The Nutritional Phenotyping of Idiopathic Pulmonary Fibrosis Through Morphofunctional Assessment: A Bicentric Cross-Sectional Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting Study
2.2. Body Composition Techniques (BIVA, NU, CT)
2.3. Functional Assessment (HGS, TUG, SPIROMETRY, DLCO)
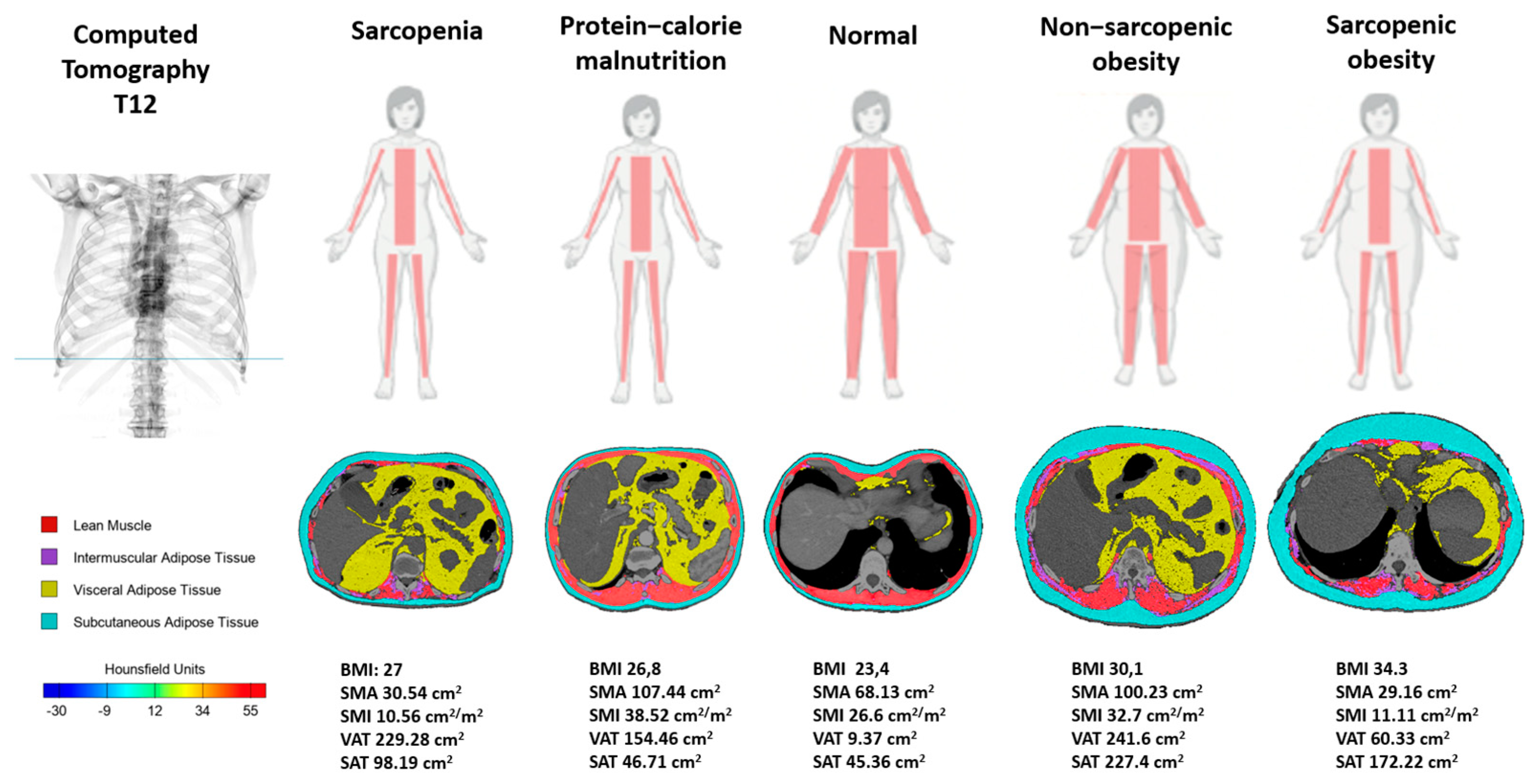
2.4. Nutritional Phenotypes Proposed (Cachexia, Malnutrition, Sarcopenia, Obesity, and Sarcopenic Obesity)
2.5. Statistical Analysis
3. Results
3.1. Morphofunctional Parameters
3.1.1. Morphofunctional Differences by Sex
3.1.2. Morphofunctional Differences Between IPF Patients and Healthy Controls
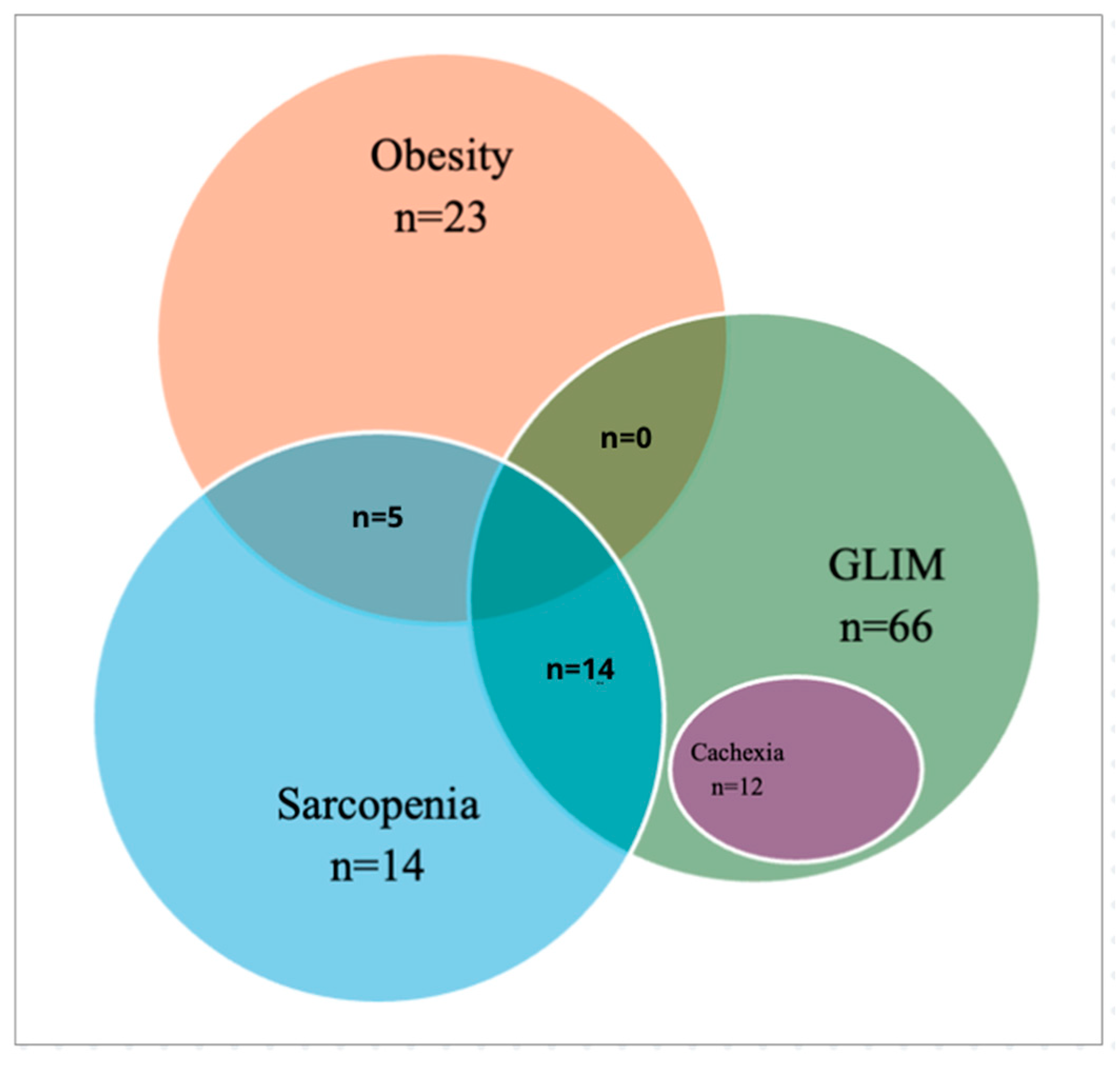
3.2. Nutritional Phenotypes
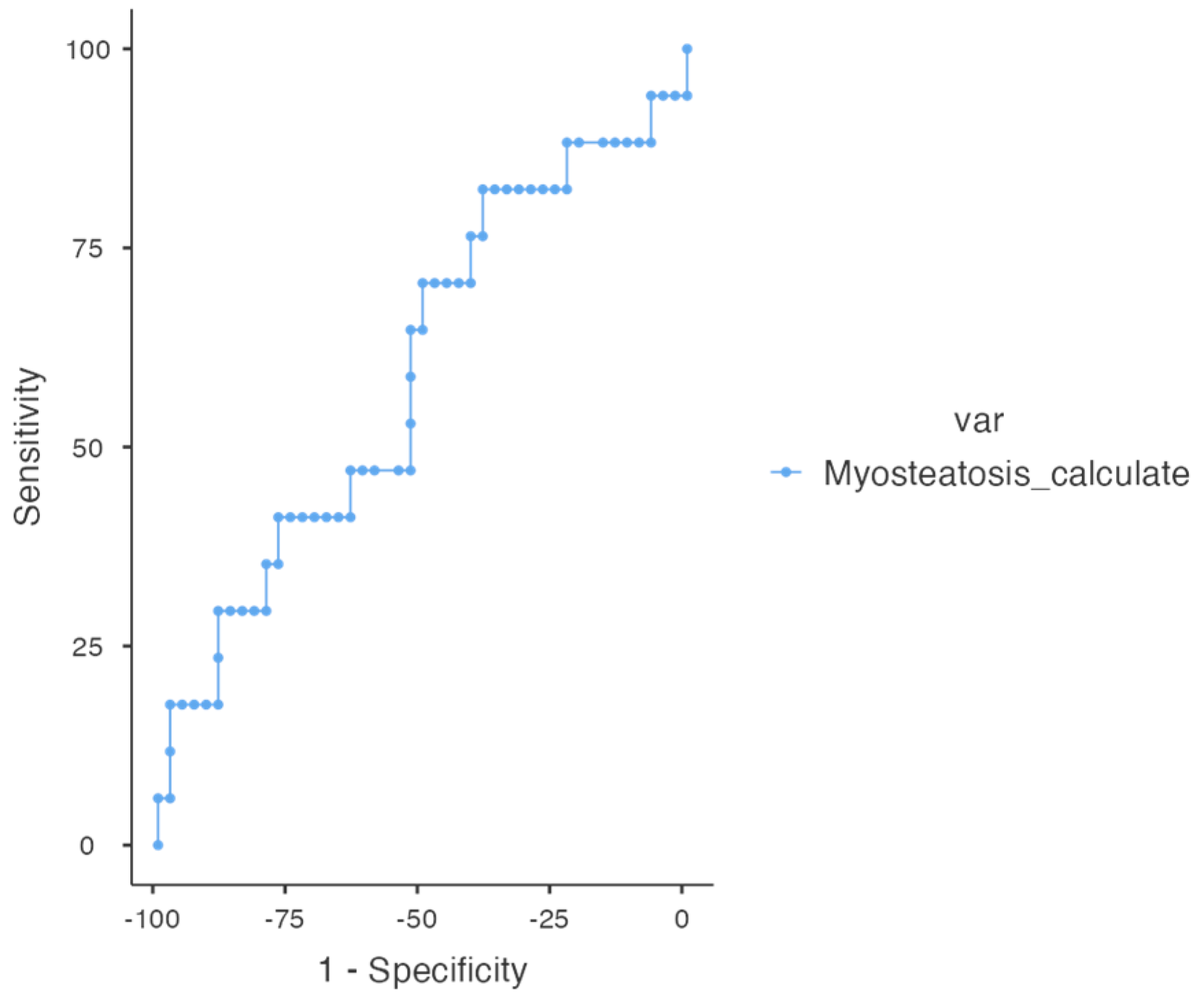
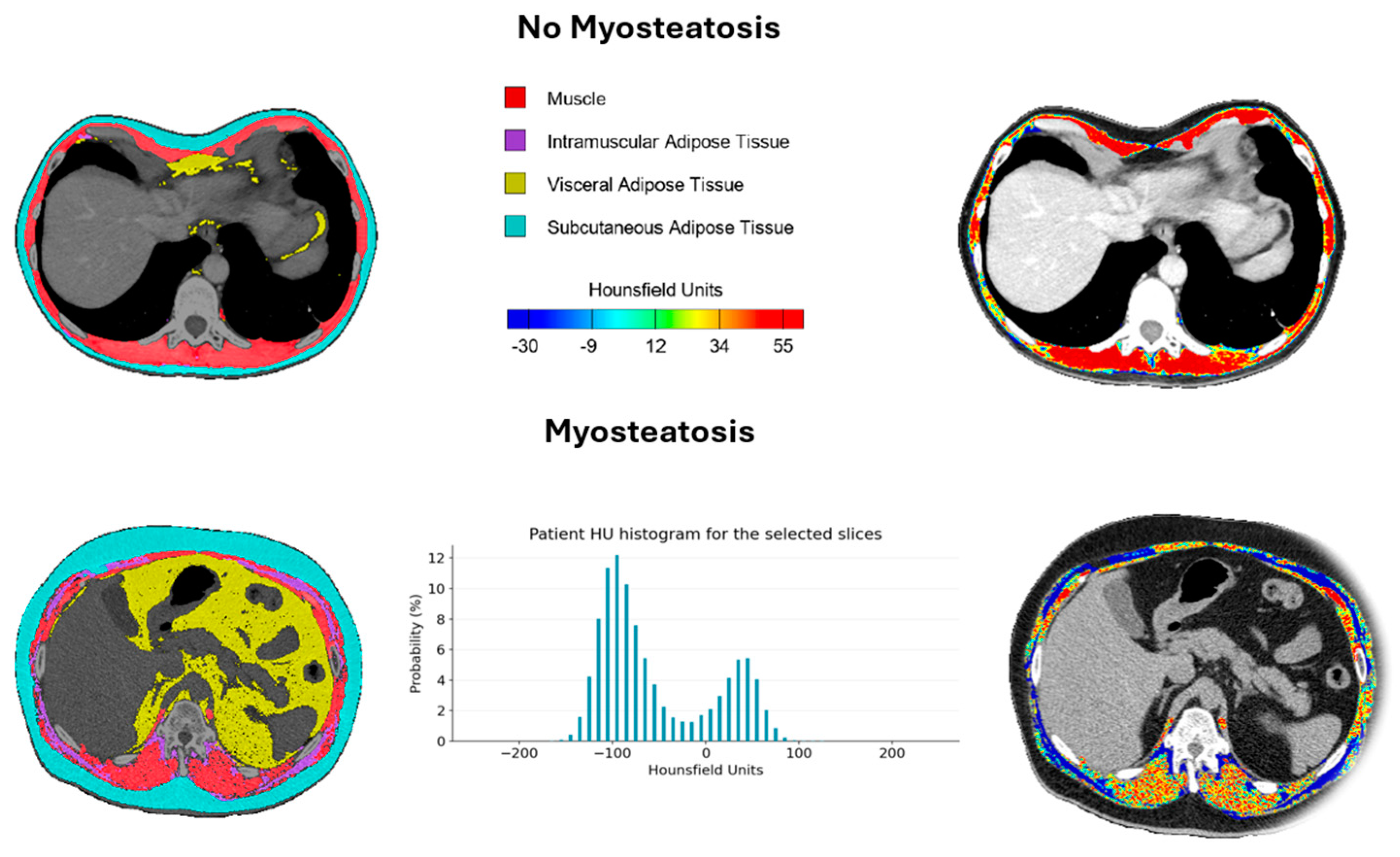
3.3. Determination of Cut-Off Point Value and Prognostic Analysis for Myoesteatosis
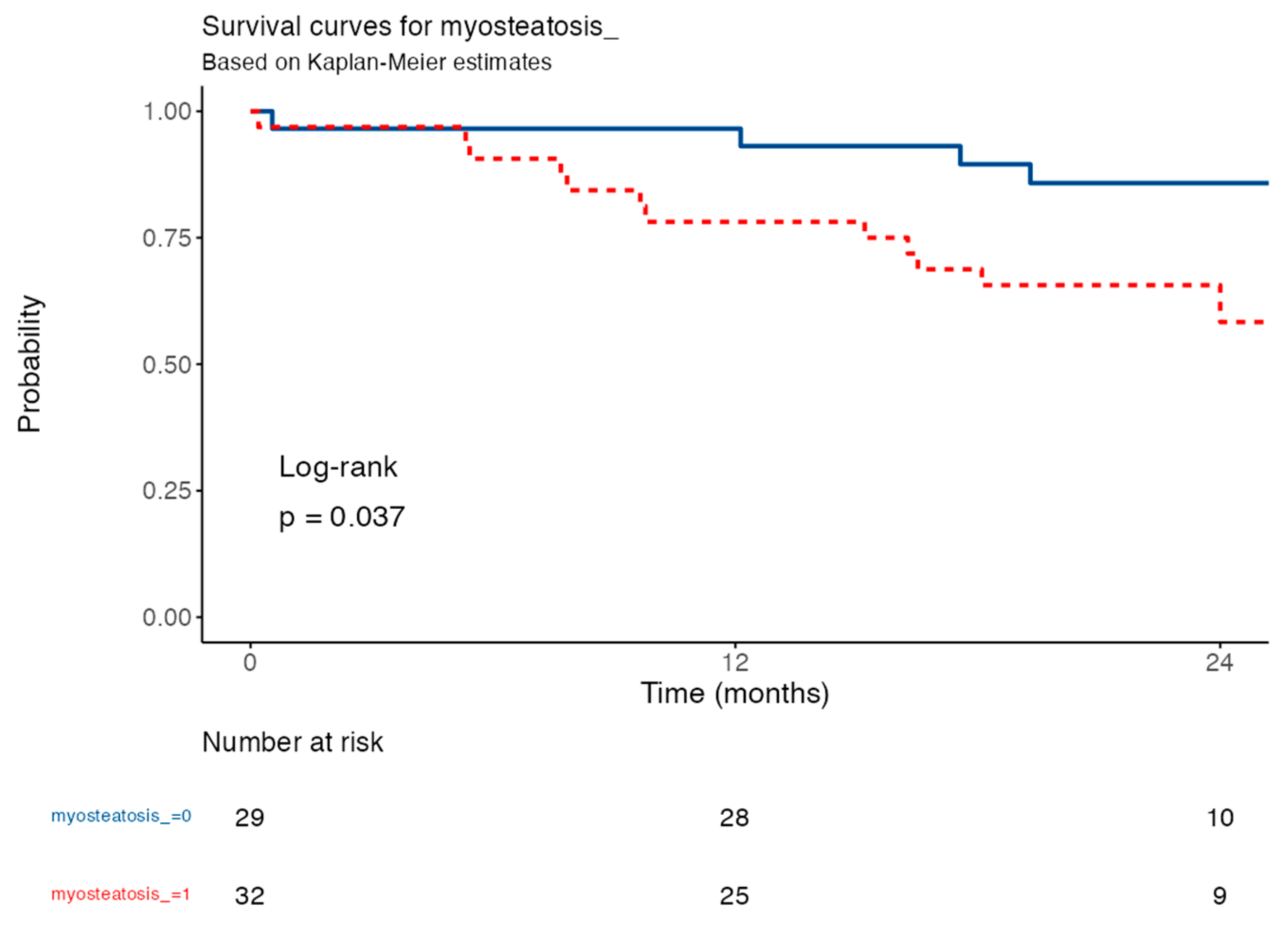
3.4. Survival Analysis Based on the Myosteatosis Cut-Off Point
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASMM | Appendicular skeletal muscle mass |
| ASMI | Appendicular skeletal muscle mass index |
| AUC | Area Under the Curve |
| BC | Body composition |
| BCM | Body cell mass |
| BIVA | Bioelectrical Impedance Vector Analysis |
| BMI | Body Mass Index |
| CI | Confidence interval |
| COPD | Chronic obstructive pulmonary diseases |
| CT | Computed tomography |
| DLCO | Diffusing capacity of the lungs for carbon monoxide |
| DOAJ | Directory of Open Access Journals |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| EASO | European Association for the Study of Obesity |
| FFM | Fat-free mass |
| FMI | Fat-free mass index |
| GLIM | Global Leadership Initiative on Malnutrition |
| HGS | Handgrip strength |
| HR | Hazard ratio |
| HU | Hounsfield unit |
| IPF | Idiopathic pulmonary fibrosis |
| IMAT | Intramuscular adipose tissue |
| LD | Linear dichroism |
| L-SAT | Leg subcutaneous adipose tissue |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MFA | Morphofunctional assessment |
| NU | Nutritional ultrasound® |
| CRP | C-reactive protein |
| Pha | Phase angle |
| SPha | Standardized phase angle |
| RF-CSA | Rectus femoris cross-sectional area |
| RF-Y-axis | Rectus femoris Y-axis |
| ROC | Receiver Operating Characteristic |
| SAT_area_T12 | Area of subcutaneous adipose tissue at the T12 computed tomography level |
| SAT_HU_T12 | Hounsfield units of subcutaneous adipose tissue at the T12 computed tomography level |
