Environmental Factors Influencing the Establishment of the Invasive Australian Redclaw Crayfish (Cherax quadricarinatus) in a Biosphere Reserve on the Central Mexican Plateau
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SGBR | Sierra Gorda Biosphere Reserve |
| TL | Total length |
| CL | Carapace length |
| VBHA | Visual-Based Habitat Assessment |
| PCA | Principal component analysis |
| NMDS | Non-metric multidimensional scaling |
| PC | Principal component |
| DO | Dissolved oxygen |
| TDS | Total dissolved solids |
| TEMP | Water temperature |
| CE | Electrical conductivity |
| EPS | Epifaunal substrate |
| EMB | Substrate embedment |
| V/D | Velocity and depth regime variations |
| SD | Sediment deposition |
| CFS | Channel flow status |
| CA | Channel alteration |
| FR | Riffle frequency |
| BS | Bank stability |
| VP | Vegetation protection |
| RW | Riparian width |
| LC | Longitudinal continuity |
| CS | Composition and structure |
| RQI | Riparian Quality Index |
| ADR | Age diversity and regeneration |
| BC | Bank condition |
| LAC | Lateral connectivity |
| VC | Vertical connectivity |
| RT | Taxon richness |
| EPT | Ephemeroptera, Plecoptera, and Trichoptera richness |
| II | Intolerant insects |
| IT | Intolerant taxa |
| FT | Fixed taxa |
| MT | Mean tolerance |
| IIBAMA | Index of biological integrity based on aquatic macroinvertebrate assemblages |
| D1 | First-order alpha diversity |
| NI | Number of individuals |
| RA | Relative abundance |
| AYU | Ayutla |
| DA | Downstream of Adjuntas |
| HIG | El Higuerón |
| SAL | El Salitrillo |
| CM | Concá Manantiales |
| PM | Puente de las Mesas |
| DRJ | Downstream of Jalpan River |
| Rs | Spearman rank order correlation |
References
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Brasdshaw, C.J.A. Courchamp F High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Wilson, J.R.U.; Richardson, D.M.; Hui, C.; Davies, S.J.; Kumschick, S.; Le Roux, J.J.; Measey, J.; Saul, W.-C. Pulliam JRC Emerging infectious diseases and biological invasions: A call for a One Health collaboration in science and management. R. Soc. Open Sci. 2019, 6, 181577. [Google Scholar] [CrossRef]
- Jackson, M.C.; Wasserman, R.J.; Grey, J.; Ricciardi, A.; Dick, J.T.A.; Alexander, M.E. Chapter Two—Novel and Disrupted Trophic Links Following Invasion in Freshwater Ecosystems. In Advances in Ecological Research, Networks of Invasion: Empirical Evidence and Case Studies; Bohan, D.A., Dumbrell, A.J., Massol, F., Eds.; Academic Press: Oxford, UK, 2017; pp. 55–97. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, I.I.I.F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Haubrock, P.J.; Bernery, C.; Cuthbert, R.N.; Liu, C.; Kourantidou, M.; Leroy, B.; Turbelin, A.J.; Kramer, A.M.; Verbrugge, L.; Diagne, C.; et al. Knowledge gaps in economic costs of invasive alien fish worldwide. Sci. Total Environ. 2022, 803, 149875. [Google Scholar] [CrossRef] [PubMed]
- Polce, C.; Cardoso, A.C.; Deriu, I.; Gervasini, E.; Tsiamis, K.; Vigiak, O.; Zulian, G.; Maes, J. Invasive alien species of policy concerns show widespread patterns of invasion and potential pressure across European. Sci. Rep. 2023, 13, 81294. [Google Scholar] [CrossRef]
- Frederico, R.G.; Salvador, G.N.; Andrade, A.; Rosa, G.R.; Torquato, G.V. Freshwater ecosystem vulnerability: Is native climatic niche good enough to predict invasion events? Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1890–1896. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Lapointe, N.W.R.; Marchetti, M.P. Non-Indigenous Fishes and Their Role in Freshwater Fish Imperilment. In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 238–269. [Google Scholar]
- Chaffin, B.C.; Garmestani, A.S.; Angeler, D.G.; Herrmann, D.L.; Stow, C.A.; Nyström, M.; Sendzimir, J.; Hopton, M.E.; Kolasa, J.; Allen, C.R. Biological invasions, ecological resilience and adaptive governance. J. Environ. Manag. Adapt. Manag. Ecosyst. Serv. 2016, 183, 399–407. [Google Scholar] [CrossRef]
- Ruiz, G.M.; Fofonoff, P.; Hines, A.H.; Grosholz, E.D. Non-indigenous species as stressors in estuarine and marine communities: Assessing invasion impacts and interactions. Limnol. Oceanogr. 1999, 44, 950–972. [Google Scholar] [CrossRef]
- Costantini, M.L.; Kabala, J.P.; Sporta-Caputi, S.; Ventura, M.; Calizza, E.; Careddu, G.; Rossi, L. Biological Invasions in Fresh Waters: Micropterus salmoides, an American Fish Conquering the World. Water 2023, 15, 3796. [Google Scholar] [CrossRef]
- Hänfling, B.; Edwards, F.; Gherardi, F. Invasive alien Crustacea: Dispersal, establishment, impact and control. BioControl 2011, 56, 573–595. [Google Scholar] [CrossRef]
- Mendoza-Alfaro, R.; Rodriguez-Almaraz, G.; Castillo-Alvarado, S.A. Riesgo de Dispersión y Posibles Impactos de los Acociles Australianos del Género Cherax en México; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico city, Mexico, 2011; 140p. [CrossRef]
