AMPK Knockout Impairs the Formation of Three-Dimensional Spheroids
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and 3D Spheroid Formation
2.2. Transmission Electron Microscopy
2.3. Reporter Assay and Western Blot
2.4. Immunohistochemistry and Immunofluorescence Analysis of 3D Spheroid Cultures
2.5. Flow Cytometry
2.6. Statistical Analysis
3. Results
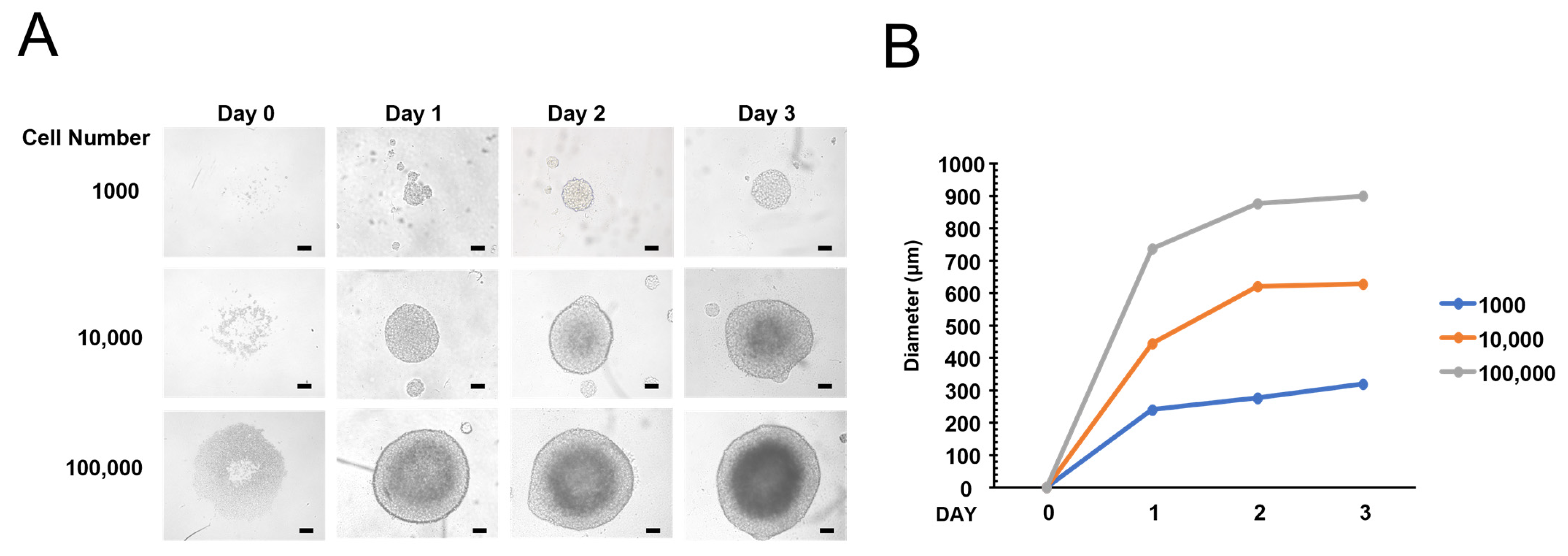
3.1. Formation of 3D Spheroid Using Ultra-Low Attachment Plate
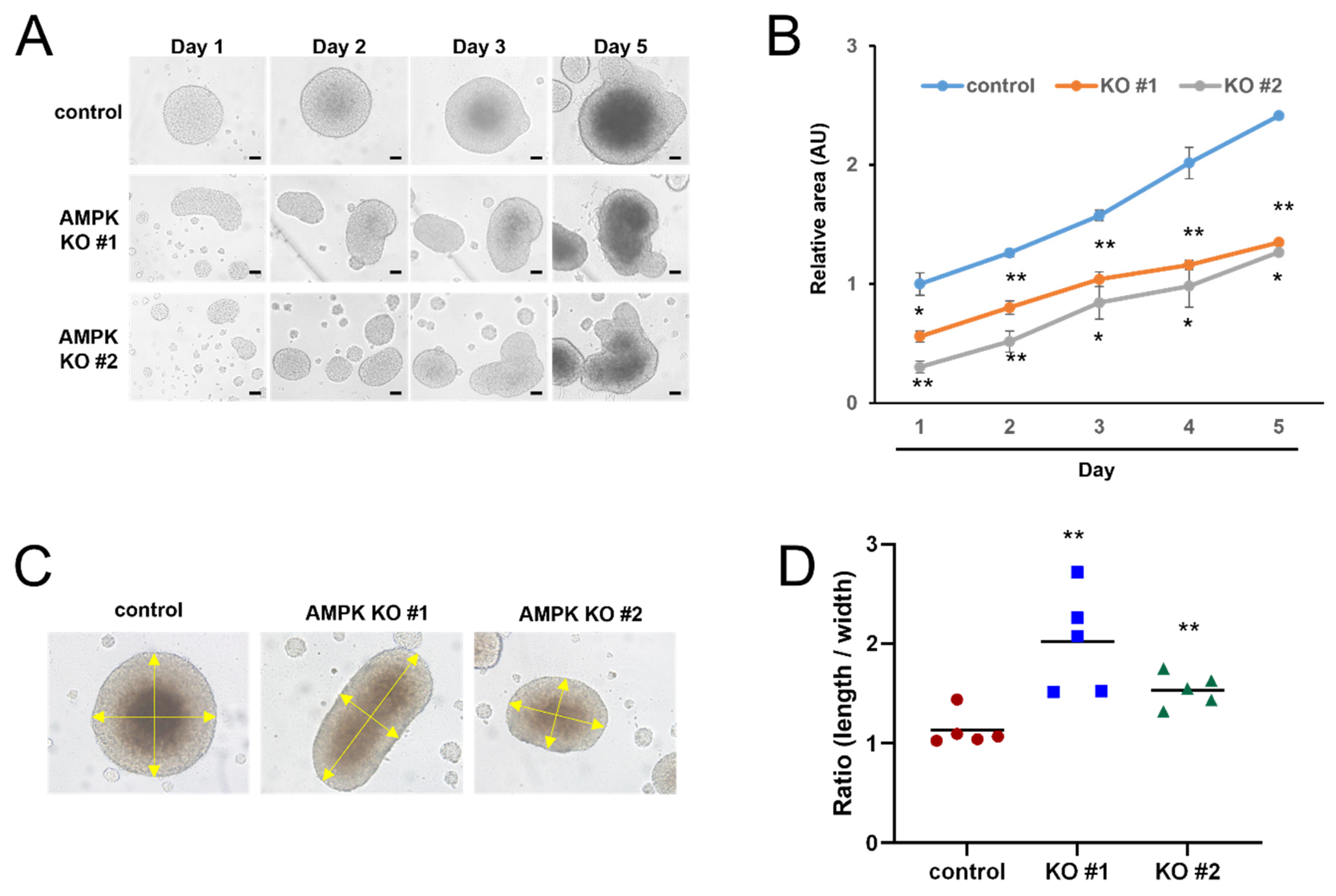
3.2. AMPK Knockout Delayed Spheroid Growth
3.3. The Expression of LC3 Protein Is Increased in AMPK Knockout Cells
3.4. AMPK Knockout Spheroids Have Enlarged Lysosomes
3.5. AMPK Knockout Increased the Apoptotic Cells in 3D Spheroid
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Muñoz, M.; Contreras, C.; Prieto, D. AMPK, metabolism, and vascular function. FEBS J. 2021, 288, 3746–3771. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, D.-H.; Kim, D.-H. Redefining the role of AMPK in autophagy and the energy stress response. Nat. Commun. 2023, 14, 2994. [Google Scholar] [CrossRef]
- Fang, C.; Pan, J.; Qu, N.; Lei, Y.; Han, J.; Zhang, J.; Han, D. The AMPK pathway in fatty liver disease. Front. Physiol. 2022, 13, 970292. [Google Scholar] [CrossRef] [PubMed]
- Göransson, O.; Kopietz, F.; Rider, M.H. Metabolic control by AMPK in white adipose tissue. Trends Endocrinol. Metab. 2023, 34, 704–717. [Google Scholar] [CrossRef]
- Roach, P.J. AMPK → ULK1 → Autophagy. Mol. Cell. Biol. 2011, 31, 3082–3084. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 29–41. [Google Scholar] [CrossRef]
- Ravi, M.; Ramesh, A.; Pattabhi, A. Contributions of 3D Cell Cultures for Cancer Research. J. Cell. Physiol. 2017, 232, 2679–2697. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Shen, L.; Lee, S.; Joo, J.C.; Hong, E.; Cui, Z.Y.; Jo, E.; Park, S.J.; Jang, H.J. Chelidonium majus Induces Apoptosis of Human Ovarian Cancer Cells via ATF3-Mediated Regulation of Foxo3a by Tip60. J. Microbiol. Biotechnol. 2022, 32, 493–503. [Google Scholar] [CrossRef]