| SMI_T12CT | Skeletal Muscle Area in computed tomography at the T12 level |
| S_SAT | Superficial abdominal adipose tissue |
| TBW | Total Body Water |
| T12-CT | Computed tomography at the T12 level |
| TLA | Three letter acronym |
| T-SAT | Total abdominal adipose tissue |
| TUG | Timed Up and Go test |
| VAT | Visceral adipose tissue |
| VAT_area_T12 | Area of visceral adipose tissue at the T12 computed tomography level |
| VAT_HU_T12 | Hounsfield units of visceral adipose tissue at the T12 computed tomography level |
References
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [PubMed]
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; De Graaff, B.; Corte, T.J.; Teoh, A.K.; Walters, E.H.; Palmer, A.J. Mortality and survival in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. ERJ Open Res. 2022, 8, 00591–02021. [Google Scholar]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [PubMed]
- Çinkooğlu, A.; Bayraktaroğlu, S.; Ufuk, F.; Unat, Ö.S.; Köse, T.; Savaş, R.; Bishop, N.M. Reduced CT-derived erector spinae muscle area: A poor prognostic factor for short- and long-term outcomes in idiopathic pulmonary fibrosis patients. Clin. Radiol. 2023, 78, 904–911. [Google Scholar]
- Ozaltin, B.; Chapman, R.; Arfeen, M.Q.U.; Fitzpatick, N.; Hemingway, H.; Direk, K.; Jacob, J. Delineating excess comorbidities in idiopathic pulmonary fibrosis: An observational study. Respir. Res. 2024, 25, 249. [Google Scholar]
- Ruaro, B.; Pozzan, R.; Confalonieri, P.; Tavano, S.; Hughes, M.; Matucci Cerinic, M.; Baratella, E.; Zanatta, E.; Lerda, S.; Geri, P.; et al. Gastroesophageal Reflux Disease in Idiopathic Pulmonary Fibrosis: Viewer or Actor? To Treat or Not to Treat? Pharmaceuticals 2022, 15, 1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, H.J.; Kim, S.; Kim, Y.J.; Kim, H.C. Epidemiology and comorbidities in idiopathic pulmonary fibrosis: A nationwide cohort study. BMC Pulm. Med. 2023, 23, 54. [Google Scholar]
- Waxman, A.B.; Elia, D.; Adir, Y.; Humbert, M.; Harari, S. Recent advances in the management of pulmonary hypertension with interstitial lung disease. Eur. Respir. Rev. 2022, 31, 210220. [Google Scholar]
- Zhu, J.; Zhou, D.; Wang, J.; Yang, Y.; Chen, D.; He, F.; Li, Y. A Causal Atlas on Comorbidities in Idiopathic Pulmonary Fibrosis. Chest 2023, 164, 429–440. [Google Scholar]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J.F. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar]