- Sagi, A.; Shoukrun, R.; Levy, T.; Barki, A.; Hulata, G.; Karplus, I. Reproduction and molt in previously spawned and first-time spawning red-claw crayfish Cherax quadricarinatus females following eyestalk ablation during the winter reproductive-arrest period. Aquaculture 1977, 56, 101–111. [Google Scholar] [CrossRef]
- Madzivanzira, T.C.; South, J.; Wood, L.E.; Nunes, A.L.; Weyl, O.L.F. A Review of Freshwater Crayfish Introductions in Africa. Rev. Fish. Sci. Aquac. 2021, 29, 218–241. [Google Scholar] [CrossRef]
- Nunes, A.L.; Zengeya, T.A.; Hoffman, A.C.; Measey, G.J.; Weyl, O.L.F. Distribution and establishment of the alien Australian redclaw crayfish, Cherax quadricarinatus, in South Africa and Swaziland. PeerJ 2017, 5, e3135. [Google Scholar] [CrossRef]
- Patoka, J.; Wardiatno, Y.; Kuříková, P.; Petrtýl, M.; Kalous, L. Cherax quadricarinatus (von Martens) has invaded Indonesian territory west of the Wallace Line: Evidences from Java. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 39. [Google Scholar] [CrossRef]
- Sze-man, Y.; Anthony, L. First record of the Australian redclaw crayfish Cherax quadricarinatus (von Martens 1868) in Hong Kong, China. BioInvasions Rec. 2020, 10, 369–377. [Google Scholar] [CrossRef]
- Álvarez, F.; Bortolini, J.L.; Villalobos, J.L.; García, L. La presencia del acocil australiano Cherax quadricarinatus (von Martens, 1868) en México. In Especies Invasoras Acuáticas: Casos de Estudio en Ecosistemas de México; Low-Pfeng, A.M., Quijón, P.A., Peters-Recagno, E.M., Eds.; Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT): Mexico City, Mexico; Instituto Nacional de Ecología y Cambio Climático (INECC): Mexico City, Mexico; University of Prince Edward Island (UPEI): Mexico City, Mexico, 2014; pp. 603–622. [Google Scholar]
- Bortolini, J.L.; Álvarez, F.; Rodríguez-Almaraz, G. On the presence of the Australian redclaw crayfish, Cherax quadricarinatus, in Mexico. Biol. Invasions 2007, 9, 615–620. [Google Scholar] [CrossRef]
- Smith, G.; Glendinning, S.; Ventura, T. Transcriptomic Changes Following Induced De-Masculinisation of Australian Red Claw Crayfish Cherax quadricarinatus. Int. J. Mol. Sci. 2023, 24, 3292. [Google Scholar] [CrossRef]
- Marufu, L.T.; Dalu, T.; Phiri, C.; Barson, M.; Simango, R.; Utete, B.; Nhiwatiwa, T. The diet of an invasive crayfish, Cherax quadricarinatus (Von Martens, 1868), in Lake Kariba, inferred using stomach content and stable isotope analyses. BioInvasions Rec. 2018, 7, 121–132. [Google Scholar] [CrossRef]
- Torres-Montoya, E.; Salomón-Soto, V.M.; Bucio-Pacheco, M.; Torres-Avendaño, J.I.; López-Ruiz, M.; Sánchez-Gonzáles, S.; Castillo-Ureta, H. Primer registro de poblaciones silvestres de Cherax quadricarinatus (Decapoda: Parastacidae) en Sinaloa, México. Rev. Mex. Biodivers. 2016, 87, 258–260. [Google Scholar] [CrossRef]
- Yiwen, Z.; Shakir, K.K.; Yeo, D.C.J. Competition between a native freshwater crab and an invasive crayfish in tropical Southeast Asia. Biol. Invasions 2019, 21, 2653–2663. [Google Scholar] [CrossRef]
- Zengeya, T.A.; Lombard, R.J.-H.; Nelwamondo, V.E.; Nunes, A.L.; Measey, J.; Weyl, O.L. Trophic niche of an invasive generalist consumer: Australian redclaw crayfish, Cherax quadricarinatus, in the Inkomati River Basin, South Africa. Austral Ecol. 2022, 47, 1480–1494. [Google Scholar] [CrossRef]
- Twardochleb, L.A.; Olden, J.D.; Larson, E.R. A global meta-analysis of the ecological impacts of nonnative crayfish Freshwater. Science 2013, 32, 1367–1382. [Google Scholar]
- Ahyong, S.T.; Yeo, D.C.J. Feral populations of the Australian Red-Claw crayfish (Cherax quadricarinatus von Martens) in water supply catchments of Singapore. Biol. Invasions 2007, 9, 943–946. [Google Scholar] [CrossRef]
- Hayakijkosol, O.; Owens, L. Investigation into the pathogenicity of reovirus to juvenile Cherax quadricarinatus. Aquaculture 2011, 316, 1–5. [Google Scholar] [CrossRef]
- Hayakijkosol, O.; Owens, L.; Picard, J. Case report of bacterial infections in a redclaw crayfish (Cherax quadricarinatus) hatchery. Aquaculture 2017, 475, 1–7. [Google Scholar] [CrossRef]
- Martín-Torrijos, L.; Correa-Villalona, A.J.; Azofeifa-Solano, J.C.; Villalobos-Rojas, F.; Wehrtmann, I.S.; Diéguez-Uribeondo, J. First Detection of the Crayfish Plague Pathogen Aphanomyces astaci in Costa Rica: European Mistakes Should Not Be Repeated. Front. Ecol. Evol. 2021, 9, 623814. [Google Scholar] [CrossRef]
- Romero, X.; Jiménez, R. Histopathological survey of diseases and pathogens present in redclaw crayfish, Cherax quadricarinatus (Von Martens), cultured in Ecuador. J. Fish Dis. 2002, 25, 653–667. [Google Scholar] [CrossRef]
- Rico-Sánchez, A.E.; Sundermann, A.; López-López, E.; Torres-Olvera, M.J.; Mueller, S.A.; Haubrock, P.J. Biological diversity in protected areas: Not yet known but already threatened. Glob. Ecol. Conserv. 2020, 22, e01006. [Google Scholar] [CrossRef]
- Carabias Lillo, J.; Provencio, E.; de la Maza Elvira, J.; Ruiz Corzo, M. Programa de Manejo Reserva de la Biosfera Sierra Gorda, 1st ed.; Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT): Mexico City, Mexico, 1999; 172p.