- Senrung, A.; Lalwani, S.; Janjua, D.; Tripathi, T.; Kaur, J.; Ghuratia, N.; Aggarwal, N.; Chhokar, A.; Yadav, J.; Chaudhary, A.; et al. 3D tumor spheroids: Morphological alterations a yardstick to anti-cancer drug response. In Vitro Models 2023, 2, 219–248. [Google Scholar] [CrossRef]
- Sant, S.; Johnston, P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today: Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef]
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 2011, 2720. [Google Scholar] [CrossRef]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, L.; Lin, Z.; Zhang, Y.; Mi, S.; Rao, L.; Xu, T. 3D bioprinted cancer cells are more tolerant to serum starvation than 2D cells due to autophagy. Mater. Today Chem. 2022, 24, 100912. [Google Scholar] [CrossRef]
- Regmi, S.; Raut, P.K.; Pathak, S.; Shrestha, P.; Park, P.-H.; Jeong, J.-H. Enhanced viability and function of mesenchymal stromal cell spheroids is mediated via autophagy induction. Autophagy 2021, 17, 2991–3010. [Google Scholar] [CrossRef]
- Bingel, C.; Koeneke, E.; Ridinger, J.; Bittmann, A.; Sill, M.; Peterziel, H.; Wrobel, J.K.; Rettig, I.; Milde, T.; Fernekorn, U.; et al. Three-dimensional tumor cell growth stimulates autophagic flux and recapitulates chemotherapy resistance. Cell Death Dis. 2017, 8, e3013. [Google Scholar] [CrossRef]
- Gomes, L.R.; Vessoni, A.T.; Menck, C.F.M. Three-dimensional microenvironment confers enhanced sensitivity to doxorubicin by reducing p53-dependent induction of autophagy. Oncogene 2015, 34, 5329–5340. [Google Scholar] [CrossRef]
- Singha, B.; Laski, J.; Ramos Valdés, Y.; Liu, E.; DiMattia, G.E.; Shepherd, T.G. Inhibiting ULK1 kinase decreases autophagy and cell viability in high-grade serous ovarian cancer spheroids. Am. J. Cancer Res. 2020, 10, 1384–1399. [Google Scholar]
- Laski, J.; Singha, B.; Wang, X.; Valdés, Y.R.; Collins, O.; Shepherd, T.G. Activated CAMKKβ-AMPK signaling promotes autophagy in a spheroid model of ovarian tumour metastasis. J. Ovarian Res. 2020, 13, 58. [Google Scholar] [CrossRef]