- Luppi, F.; Kalluri, M.; Faverio, P.; Kreuter, M.; Ferrara, G. Idiopathic pulmonary fibrosis beyond the lung: Understanding disease mechanisms to improve diagnosis and management. Respir. Res. 2021, 22, 109. [Google Scholar]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [PubMed]
- Faverio, P.; Bocchino, M.; Caminati, A.; Fumagalli, A.; Gasbarra, M.; Iovino, P.; Petruzzi, A.; Scalfi, L.; Sebastiani, A.; Stanziola, A.A.; et al. Nutrition in Patients with Idiopathic Pulmonary Fibrosis: Critical Issues Analysis and Future Research Directions. Nutrients 2020, 12, 1131. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Pison, C.; Mölken, M.R.-V.; Slinde, F.; Steiner, M.; et al. Nutritional assessment and therapy in COPD: A European Respiratory Society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 10, 207–217. [Google Scholar]
- Jouneau, S.; Rousseau, C.; Lederlin, M.; Lescoat, A.; Kerjouan, M.; Chauvin, P.; Luque-Paz, D.; Guillot, S.; Oger, E.; Vernhet, L.; et al. Malnutrition and decreased food intake at diagnosis are associated with hospitalization and mortality of idiopathic pulmonary fibrosis patients. Clin. Nutr. 2022, 41, 1335–1342. [Google Scholar]
- Mochizuka, Y.; Suzuki, Y.; Kono, M.; Hasegawa, H.; Hashimoto, D.; Yokomura, K.; Inoue, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; et al. Geriatric Nutritional Risk Index is a predictor of tolerability of antifibrotic therapy and mortality risk in patients with idiopathic pulmonary fibrosis. Respirology 2023, 28, 775–783. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar]
- Herault, A.; Lévêque, E.; Draye-Carbonnier, S.; Decazes, P.; Zduniak, A.; Modzelewski, R.; Libraire, J.; Achamrah, N.; Ménard, A.-L.; Lenain, P.; et al. High prevalence of pre-existing sarcopenia in critically ill patients with hematologic malignancies admitted to the intensive care unit for sepsis or septic shock. Clin. Nutr. ESPEN 2023, 55, 373–383. [Google Scholar] [CrossRef]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Manini, T.; Patel, S.M.; Newman, A.; Travison, T.; Kiel, D.P.; Santanasto, A.J.; Xue, Q.; Shardell, M.; Duchowny, K.; et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J. Am. Geriatr. Soc. 2020, 68, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Fumagalli, A.; Conti, S.; Madotto, F.; Bini, F.; Harari, S.; Mondoni, M.; Oggionni, T.; Barisione, E.; Ceruti, P.; et al. Sarcopenia in idiopathic pulmonary fibrosis: A prospective study exploring prevalence, associated factors and diagnostic approach. Respir. Res. 2022, 23, 228. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.; Bodduluri, S.; O’Hare, L.; Blumhoff, S.; Acosta Lara, M.D.P.; De Andrade, J.A.; Kim, Y.-I.; Luckhardt, T.; McDonald, M.; Kulkarni, T. Association of musculoskeletal involvement with lung function and mortality in patients with idiopathic pulmonary fibrosis. Respir. Res. 2024, 25, 81. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Morley, J.E.; Thomas, D.R.; Wilson, M.M.G. Cachexia: Pathophysiology and clinical relevance1,2. Am. J. Clin. Nutr. 2006, 83, 735–743. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Imbimbo, G.; Jager-Wittenaar, H.; Cederholm, T.; Rothenberg, E.; Di Girolamo, F.G.; Amabile, M.I.; Sealy, M.; Schneider, S.; Barazzoni, R.; et al. Disease-related malnutrition with inflammation and cachexia. Clin. Nutr. 2023, 42, 1475–1479. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, S.; Pan, B.; Xie, W.; Meng, J. Ectopic and visceral fat deposition in aging, obesity, and idiopathic pulmonary fibrosis: An interconnected role. Lipids Health Dis. 2023, 22, 201. [Google Scholar] [CrossRef]
- Jing, X.; Tan, L.; Fu, H.; Yang, L.; Yang, M. Associations of ADL Disability with Trunk Muscle Mass and Muscle Quality Indicators Measured by Opportunistic Chest Computed Tomography Imaging Among Older Inpatients. Front. Med. 2021, 8, 743698. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Liu, H.; Zhu, L.; Wang, D.; Xie, F.; Shi, L.; Mei, J.; Jiang, X.; Zeng, Q.; Hu, P.; et al. Myosteatosis predicting risk of transition to severe COVID-19 infection. Clin. Nutr. 2022, 41, 3007–3015. [Google Scholar]
- García-Almeida, J.M.; García-García, C.; Ballesteros-Pomar, M.D.; Olveira, G.; Lopez-Gomez, J.J.; Bellido, V.; Lesmes, I.B.; Burgos, R.; Sanz-Paris, A.; Matia-Martin, P.; et al. Expert Consensus on Morphofunctional Assessment in Disease-Related Malnutrition. Grade Review and Delphi Study. Nutrients 2023, 15, 612. [Google Scholar] [CrossRef]
- García, C.G.; Almeida, J.M.G.; Aguilar, I.M.V.; Castañeda, V.B.; Guerrero, D.B. Morphofunctional assessment of patient nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. Available online: https://www.nutricionhospitalaria.org/articles/03378/show (accessed on 1 December 2024).
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef] [PubMed]