- Carabias Lillo, J.; Provencio, E.; Rosas Hernández, M.I.; Ramírez Reivich, X. Áreas Naturales Protegidas de México con Decretos Federales, 1st ed.; Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT): Mexico City, Mexico; Red para el Desarrollo Sostenible, A.C.: Mexico City, Mexico, 2000; 866p.
- Rodríguez-Cruz, L.D.; Torres-Olvera, M.J.; Durán-Rodríguez, O.Y.; Juan Pablo, R.H. The invasive Australian redclaw crayfish Cherax quadricarinatus Von Martens, 1868: A new threat for biodiversityin the Sierra Gorda Biosphere Reserve, Central Mexican Plateau. BioInvasions Rec. 2023, 12, 819–828. [Google Scholar] [CrossRef]
- Arias-Rodríguez, A.; Torralba-Burrial, A. First record of the redclaw crayfish Cherax quadricarinatus (Von Martens, 1868) on the Iberian Peninsula. Limnetica 2021, 40, 33–42. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Alvarez, S.H.; Ibáñez, A.L. Comparative morphometrics and relative growth of Cherax quadricarinatus (Von Martens, 1868) males and females. Crustaceana 2014, 87, 674–685. [Google Scholar] [CrossRef]
- Sedik, Y.; Rumahlatu, D.; Irawan, B.; Soegianto, A. Morphometric characteristics of crayfish, Cherax gherardiae, from Maybrat, West Papua, Indonesia. Arch. Pol. Fish. 2019, 26, 223–230. [Google Scholar] [CrossRef]
- Rigg, D.P.; Saymour, J.E.; Courtney, R.L.; Jones, C.M. A review of juvenile redclaw crayfish Cherax quadricarinatus (von Martens, 1898) aquaculture: Global production practices and innovation. Freshw. Crayfish 2020, 25, 13–30. [Google Scholar] [CrossRef]
- Masser, M.P.; Rouse, D.B. Australian Red Claw Crayfish; SRAC Publication No. 244; Southern Regional Aquaculture Center (SRAC): Stoneville, MI, USA, 1997; 8p, Available online: https://srac.msstate.edu/pdfs/Fact%20Sheets/244%20Australian%20Red%20Claw%20Crayfish.pdf (accessed on 20 September 2024).
- Vazquez, F.J.; López-Greco, L.S. Intersex females in the red claw crayfish, Cherax quadricarinatus (Decapoda: Parastacidae). Rev. Biol. Trop. 2007, 55, 25–31. [Google Scholar] [CrossRef]
- Moncayo-Estrada, R.; Lyons, J.; Ramirez-Herrejon, J.P.; Escalera-Gallardo, C.; Campos-Campos, O. Status and trends in biotic integrity in a sub-tropical river drainage: Analysis of the fish assemblage over a three decade period. River Res. Appl. 2015, 31, 808–824. [Google Scholar] [CrossRef]
- NMX-AA-159-SCFI-2012; Norma Mexicana que Establece el Procedimiento para la Determinación del Caudal Ecológico en Cuencas Hidrológicas. Diario Oficial de la Federación: México City, México, 2012.
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; U.S. Environmental Protection Agency: Washington, DC, USA; Office of Water: Washington, DC, USA, 1999.
- González del Tánago, M.; García de Jalón, D. Riparian Quality Index (RQI): A methodology for characterising and assessing the environmental conditions of riparian zones. Limnetica 2011, 30, 235–254. [Google Scholar] [CrossRef]
- Pérez-Munguía, R.M.; Pineda-López, R.; Torres García, U.T.; López, R.P. Diseño de un índice de integridad biótica, para ríos y arroyos del centro de México, usando las asociaciones de macroinvertebrados. Entomol. Mex. 2005, 4, 241–245. [Google Scholar]
- Torres-Olvera, M.J.; Durán-Rodríguez, O.Y.; Torres-García, U.T.; Pineda-López, R.; Ramírez-Herrejón, J.P. Validation of an index of biological integrity based on aquatic macroinvertebrates assemblages in two subtropical basins of central Mexico. Lat. J. Aquat. Res. 2018, 46, 945–960. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insects of North America, 4th ed.; Kendall Hunt Publishing Company: Dubuque, IA, USA, 2008. [Google Scholar]
- Springer, M.; Ramírez, A.; Hanson, P. Macroinvertebrados de Agua Dulce de Costa Rica I. Rev. Biol. Trop. 2010, 58 (Suppl. S4), 1–238. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Basualdo, C.V. Choosing the best non-parametric richness estimator for benthic macroinvertebrates databases. Rev. Soc. Entomológica Argent. 2011, 70, 27–38. [Google Scholar]