- El-Araby, M.E.; Khan, M.I.; Muhammad, Y.A.; Razeeth Shait Mohammed, M.; Dalhat, M.H.; Alharbi, M.A.; Mass, S.; Abou Gharbia, M.; Khayat, M.T.; Omar, A.M. The Relationships of AMPK Allosteric Inhibitors with Their Activities against Breast Cancer in 2D and 3D Models. ChemistrySelect 2023, 8, e202302979. [Google Scholar] [CrossRef]
- Jang, M.; Park, R.; Kim, H.; Namkoong, S.; Jo, D.; Huh, Y.H.; Jang, I.-S.; Lee, J.I.; Park, J. AMPK contributes to autophagosome maturation and lysosomal fusion. Sci. Rep. 2018, 8, 12637. [Google Scholar] [CrossRef]
- Jo, D.; Park, R.; Kim, H.; Jang, M.; Lee, E.-J.; Jang, I.-S.; Park, J. AMP-activated protein kinase regulates the expression of human telomerase reverse transcriptase. PLoS ONE 2018, 13, e0207864. [Google Scholar] [CrossRef]
- Jang, M.; Park, R.; Yamamoto, A.; Park, Y.-I.; Park, Y.; Lee, S.; Park, J. AMPK inhibitor, compound C, inhibits coronavirus replication in vitro. PLoS ONE 2023, 18, e0292309. [Google Scholar] [CrossRef]
- Napolitano, G.; Ballabio, A. TFEB at a glance. J. Cell Sci. 2016, 129, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Young, N.P.; Kamireddy, A.; Van Nostrand, J.L.; Eichner, L.J.; Shokhirev, M.N.; Dayn, Y.; Shaw, R.J. AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes. Genes. Dev. 2016, 30, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; El-Houjeiri, L.; Zirden, L.C.; Puustinen, P.; Blanchette, P.; Jeong, H.; Dejgaard, K.; Siegel, P.M.; Pause, A. AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy 2021, 17, 3957–3975. [Google Scholar] [CrossRef]
- Parcon, P.A.; Balasubramaniam, M.; Ayyadevara, S.; Jones, R.A.; Liu, L.; Shmookler Reis, R.J.; Barger, S.W.; Mrak, R.E.; Griffin, W.S.T. Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to CLEAR motifs. Alzheimer’s Dement. 2018, 14, 230–242. [Google Scholar] [CrossRef]
- Namkoong, S.; Lee, K.I.; Lee, J.I.; Park, R.; Lee, E.-J.; Jang, I.-S.; Park, J. The integral membrane protein ITM2A, a transcriptional target of PKA-CREB, regulates autophagic flux via interaction with the vacuolar ATPase. Autophagy 2015, 11, 756–768. [Google Scholar] [CrossRef]