- De Palo, T.; Messina, G.; Edefonti, A.; Perfumo, F.; Pisanello, L.; Peruzzi, L.; Di Iorio, B.; Mignozzi, M.; Vienna, A.; Conti, G.; et al. Normal values of the bioelectrical impedance vector in childhood and puberty. Nutrition 2000, 16, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Palmas, F.; Ciudin, A.; Guerra, R.; Eiroa, D.; Espinet, C.; Roson, N.; Burgos, R.; Simó, R. Comparison of computed tomography and dual-energy X-ray absorptiometry in the evaluation of body composition in patients with obesity. Front. Endocrinol. 2023, 14, 1161116. [Google Scholar] [CrossRef]
- Soria-Utrilla, V.; Sánchez-Torralvo, F.J.; Palmas-Candia, F.X.; Fernández-Jiménez, R.; Mucarzel-Suarez-Arana, F.; Guirado-Peláez, P.; Olveira, G.; García-Almeida, J.M.; Burgos-Peláez, R. AI-Assisted Body Composition Assessment Using CT Imaging in Colorectal Cancer Patients: Predictive Capacity for Sarcopenia and Malnutrition Diagnosis. Nutrients 2024, 16, 1869. [Google Scholar] [CrossRef]
- Fernández-Jiménez, R.; Sanmartín-Sánchez, A.; Cabrera-César, E.; Espíldora-Hernández, F.; Vegas-Aguilar, I.; Amaya-Campos, M.D.M.; Palmas-Candia, F.X.; Claro-Brandner, M.; Olivares-Alcolea, J.; Simón-Frapolli, V.J.; et al. IA-Body Composition CT at T12 in Idiopathic Pulmonary Fibrosis: Diagnosing Sarcopenia and Correlating with Other Morphofunctional Assessment Techniques. Nutrients 2024, 16, 2885. [Google Scholar] [CrossRef]
- Brath, M.S.G.; Sahakyan, M.; Mark, E.B.; Frøkjær, J.B.; Rasmussen, H.H.; Østergaard, L.R.; Weinreich, U.M. Association between thoracic and third lumbar CT-derived muscle mass and density in Caucasian patients without chronic disease: A proof-of-concept study. Eur. Radiol Exp. 2023, 7, 26. [Google Scholar]
- Molwitz, I.; Ozga, A.K.; Gerdes, L.; Ungerer, A.; Köhler, D.; Ristow, I.; Leiderer, M.; Adam, G.; Yamamura, J. Prediction of abdominal CT body composition parameters by thoracic measurements as a new approach to detect sarcopenia in a COVID-19 cohort. Sci. Rep. 2022, 12, 6443. [Google Scholar] [PubMed]
- Nemec, U.; Heidinger, B.; Sokas, C.; Chu, L.; Eisenberg, R.L. Diagnosing Sarcopenia on Thoracic Computed Tomography. Acad. Radiol. 2017, 24, 1154–1161. [Google Scholar]
- Salhöfer, L.; Bonella, F.; Meetschen, M.; Umutlu, L.; Forsting, M.; Schaarschmidt, B.M.; Opitz, M.K.; Kleesiek, J.; Hosch, R.M.; Koitka, S.; et al. Automated 3D-Body Composition Analysis as a Predictor of Survival in Patients with Idiopathic Pulmonary Fibrosis. J. Thorac. Imaging 2024, 40, e0823. Available online: https://journals.lww.com/10.1097/RTI.0000000000000803 (accessed on 12 December 2024).
