- Lokman, M.S.; Althagafi, H.A.; Alharthi, F.; Habotta, O.A.; Hassan, A.A.; Elhefny, M.A.; Al Sberi, H.; Theyab, A.; Mufti, A.H.; Alhazmi, A.; et al. Protective effect of quercetin against 5-fluorouracil-induced cardiac impairments through activating Nrf2 and inhibiting NF-kappaB and caspase-3 activities. Environ. Sci. Pollut. Res. Int. 2023, 30, 17657–17669. [Google Scholar] [CrossRef] [PubMed]
- Farooq, J.; Sultana, R.; James, J.P.; Fathima, C.Z.; Almutairy, A.F.; Hussain, A.S.M. Efficacy of Thymoquinone and Hesperidin in Attenuating Cardiotoxicity from 5-Fluorouracil: Insights from In Vivo and In Silico Studies. Toxics 2024, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.N.; Sallam, N.; El-Abhar, H.S. Activated ROCK/Akt/eNOS and ET-1/ERK pathways in 5-fluorouracil-induced cardiotoxicity: Modulation by simvastatin. Sci. Rep. 2020, 10, 14693. [Google Scholar] [CrossRef]
- Pontoppidan, P.E.; Shen, R.L.; Cilieborg, M.S.; Jiang, P.; Kissow, H.; Petersen, B.L.; Thymann, T.; Heilmann, C.; Muller, K.; Sangild, P.T. Bovine Colostrum Modulates Myeloablative Chemotherapy-Induced Gut Toxicity in Piglets. J. Nutr. 2015, 145, 1472–1480. [Google Scholar] [CrossRef]
- Shen, R.L.; Rathe, M.; Jiang, P.; Pontoppidan, P.E.; Heegaard, P.M.; Muller, K.; Sangild, P.T. Doxorubicin-Induced Gut Toxicity in Piglets Fed Bovine Milk and Colostrum. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 698–707. [Google Scholar] [CrossRef]
- Karabacak, M.; Kanbur, M.; Eraslan, G.; Silig, Y.; Soyer Sarica, Z.; Tekeli, M.Y.; Tas, A. The effects of colostrum on some biochemical parameters in the experimental intoxication of rats with paracetamol. Environ. Sci. Pollut. Res. Int. 2018, 25, 23897–23908. [Google Scholar]
- Abdelmeguid, N.E.; Khalil, M.I.; Badr, N.S.; Alkhuriji, A.F.; El-Gerbed, M.S.A.; Sultan, A.S. Ameliorative effects of colostrum against DMBA hepatotoxicity in rats. Saudi J. Biol. Sci. 2021, 28, 2254–2266. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dörtbudak, M.B.; Demircioğlu, M.; Demircioğlu, İ.; Nicotra, M.; Di Cerbo, A. Pathological Investigation of the Effect of Bovine Colostrum Against 5-FU-Induced Liver, Kidney, and Heart Toxicity in Rats. Life 2025, 15, 564. https://doi.org/10.3390/life15040564
Dörtbudak MB, Demircioğlu M, Demircioğlu İ, Nicotra M, Di Cerbo A. Pathological Investigation of the Effect of Bovine Colostrum Against 5-FU-Induced Liver, Kidney, and Heart Toxicity in Rats. Life. 2025; 15(4):564. https://doi.org/10.3390/life15040564
Chicago/Turabian StyleDörtbudak, Muhammet Bahaeddin, Muhammed Demircioğlu, İsmail Demircioğlu, Mario Nicotra, and Alessandro Di Cerbo. 2025. "Pathological Investigation of the Effect of Bovine Colostrum Against 5-FU-Induced Liver, Kidney, and Heart Toxicity in Rats" Life 15, no. 4: 564. https://doi.org/10.3390/life15040564
APA StyleDörtbudak, M. B., Demircioğlu, M., Demircioğlu, İ., Nicotra, M., & Di Cerbo, A. (2025). Pathological Investigation of the Effect of Bovine Colostrum Against 5-FU-Induced Liver, Kidney, and Heart Toxicity in Rats. Life, 15(4), 564. https://doi.org/10.3390/life15040564