- Martínez-Sanz, C.; García-Criado, F.; Aláez, C.F.; Aláez, M.F. Assessment of richness estimation methods on macroinvertebrate communities of mountain ponds in Castilla y León (Spain). Ann. Limnol. Int. J. Limnol. 2010, 46, 101–110. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists, 1st ed.; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper DA, T.; Ryan, P.D. PAST—Palaeontological Statistics Software Package for Education and Data Analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Carbajal-Becerra, O.; Olvera-Rodríguez, K.J.; de Souza, G.M.; Durán-Rodríguez, O.Y.; Ramírez-García, A.; Ramírez-Herrejón, J.P. Trophic strategies of the invasive Twospot livebearer (Pseudoxiphophorus bimaculatus, Teleostei: Poeciliidae) in a gradient of environmental quality in central Mexico. Neotrop. Ichthyol. 2020, 18, e190080. [Google Scholar] [CrossRef]
- Durán-Rodríguez, O.Y.; Moncayo-Estrada, R.; Torres-Olvera, M.J.; Pineda-López, R.F.; Ramírez-Herrejón, J.P. Invasion stage of the exotic snail Melanoides tuberculata related to environmental and biological factors in a subtropical river drainage. J. Freshw. Ecol. 2024, 39, 2403368. [Google Scholar] [CrossRef]
- Ramírez-Herrejón, J.P.; Mercado-Silva, N.; Balart, E.F.; Moncayo-Estrada, R.; Mar-Silva, V.; Caraveo-Patino, J. Environmental degradation in a eutrophic shallow lake is not simply due to abundance of non-native. Cyprinus Carpioenvironmental Manag. 2015, 56, 603–617. [Google Scholar] [CrossRef]
- Nakayama, S.M.M.; Ikenaka, Y.; Muzandu, K.; Choongo, K.; Oroszlany, B.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Heavy Metal Accumulation in Lake Sediments, Fish (Oreochromis niloticus and Serranochromis thumbergi), and Crayfish (Cherax quadricarinatus) in Lake Itezhi-tezhi and Lake Kariba, Zambia. Arch. Environ. Contam. Toxicol. 2010, 59, 291–300. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Oficialdegui, F.J.; Zeng, Y.; Patoka, J.; Yeo, D.C.J.; Kouba, A. The redclaw crayfish: A prominent aquaculture species with invasive potential in tropical and subtropical biodiversity hotspots. Rev. Aquac. 2021, 13, 1488–1530. [Google Scholar] [CrossRef]
- Snovsky, G.; Galil, B. The Australian redclaw crayfish Cherax quadricarinatus (von Martens, 1868) (Crustacea: Decapoda: Parastacidae) in the Sea of Galilee, Israel. Aquat. Invasions 2011, 6 (Suppl. S1), S29–S231. [Google Scholar] [CrossRef]
- Bugnot, A.B.; López-Greco, L.S. Sperm production in the red claw crayfish Cherax quadricarinatus (Decapoda, Parastacidae). Aquaculture 2009, 295, 292–299. [Google Scholar] [CrossRef]
- Levine, J.M.; D’Antonio, C.M. Elton revisited: A review of evidence linking diversity and invasibility. Oikos 1999, 87, 15–26. [Google Scholar] [CrossRef]
- Durán-Rodríguez, O.Y.; Valencia-Espinosa, J.A.; Torres-Olvera, M.J.; Pineda-López, R.F.; Jones, R.W.; Ramírez-Herrejón, J.P. Spatial and temporal organization of aquatic insect assemblages in two subtropical river drainages. Hidrobiológica 2022, 32, 127–140. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Giling, D.; Reich, P.; Thompson, R.M. Loss of riparian vegetation alters the ecosystem role of a freshwater crayfish (Cherax destructor) in an Australian intermittent lowland stream. J. N. Am. Benthol. Soc. 2009, 28, 626–637. [Google Scholar] [CrossRef]
- Pavasovic, A. Evaluation of the Nutritional Requirements of Redclaw Crayfish, Cherax quadricarinatus. Ph.D. Thesis, Queensland University of Technology, Queensland, Australia, 2008. Available online: https://eprints.qut.edu.au/16615/ (accessed on 20 September 2024).