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech. Rep. Ser. 2000, 894, 1–252. [Google Scholar]
- Cox, I.A.; Borchers Arriagada, N.; De Graaff, B.; Corte, T.J.; Glaspole, I.; Lartey, S.; Walters, E.H.; Palmer, A.J. Health-related quality of life of patients with idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Eur. Respir. Rev. 2020, 29, 200154. [Google Scholar]
- Kanjrawi, A.A.; Mathers, L.; Webster, S.; Corte, T.J.; Carey, S. Nutritional status and quality of life in interstitial lung disease: A prospective cohort study. BMC Pulm. Med. 2021, 21, 51. [Google Scholar]
- Iwanami, Y.; Ebihara, K.; Nakao, K.; Kubo, R.; Miyagi, M.; Nakamura, Y.; Sakamoto, S.; Kishi, K.; Okuni, I.; Ebihara, S. Impact of Controlling Nutritional Status Score on Mortality in Elderly Patients with Idiopathic Pulmonary Fibrosis. JCM 2024, 13, 2825. [Google Scholar]
- Kondrup, J. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.-L. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar]
- Alahmad, M.A.M.; Gibson, C.A. The impact of pulmonary cachexia on inpatient outcomes: A national study. Ann. Thorac. Med. 2023, 18, 156–161. [Google Scholar] [PubMed]
- Fernández-Jiménez, R.; Cabrera Cesar, E.; Sánchez García, A.; Espíldora Hernández, F.; Vegas-Aguilar, I.M.; Amaya-Campos, M.D.M.; Cornejo-Pareja, I.; Guirado-Peláez, P.; Simón-Frapolli, V.; Murri, M.; et al. Rectus Femoris Cross-Sectional Area and Phase Angle asPredictors of 12-Month Mortality in Idiopathic Pulmonary Fibrosis Patients. Nutrients 2023, 15, 4473. [Google Scholar] [CrossRef]
- Awano, N.; Inomata, M.; Kuse, N.; Tone, M.; Yoshimura, H.; Jo, T.; Takada, K.; Sugimoto, C.; Tanaka, T.; Sumikawa, H.; et al. Quantitative computed tomography measures of skeletal muscle mass in patients with idiopathic pulmonary fibrosis according to a multidisciplinary discussion diagnosis: A retrospective nationwide study in Japan. Respir. Investig. 2020, 58, 91–101. [Google Scholar]
- Nakano, A.; Ohkubo, H.; Taniguchi, H.; Kondoh, Y.; Matsuda, T.; Yagi, M.; Furukawa, T.; Kanemitsu, Y.; Niimi, A. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci. Rep. 2020, 10, 2312. [Google Scholar] [PubMed]
- Woolcott, O.O.; Bergman, R.N. Defining cutoffs to diagnose obesity using the relative fat mass (RFM): Association with mortality in NHANES 1999–2014. Int. J. Obes. 2020, 44, 1301–1310. [Google Scholar]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar]
- Faverio, P.; Fumagalli, A.; Conti, S.; Madotto, F.; Bini, F.; Harari, S.; Mondoni, M.; Oggionni, T.; Barisione, E.; Ceruti, P. Nutritional assessment in idiopathic pulmonary fibrosis: A prospective multicentre study. ERJ Open Res. 2022, 8, 00443–02021. [Google Scholar]
- Wunderle, C.; Gomes, F.; Schuetz, P.; Stumpf, F.; Austin, P.; Ballesteros-Pomar, M.D.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; et al. ESPEN practical guideline: Nutritional support for polymorbid medical inpatients. Clin. Nutr. 2024, 43, 674–691. [Google Scholar]
- Wunderle, C.; Gomes, F.; Schuetz, P.; Stumpf, F.; Austin, P.; Ballesteros-Pomar, M.D.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; et al. ESPEN guideline on nutritional support for polymorbid medical inpatients. Clin. Nutr. 2023, 42, 1545–1568. [Google Scholar]
| Variables | Females (n = 13) | Males (n = 69) | p-Value |
|---|---|---|---|
| Weight (kg) | 76.4 ± 9.3 | 79.9 ± 12.7 | 0.341 |
| Height (cm) | 159.0 ± 4.8 | 170.9 ± 7.0 | <0.001 |
| BMI (kg/m2) | 30.1 ± 2.7 | 27.3 ± 3.4 | 0.004 |
| BIVA Parameters | |||
| Rz (Ω) | 562.7 ± 62.7 | 515.5 ± 60.2 | 0.029 |
| Xc (Ω) | 44.5 ± 5.7 | 44.1 ± 8.3 | 0.859 |
| FFM (kg) | 45.7 ± 3.2 | 56.9 ± 6.6 | <0.001 |
| BCM (kg) | 20.7 ± 2.4 | 27.0 ± 4.9 | <0.001 |
| TBW (L) | 34.0 ± 2.5 | 42.5 ± 5.4 | <0.001 |
| FM (kg) | 30.7 ± 8.0 | 23.0 ± 7.5 | 0.003 |
| Pha (°) | 4.5 ± 0.5 | 4.9 ± 0.8 | 0.097 |
| NAK (ratio) | 1.08 ± 0.11 | 1.18 ± 0.19 | 0.089 |
| Hydration (%) | 74.3 ± 1.9 | 74.7 ± 2.4 | 0.482 |
| Nutrition (score) | 624.0 ± 73.2 | 791.1 ± 162.1 | <0.001 |
| Nutritional Ultrasound (NU) | |||
| RF-CSA (cm2) | 2.4 ± 0.4 | 3.6 ± 1.0 | <0.001 |
| RF-Y-Axis (cm) | 1.0 ± 0.2 | 1.1 ± 0.3 | 0.026 |
| L-SAT (cm) | 1.7 ± 0.6 | 0.6 ± 0.3 | <0.001 |
| T-SAT (cm) | 2.4 ± 0.7 | 1.6 ± 0.7 | <0.001 |
| S-SAT (cm) | 1.1 ± 0.3 | 0.7 ± 0.2 | <0.001 |
| VAT (cm2) | 0.8 ± 0.5 | 0.6 ± 0.3 | 0.043 |
| Functional Tests | |||
| HGS (kg) | 19.2 ± 4.9 | 35.7 ± 8.4 | <0.001 |
| TUG (s) | 9.9 ± 2.7 | 7.3 ± 1.8 | <0.001 |