- Li, X.; Darzynkiewicz, Z. Cleavage of Poly(ADP-Ribose) Polymerase Measured in Situ in Individual Cells: Relationship to DNA Fragmentation and Cell Cycle Position during Apoptosis. Exp. Cell Res. 2000, 255, 125–132. [Google Scholar] [CrossRef]
- Hurley, J.H.; Young, L.N. Mechanisms of Autophagy Initiation. Annu. Rev. Biochem. 2017, 86, 225–244. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of AMPK in autophagy. Front. Physiol. 2022, 13, 1015500. [Google Scholar] [CrossRef]
- Shiflett, S.L.; Kaplan, J.; Ward, D.M. Chediak–Higashi Syndrome: A Rare Disorder of Lysosomes and Lysosome Related Organelles. Pigment Cell Res. 2002, 15, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Yim, W.W.-Y.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-L.; Perillo, W.; Liou, D.; Marambaud, P.; Wang, P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J. Surg. Oncol. 2012, 106, 680–688. [Google Scholar] [CrossRef]
- Liu, X.; Chhipa, R.R.; Nakano, I.; Dasgupta, B. The AMPK Inhibitor Compound C Is a Potent AMPK-Independent Antiglioma Agent. Mol. Cancer Ther. 2014, 13, 596–605. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-I.; Park, R.; Lee, S.; Lee, C.; Yoo, I.; Ka, H.; Huh, Y.H.; Hong, J.; Park, J. AMPK Knockout Impairs the Formation of Three-Dimensional Spheroids. Life 2025, 15, 525. https://doi.org/10.3390/life15040525
Park Y-I, Park R, Lee S, Lee C, Yoo I, Ka H, Huh YH, Hong J, Park J. AMPK Knockout Impairs the Formation of Three-Dimensional Spheroids. Life. 2025; 15(4):525. https://doi.org/10.3390/life15040525
Chicago/Turabian StylePark, Yea-In, Rackhyun Park, Siyun Lee, Chunghyeon Lee, Inkyu Yoo, Hakhyun Ka, Yang Hoon Huh, Jongkwang Hong, and Junsoo Park. 2025. "AMPK Knockout Impairs the Formation of Three-Dimensional Spheroids" Life 15, no. 4: 525. https://doi.org/10.3390/life15040525
APA StylePark, Y.-I., Park, R., Lee, S., Lee, C., Yoo, I., Ka, H., Huh, Y. H., Hong, J., & Park, J. (2025). AMPK Knockout Impairs the Formation of Three-Dimensional Spheroids. Life, 15(4), 525. https://doi.org/10.3390/life15040525