- Baudry, T.; Smith-Ravin, J.; Arqué, A.; Goût, J.P.; Cucherousset, J.; Paillisson, J.-M.; Grandjean, F. Trophic niche of the invasive Cherax quadricarinatus and extent of competition with native shrimps in insular freshwater food webs. Biol. Invasions 2024, 26, 3227–3241. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; De Sostoa, A.; Sánchez-Espada, S. Factors affecting the establishment of the invasive crayfish Procambarus clarkii (Crustacea, Decapoda) in the Mediterranean rivers of the northeastern Iberian Peninsula. Hydrobiologia 2013, 703, 33–45. [Google Scholar] [CrossRef]
- Gil-Sanchez, J.M.; Alba-Tercedor, J. Ecology of the native and introduced crayfishes Austropotamobius pallipes and Procambarus clarkii in southern Spain and implications for conservation of the native species. Biol. Conserv. 2002, 105, 75–80. [Google Scholar] [CrossRef]
- Kurniawan, A.; Adibrata, S.; Lingga, R.; Setiadi, J.; Hidayah, R.S.N.; Wulandari, U.A. Dietary shift for juvenile freshwater redclaw crayfish (Cherax quadricarinatus): A review. AACL Bioflux 2024, 17, 2659–2672. [Google Scholar]
- Garcia, C.; Montgomery, E.; Krug, J.; Dagit, R. Removal efforts and ecosystem effects of invasive red swamp crayfish (Procambarus clarkii) in Topanga Creek, California. Bull. South. Calif. Acad. Sci. 2015, 114, 12–21. [Google Scholar] [CrossRef][Green Version]
- Kuhlmann, M.L. Invasion-related change in crayfish density affects a stream macroinvertebrate community. Northeast Nat. 2016, 23, 434–453. [Google Scholar] [CrossRef]
- Pintor, L.M.; Sih, A. Scale dependent effects of native prey diversity, prey biomass and natural disturbance on the invasion success of an exotic predator. Biol. Invasions 2011, 13, 1357–1366. [Google Scholar] [CrossRef]
- Vorob’eva, L.V.; Borisov, R.R.; Kovacheva, N.P.; Pyatikopova, O.V. Food Spectrum of the Australian Redclaw Crayfish Cherax quadricarinatus (Von Martens, 1868) (Decapoda, Parastacidae) in the Ponds of Astrakhan Oblast Russian. J. Biol. Invasions 2024, 15, 146–157. [Google Scholar] [CrossRef]
- Bonvillain, C.P.; Rutherford, D.A.; Kelso, W.E.; Murphy, C.E. Biotic and abiotic influences on population characteristics of Procambarus clarkii in the Atchafalaya River Basin, Louisiana. Freshw. Crayfish 2013, 19, 125–136. [Google Scholar] [CrossRef]
- Green, S.J.; Grosholz, E.D. Functional eradication as a framework for invasive species control. Front. Ecol. Environ. 2021, 19, 98–107. [Google Scholar] [CrossRef]
- Alda. Form alternation of the gonopod and chela from breeding to non-breeding season in males of the crayfish Cambaroides dauricus (Decapoda: Cambaroididae). Zool. Stud. 2024, 63, 24. [Google Scholar] [CrossRef]
- Hamasaki, K.; Osabe, N.; Nishimoto, S.; Dan, S.; Kitada, S. Sexual Dimorphism and Reproductive Status of the Red Swamp Crayfish Procambarus clarkia. Zool. Stud. 2020, 59, e7. [Google Scholar] [CrossRef]


| Site | Shore Line | Riparian Vegetation | Substrate | Habitat | Main Impacts |
|---|---|---|---|---|---|
| PM | Banks significantly modified by human action. | Average width of riparian corridor significantly altered by human action. Riparian vegetation appears in small patches covering less than 30% of segment length. | Particle stratification that provides diversity of niche space. Akal (60%), psammal (20%), macrolithal (10%), mesolithal (5%), megalithal (3%), microlithal (2%). | Overhanging vegetation, canopy cover shading (3%), submerged vegetation, woody debris, undercut banks, and boulders. HMU: glide (80%), ruffle (10%), run (5%), and backwater (5%). HS: shallow fast (75%), wading fast (10%), shallow slow (5%), wading flows (5%), shallow flows (3%), and shallow slow (2%). | Natural area with moderate human impact (road, livestock, and tourism). |