| Variable | IPF (Mean ± SD) | Control (Mean ± SD) | p-Value |
|---|---|---|---|
| Patients (n) | 82 | 32 | |
| Gender (Male) | 84.1% (69/82) | 68.75% (22/32) | p = 0.66 |
| Age | 71.1 ± 7.35 | 70.2 ± 5.19 | p = 0.526 |
| Weight (kg) | 79.39 ± 12.28 | 72.98 ± 13.21 | 0.016 |
| Height (cm) | 169.05 ± 7.98 | 165.91 ± 9.12 | 0.072 |
| BMI (kg/m2) | 27.73 ± 3.45 | 26.35 ± 3.29 | 0.054 |
| BIVA Parameters | |||
| Rz (Ω) | 522.96 ± 62.65 | 520.47 ± 84.74 | 0.352 |
| Xc (Ω) | 44.18 ± 7.92 | 49.86 ± 5.12 | <0.001 |
| FFM (kg) | 55.17 ± 7.45 | 53.92 ± 10.36 | 0.940 |
| BCM (kg) | 26.03 ± 5.11 | 27.82 ± 6.51 | 0.124 |
| TBW (L) | 41.19 ± 5.95 | 39.62 ± 7.67 | 0.247 |
| FM (kg) | 24.22 ± 8.05 | 19.05 ± 5.66 | 0.001 |
| Pha (°) | 4.85 ± 0.77 | 5.54 ± 0.61 | <0.001 |
| NAK (ratio) | 1.16 ± 0.19 | 1.07 ± 0.08 | 0.009 |
| Hydration (%) | 74.61 ± 2.36 | 73.44 ± 0.23 | 0.006 |
| Nutrition (score) | 764.61 ± 163.19 | 832.97 ± 183.75 | 0.055 |
| Spha (°) | −0.92 ± 0.94 | −0.26 ± 0.48 | <0.001 |
| Nutritional Ultrasound (NU) | |||
| RF-CSA (cm2) | 3.37 ± 1.00 | 4.58 ± 1.61 | <0.001 |
| RF-Y-Axis (cm) | 1.12 ± 0.28 | 1.40 ± 0.34 | <0.001 |
| L-SAT (cm) | 0.81 ± 0.53 | 0.83 ± 0.46 | 0.845 |
| T-SAT (cm) | 1.70 ± 0.73 | 1.60 ± 0.64 | 0.498 |
| S-SAT (cm) | 0.74 ± 0.30 | 0.70 ± 0.30 | 0.538 |
| VAT (cm) | 0.66 ± 0.30 | 0.66 ± 0.28 | 0.907 |
| Functional Tests | |||
| HGS (kg) | 33.11 ± 10.02 | 32.56 ± 8.03 | 0.785 |
| TUG (s) | 7.72 ± 2.17 | 5.79 ± 1.12 | <0.001 |
| Nutritional Phenotype | Criteria | Counts (n) | % of Total |
|---|---|---|---|
| Cachexia, Evans’ Criteria | 11 | 13.4% | |
| GLIM Cachexia | 44 | 53.7% | |
| Low-Intake Cachexia | 46 | 56.1% | |
| Inflammation Cachexia (CRP) | 32 | 39.0% | |
| Low Muscle Mass (Low ASMI/ASMM) | 52 | 63.4% | |
| Sarcopenia, EWGSOP2 Criteria | 14 | 16.5% | |
| Low Muscle Mass (Low ASMI/ASMM) | 55 | 64.7% | |
| Dinapenia | 19 | 22.4% | |
| Dysfunctionality (Severe Sarcopenia) | 0 | 0% | |
| Disease-Related Malnutrition, GLIM Criteria | |||
| No GLIM Malnutrition | 16 | 18.8% | |
| Moderate Malnutrition | 61 | 71.8% | |
| Lost Weight (Moderate) | 21 | 25.6% | |
| BMI (Moderate) | 23 | 28.0% | |
| Low Muscle Mass (Low ASMI/ASMM) | 52 | 63.4% | |
| Severe Malnutrition | 8 | 9.4% | |
| Lost Weight (Severe) | 22 | 26.8% | |
| Low Muscle Mass (Low ASMI/ASMM) | 52 | 63.4% | |
| BMI (Severe) | 0 | 0.0% | |
| Obesity and Sarcopenic Obesity | |||
| Obesity, WHO Criteria (BMI ≥ 30) | 23 | 28.0% | |
| Sarcopenic Obesity, ESPEN/EASO Criteria | 5.79 ± 1.12 | <0.001 | |
| Dinapenia | 19 | 22.4% | |
| High Fat Mass (%) | 33 | 40.2% | |
| SMM/KG | 35 | 41.2% | |
| Dysfunctionality (Severe Sarcopenia) | 0 | 0% |
| Parameter | Non-Cachexia (n = 65) | Cachexia (n = 17) | p | Non-Sarcopenia (n = 70) | Sarcopenia (n = 12) | p | Non-Malnutrition (n = 16) | Malnutrition, GLIM (n = 69) | p |
|---|---|---|---|---|---|---|---|---|---|
| Phase Angle (Pha) | 4.91 ± 0.73 | 4.61 ± 0.89 | 0.173 | 4.90 ± 0.73 | 4.53 ± 0.93 | 0.156 | 5.17 ± 0.86 | 4.75 ± 0.72 | 0.046 |
| Standardized Phase Angle (Spha) | −0.87 ± 0.96 | −1.11 ± 0.88 | 0.397 | −0.91 ± 0.91 | −0.96 ± 1.15 | 0.572 | −0.75 ± 1.05 | −1.01 ± 0.93 | 0.335 |
| Fat-Free Mass (FFM, kg) | 56.41 ± 7.55 | 50.45 ± 4.79 | 0.002 | 56.24 ± 7.27 | 48.93 ± 5.20 | 0.001 | 61.04 ± 5.03 | 53.59 ± 7.32 | <0.001 |
| Body Cell Mass (BCM, kg) | 26.84 ± 5.06 | 22.91 ± 4.09 | 0.007 | 26.72 ± 4.91 | 21.97 ± 4.47 | 0.003 | 29.82 ± 4.01 | 24.96 ± 4.93 | <0.001 |
| NAK (ratio) | 1.16 ± 0.19 | 1.18 ± 0.19 | 0.672 | 1.16 ± 0.18 | 1.19 ± 0.23 | 0.906 | 1.16 ± 0.27 | 1.17 ± 0.17 | 0.922 |
| RF-CSA (cm2) | 3.51 ± 1.04 | 2.85 ± 0.65 | 0.014 | 3.48 ± 1.02 | 0.73 ± 0.62 | 0.019 | 3.67 ± 0.91 | 3.27 ± 1.01 | 0.151 |
| RF-Y-Axis (cm) | 1.16 ± 0.29 | 0.99 ± 0.18 | 0.012 | 1.15 ± 0.28 | 0.98 ± 0.21 | 0.018 | 1.20 ± 0.26 | 1.10 ± 0.28 | 0.181 |
| Handgrip Strength (HGS, kg) | 34.83 ± 9.65 | 26.53 ± 8.83 | 0.004 | 35.40 ± 8.81 | 19.72 ± 4.75 | <0.001 | 37.00 ± 8.64 | 31.95 ± 10.04 | 0.067 |
| TUG (s) | 7.63 ± 2.27 | 8.04 ± 1.77 | 0.258 | 7.53 ± 2.10 | 8.87 ± 2.34 | 0.048 | 7.00 ± 1.47 | 7.86 ± 2.24 | 0.076 |
| SMI_T12CT (kg/m2) | 27.67 ± 7.07 | 22.36 ± 5.01 | 0.018 | 27.20 ± 6.93 | 21.98 ± 5.74 | 0.061 | 31.38 ± 9.01 | 24.79 ± 5.54 | 0.002 |
| Muscle Area T12 (cm2) | 79.76 ± 22.64 | 62.49 ± 12.87 | 0.015 | 78.17 ± 22.03 | 61.53 ± 15.63 | 0.071 | 91.48 ± 26.48 | 70.48 ± 18.18 | 0.001 |
| Parameter | Non-Obese (n = 62) | Obese (n = 23) | p | Non-Sarcopenic Obese (n = 77) | Sarcopenic Obese (n = 5) | p |
|---|---|---|---|---|---|---|
| Pha | 4.69 ± 0.70 | 5.18 ± 0.84 | 0.008 | 4.86 ± 0.75 | 4.24 ± 0.89 | 0.077 |
| Spha | −1.20 ± 0.97 | −0.31 ± 0.49 | <0.001 | −0.94 ± 0.95 | −1.21 ± 1.07 | 0.546 |
| FFM | 53.83 ± 6.75 | 58.11 ± 8.68 | 0.019 | 55.43 ± 7.35 | 47.92 ± 7.15 | 0.029 |
| BCM | 24.83 ± 4.26 | 28.69 ± 6.20 | 0.002 | 26.21 ± 4.97 | 20.52 ± 4.98 | 0.015 |
| NAK | 1.22 ± 0.18 | 1.02 ± 0.12 | <0.001 | 1.17 ± 0.19 | 1.14 ± 0.19 | 0.733 |
| RF-CSA | 3.27 ± 0.92 | 3.56 ± 1.19 | 0.239 | 3.38 ± 1.01 | 2.87 ± 0.55 | 0.300 |
| RF-Y-Axis | 1.06 ± 0.25 | 1.26 ± 0.30 | 0.004 | 1.12 ± 0.28 | 1.06 ± 0.25 | 0.659 |
| HGS | 32.57 ± 9.05 | 33.78 ± 12.20 | 0.621 | 33.95 ± 9.28 | 16.20 ± 1.79 | <0.001 |
| TUG | 7.49 ± 2.01 | 8.23 ± 2.41 | 0.160 | 7.53 ± 2.03 | 10.36 ± 2.26 | 0.003 |
| SMI_T12CT | 24.53 ± 5.60 | 30.17 ± 8.20 | 0.003 | 26.48 ± 6.83 | 22.11 ± 7.78 | 0.344 |
| Muscle_area_T12 | 69.92 ± 17.45 | 86.98 ± 26.54 | 0.004 | 76.06 ± 21.70 | 59.19 ± 18.52 | 0.227 |
| Parameter | Non-Obese (n = 62) | Obese (n = 23) | p | Non-Sarcopenic Obese (n = 77) | Sarcopenic Obese (n = 5) | p |
|---|---|---|---|---|---|---|
| FMI | 7.29 ± 1.96 | 11.53 ± 2.46 | <0.001 | 8.18 ± 2.67 | 12.52 ± 2.13 | 0.002 |
| L-SAT | 0.66 ± 0.38 | 1.12 ± 0.70 | <0.001 | 0.75 ± 0.48 | 1.43 ± 0.81 | 0.031 |
| T-SAT | 1.61 ± 0.70 | 1.85 ± 0.77 | 0.177 | 1.63 ± 0.71 | 2.40 ± 0.59 | 0.024 |
| S-SAT | 0.70 ± 0.29 | 0.83 ± 0.31 | 0.078 | 0.71 ± 0.29 | 1.03 ± 0.18 | 0.013 |
| VAT | 0.64 ± 0.27 | 0.67 ± 0.39 | 0.694 | 0.64 ± 0.30 | 0.84 ± 0.36 | 0.177 |
| TUG | 7.49 ± 2.01 | 8.23 ± 2.41 | 0.160 | 7.53 ± 2.03 | 10.36 ± 2.26 | 0.006 |
| IMAT_perc_T12 | 1.79 ± 0.60 | 2.05 ± 0.88 | 0.186 | 1.83 ± 0.68 | 2.47 ± 0.88 | 0.166 |
| IMAT_HU_T12 | −63.86 ± 5.70 | −64.07 ± 4.72 | 0.889 | −63.71 ± 5.34 | −66.93 ± 6.08 | 0.251 |
| VAT_area_T12 | 167.86 ± 86.98 | 199.01 ± 63.86 | 0.176 | 178.76 ± 83.97 | 152.73 ± 25.62 | 0.542 |
| VAT_HU_T12 | −96.73 ± 6.66 | −99.92 ± 4.77 | 0.071 | −97.71 ± 6.28 | −97.13 ± 7.56 | 0.861 |
| SAT_area_T12 | 101.15 ± 49.43 | 162.72 ± 49.34 | <0.001 | 115.97 ± 56.04 | 166.98 ± 46.43 | 0.046 |
| SAT_HU_T12 | −96.53 ± 9.71 | −103.37 ± 7.95 | 0.011 | −98.31 ± 9.95 | −101.97 ± 3.60 | 0.469 |
| Parameter | All | HR (Multivariable) | Months | Survival % | 95% CI |
|---|---|---|---|---|---|
| Absence of myoesteatosis | 29 (47.5%) | 12 | 96.6% | 100% | |
| Myoesteatosis ≥ 15% | 32 (52.5%) | 3.13 (1.01–9.70), p = 0.049 | 12 | 65% | 93.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartín-Sánchez, A.; Fernández-Jiménez, R.; Cabrera-César, E.; Espíldora-Hernández, F.; Vegas-Aguilar, I.; Amaya-Campos, M.d.M.; Palmas-Candia, F.X.; Olivares-Alcolea, J.; Simón-Frapolli, V.J.; Cornejo-Pareja, I.; et al. The Nutritional Phenotyping of Idiopathic Pulmonary Fibrosis Through Morphofunctional Assessment: A Bicentric Cross-Sectional Case–Control Study. Life 2025, 15, 516. https://doi.org/10.3390/life15040516
Sanmartín-Sánchez A, Fernández-Jiménez R, Cabrera-César E, Espíldora-Hernández F, Vegas-Aguilar I, Amaya-Campos MdM, Palmas-Candia FX, Olivares-Alcolea J, Simón-Frapolli VJ, Cornejo-Pareja I, et al. The Nutritional Phenotyping of Idiopathic Pulmonary Fibrosis Through Morphofunctional Assessment: A Bicentric Cross-Sectional Case–Control Study. Life. 2025; 15(4):516. https://doi.org/10.3390/life15040516
Chicago/Turabian StyleSanmartín-Sánchez, Alicia, Rocío Fernández-Jiménez, Eva Cabrera-César, Francisco Espíldora-Hernández, Isabel Vegas-Aguilar, María del Mar Amaya-Campos, Fiorella Ximena Palmas-Candia, Josefina Olivares-Alcolea, Víctor José Simón-Frapolli, Isabel Cornejo-Pareja, and et al. 2025. "The Nutritional Phenotyping of Idiopathic Pulmonary Fibrosis Through Morphofunctional Assessment: A Bicentric Cross-Sectional Case–Control Study" Life 15, no. 4: 516. https://doi.org/10.3390/life15040516
APA StyleSanmartín-Sánchez, A., Fernández-Jiménez, R., Cabrera-César, E., Espíldora-Hernández, F., Vegas-Aguilar, I., Amaya-Campos, M. d. M., Palmas-Candia, F. X., Olivares-Alcolea, J., Simón-Frapolli, V. J., Cornejo-Pareja, I., Sánchez-García, A., Murri, M., Guirado-Peláez, P., Vidal-Suárez, Á., Garrido-Sánchez, L., Tinahones, F. J., Velasco-Garrido, J. L., & García-Almeida, J. M. (2025). The Nutritional Phenotyping of Idiopathic Pulmonary Fibrosis Through Morphofunctional Assessment: A Bicentric Cross-Sectional Case–Control Study. Life, 15(4), 516. https://doi.org/10.3390/life15040516