| HIG | Banks moderately modified by human action in their form and processes. | Average width of riparian corridors significantly reduced by human action, with average width less than 1 active channel width. Riparian corridor moderately fragmented with 50% of natural coverage including several strata. | Particles (25–30%) surrounded by fine sediment. Mesolithal (40%), psammal (40%), microlithal (10%), akal (10%). | Overhanging vegetation, canopy cover shading (3%), shallow margins, undercut banks, boulders, and woody debris. HMU: pool (40%), run (40%), ruffle (10%), and backwater (10%) HS: wading flows (40%), deep slow (40%), shallow fast (10%), and shallow slow (10%). | Natural area with minimal human impact. |
| SAL | Banks severely altered by human action. Channel margins connected to urbanized areas and roads. | Average width of riparian corridors severely reduced due to human action. Few riparian woody species; herbaceous communities predominate due to human actions. | Particles (>75%) surrounded by fine sediment. Psammal (90%), mesolithal (5%), macrolithal (5%). | Overhanging vegetation, canopy cover shading (2%), undercut banks, and woody debris. HMU: glide (95%) and ruffle (5%). HS: shallow slow (90%), shallow flows (5%), and shallow fast (5%). | Area impacted by sand dredging and roads. |
| DA | Banks moderately modified by human action in their form and processes. | Moderately restricted by human action. Average width of around 3 times active channel width. Riparian corridors moderately fragmented with 60% of natural coverage including several strata. | Particles (30–40%) surrounded by fine sediment. Megalithal (70%), psammal (20%), macrolithal (10%). | Overhanging vegetation, canopy cover shading (5%) boulders, and shallow margins. HMU: run (50%), rapid (25%), pool (20%), and backwater (5%). HS: wading fast (40%), deep fast (25%), deep flows (15%), wading flows (15%), and shallow slow (5%). | Natural area with moderate human impact (tourism, livestock). |
| DRJ | Banks moderately modified by human action in their form and processes. | Continuity and coverage of riparian corridor in natural conditions including a mix of species corresponding to native vegetation associations of river segment, with different strata. | Particle stratification that provides diversity of niche space. Mesolithal (70%), macrolithal (20%), pelal (10%). | Overhanging vegetation, canopy cover shading (50%), shallow margin both left and right, submerged vegetation, undercut banks, and boulders. HMU: run (60%), rapid (20%), pool (10%), and fast run (10%). HS: wading flows (60%), shallow fast (20%), wading fast (10%), deep slow (5%), and deep flows (5%). | Natural area with minimal human impact. |
| CM | Banks severely altered by human action. | Average width of riparian corridor significantly altered by human action. Riparian vegetation is reduced to isolated trees or shrubs, leaving large open areas), including only one stratum. | Particles (>75%) surrounded by fine sediment. Microlithal (50%), debris (20%), pelal (15%), psammal (5%), akal (5%), mesolithal (5%). | Overhanging vegetation, canopy cover shading (70%), undercut banks, and submerged vegetation. HMU: pool (55%), glide (40%), and riffle (5%). HS: wading slow (55%), wading flows (40%), and shallow fast (5%). | Highly impacted by tourism activities. |
| AYU | Banks moderately modified by human action in their form and processes. | Moderately restricted by human action. In valley surrounded by vegetation. | Particle stratification that provides diversity of niche space. Megalithal (60%), macrolithal (20%), mesolithal (15%), microlithal (5%). | Overhanging vegetation, canopy cover shading (20%), shallow margin left, boulders, and woody debris. HMU: glide (60%), pool (20%), rapid (10%), and run (10%). HS: wading flows (60%), wading slow (20%), wading fast (10%), and deep slow (10%). | Natural area with moderate human impact (tourism). |
| Index | Variable | Acronym | Variable Description |
|---|---|---|---|
| RQI Riparian Quality Index (González-del-Tánago and García-de-Jalón, 2011) [46] An index developed to assess and characterize the ecological status of riparian systems. | Riparian width | RW * | Assesses restrictions to the riparian corridor caused by human influence. When there are no restrictions, the riparian width has its natural borders, and vegetation covers all land that is between the channel and adjacent slopes. |
| Longitudinal continuity | LC * | An estimation of the intensity of fragmentation of the riparian vegetated area based on the size and frequency of open areas created by human actions (i.e., land-use). | |
| Composition and structure of riparian vegetation | CS * | This variable helps assess the condition of riparian composition by evaluating the vegetation’s natural succession stages. | |
| Age diversity and regeneration | ADR | This variable refers to the age classes of woody species in the riparian zone. It helps to evaluate the regeneration of woody species. | |
| Bank condition | BC | This variable helps to assess the heterogeneity of the water shoreline, stability of banks, and changes in erosion and sedimentation. | |
| Lateral connectivity | LAC | The variable assesses how much the flow regulation has been altered by morphological changes in the margins of the river or by channelization works that prevent the occurrence of natural bank flooding. | |
| Vertical connectivity | VC | The level of alterations to the soil surface that reduce natural infiltration and alterations to substrata that reduce alluvial permeability, subsurface flows, and groundwater connectivity. | |
| VBHA Visual-Based Habitat Assessment (Barbour et al., 1999) [45] A qualitative index to visually evaluate the environmental condition of rivers and streams by assessing their physical and ecological characteristics. | Epifaunal substrate | EPS | The abundance and diversity of submerged structures in a stream (such as cobbles, rocks, logs, and undercut banks) that shape habitat complexity. These features provide refuge, feeding grounds, and spawning sites for macrofauna. |
| Substrate embedment | EMB | The degree to which rocks and snags are buried in streambed sediments. Higher embeddedness reduces the surface area for shelter, spawning, and egg incubation. | |
| Velocity and depth regime variations | V/D | The presence of diverse flow patterns (slow–deep, slow–shallow, fast–deep, and fast–shallow) enhancing habitat complexity. | |
| Sediment deposition | SD | The accumulation of sediment in pools and the alteration of river bottoms. Excessive deposition indicates instability and reduces habitat suitability. | |
| Channel flow status | CFS | Refers to the degree to which the stream channel is filled with water. Changes in flow, caused by factors such as channel widening or flow reduction, limit suitable habitats for aquatic organisms. | |
| Channel alteration | CA | Refers to significant changes in the stream’s shape, often due to human activities like straightening, deepening, or diverting for flood control or irrigation. | |
| Riffle frequency | FR | Measures the occurrence of riffles, which contribute to habitat diversity and fauna richness. More frequent riffles enhance habitat quality. | |
| Bank stability | BS * | Assesses the degree of bank erosion and potential for collapse. Steep, unstable banks with crumbling soil, exposed roots, or lack of vegetation indicate sediment movement issues and reduced habitat quality. | |
| Vegetation protection | VP * | Refers to the extent of vegetation on stream banks and the adjacent riparian zone. The presence of native vs. exotic plants and the impact of grazing or urbanization on vegetation are also considered. | |
| IIBAMA Index of biological integrity based on aquatic macroinvertebrate assemblages (Pérez-Munguía and Pineda-López, 2005; Torres-Olvera, et al., 2018) [47,48] This index was developed to estimate the environmental condition of rivers and streams in central México. The index is based on families of aquatic macroinvertebrates as indicators of degradation in river ecosystems. | Taxon richness | RT | The number of aquatic macroinvertebrate families in a sample. Higher taxa richness may indicate habitat heterogeneity, which is related to refuge availability and an increased speciation likelihood. |
| Ephemeroptera, Plecoptera, and Trichoptera richness | EPT | Families in these Orders (excluding Baetidae) are associated with the transformation of organic matter into nutrients and are sensitive to environmental stress. | |
| Intolerant insects | II | Aquatic insect families that are sensitive to environmental degradation. The absence of sensitive insects is an indicator of alterations in environmental conditions (i.e., dissolved oxygen, temperature, water level). | |
| Intolerant taxa | IT | Refers to variables such as variable II plus other taxa of macroinvertebrates that are not tolerant. | |
| Mean tolerance | MT | Corresponds to the average of the tolerance values present in the sample. | |
| Fixed taxa | FT | Corresponds to the number of taxa that have life habits fixed to the substrate. |
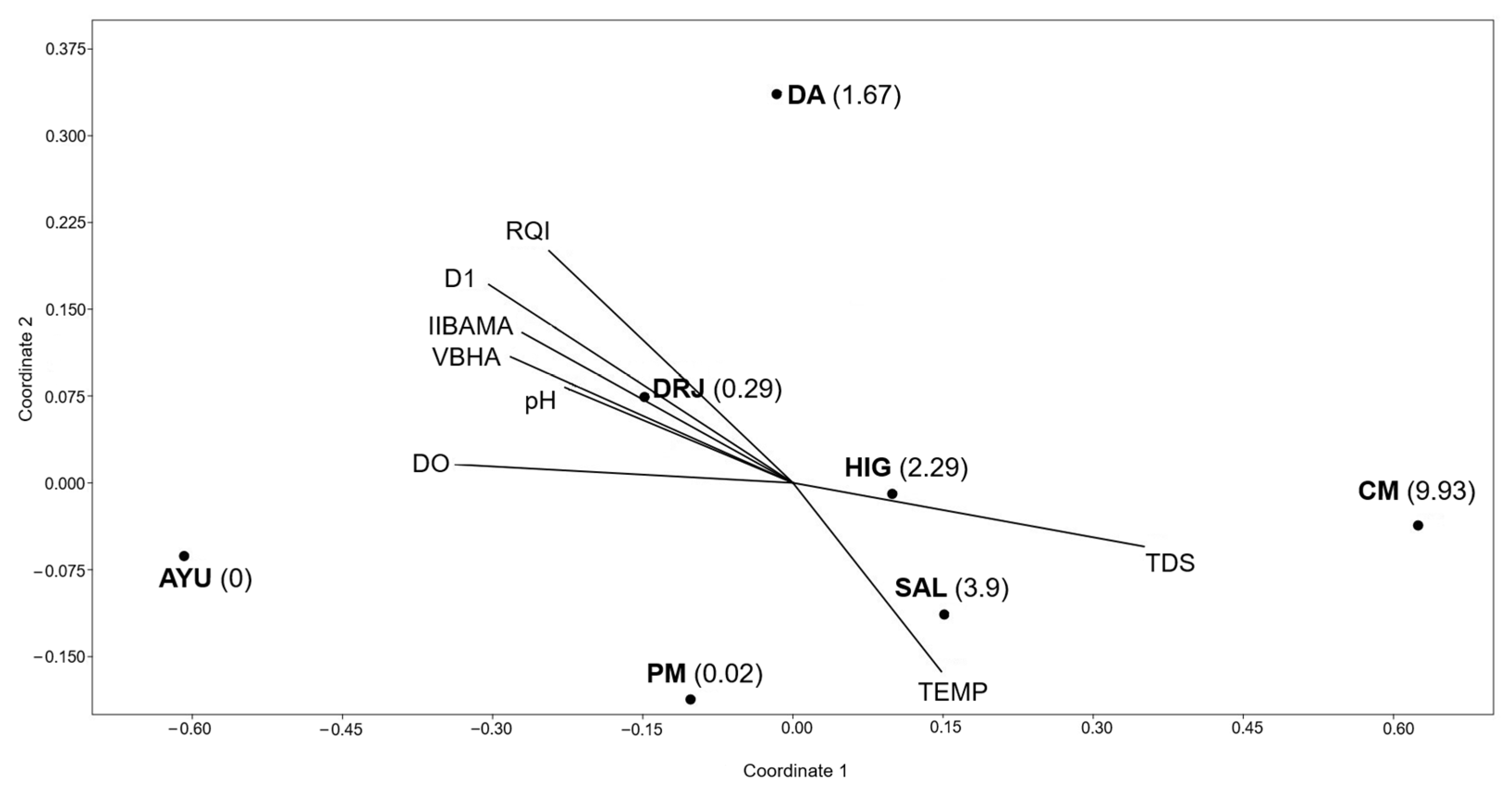
| Study Site | NI | RA | Global Density | Average Density |
|---|---|---|---|---|
| AYU | 0 | 0 | 0 | 0 |
| DA | 10 ± 14.3 | 5.7 | 1.67 | 0.24 ± 0.3 |
| HIG | 13.7 ± 12.5 | 9.4 | 2.29 | 0.33 ± 0.29 |
| SAL | 23.4 ± 19.6 | 24.5 | 3.9 | 0.56 ± 0.47 |
| CM | 59.6 ± 47.5 | 58.5 | 9.92 | 1.4 ± 1.1 |
| PM | 0.14 ± 0.38 | 0 | 0.02 | 0.003 ± 0.009 |
| DRJ | 1.7 ± 1.4 | 1.9 | 0.29 | 0.04 ± 0.03 |
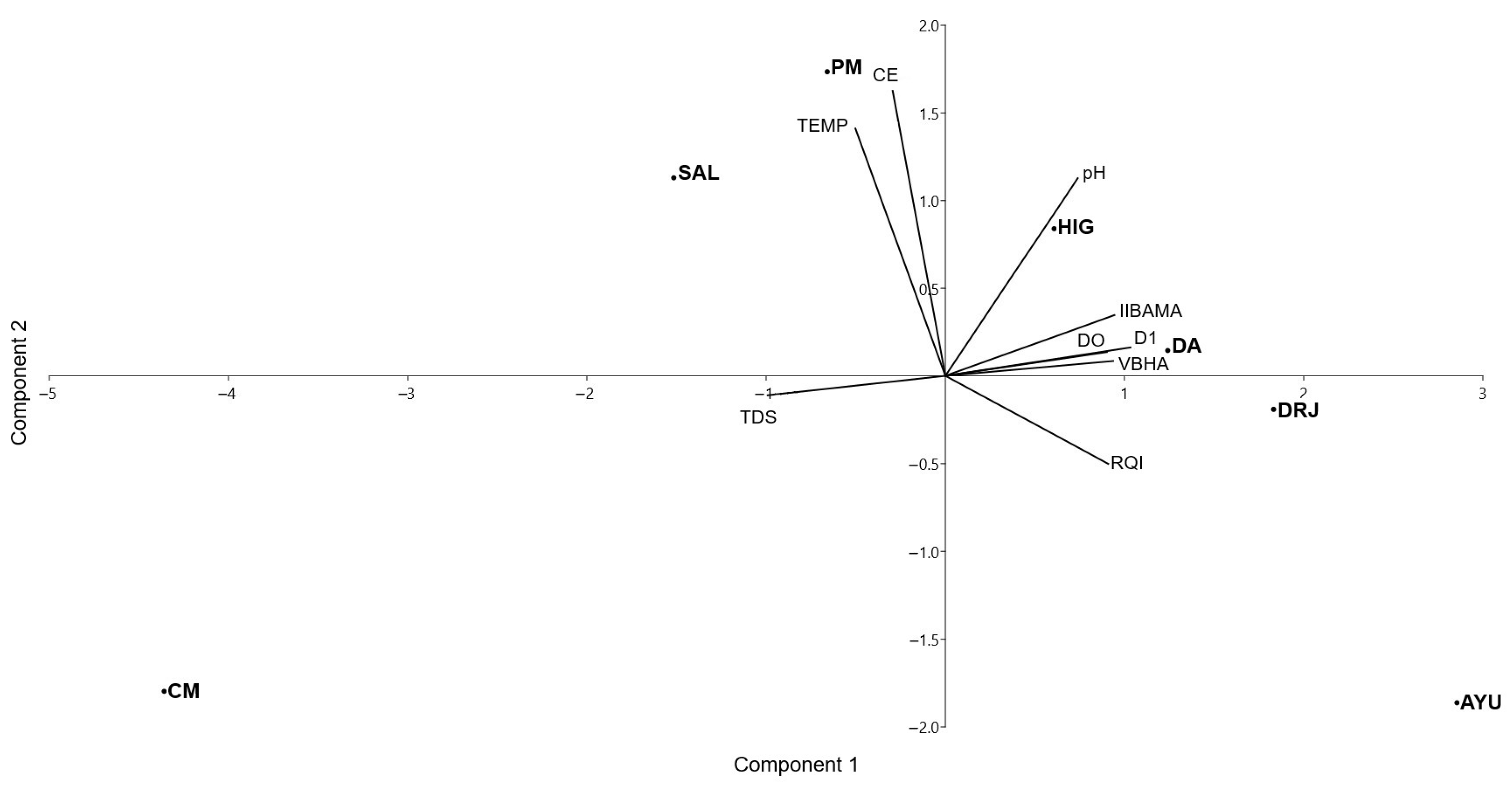
| PC 1 | PC 2 | |
|---|---|---|
| Eigenvalue | 19.5955 | 6.3525 |
| Percentage variance | 59.38 | 19.25 |
| pH | 0.1775 | −0.2208 |
| CE | −0.0199 | −0.3479 |
| TEMP | −0.0798 | −0.2378 |
| TDS | −0.2030 | −0.0157 |
| DO | 0.1806 | −0.0281 |
| EPS | 0.0641 | 0.3090 |
| EMB | 0.1593 | 0.1572 |
| V/D | 0.2079 | −0.0577 |
| SD | −0.0067 | 0.3559 |
| CFS | 0.0278 | 0.3645 |
| CA | 0.2098 | −0.1047 |
| FR | 0.2134 | −0.0821 |
| BS | 0.2138 | 0.0690 |
| VP | 0.0628 | 0.1302 |
| VBHA | 0.2075 | 0.1048 |
| RW | 0.1485 | 0.1704 |
| LC | 0.1423 | 0.1356 |
| CS | 0.1445 | 0.1773 |
| ADR | 0.1752 | −0.2115 |
| BC | 0.2030 | 0.0774 |
| LAC | 0.1214 | −0.2405 |
| VC | 0.1996 | 0.0009 |
| RQI | 0.1947 | 0.1637 |
| RT | 0.1841 | 0.1378 |
| EPT | 0.1857 | −0.1912 |
| II | 0.2151 | −0.0181 |
| IT | 0.2178 | 0.0024 |
| MT | −0.1757 | 0.2107 |
| FT | 0.2196 | −0.0109 |
| IIBAMA | 0.2132 | 0.0313 |
| D1 | 0.2199 | 0.0157 |
| Variable | rs | p |
|---|---|---|
| pH | −0.0541 | 0.9084 |
| CE | 0.2143 | 0.6445 |
| TEMP | 0.3214 | 0.4821 |
| TDS | 0.75 | 0.0522 |
| DO | −0.3929 | 0.3833 |
| EPS | −0.6667 | 0.1019 |
| EMB | −0.7857 | 0.0362 a |
| V/D | −0.4364 | 0.3276 |
| SD | −0.1081 | 0.8175 |
| CFS | −0.3368 | 0.4601 |
| CA | −0.5455 | 0.2053 |
| FR | −0.393 | 0.3832 |
| BS | −0.7092 | 0.0743 |
| VP | −0.4491 | 0.3121 |
| VBHA | −0.6786 | 0.0938 |
| RW | −0.5455 | 0.2053 |
| LC | −0.3143 | 0.5441 |
| CS | −0.4505 | 0.3104 |
| ADR | 0.0741 | 0.8745 |
| BC | −0.393 | 0.3832 |
| LAC | 0 | 1 |
| VC | −0.2594 | 0.5742 |
| RQI | −0.7027 | 0.0782 |
| RT | −1 | <0.0001 a |
| EPT | 0 | 1 |
| II | −0.4364 | 0.3276 |
| IT | −0.593 | 0.1605 |
| MT | 0 | 1 |
| FT | −0.6365 | 0.1243 |
| IIBAMA | −0.4546 | 0.3054 |
| D1 | −0.70273 | 0.089683 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Rodríguez, O.Y.; García-Ávila, D.A.; Valencia-Espinosa, J.A.; Arroyo-Reséndiz, E.; Torres-Olvera, M.J.; Ramírez-Herrejón, J.P. Environmental Factors Influencing the Establishment of the Invasive Australian Redclaw Crayfish (Cherax quadricarinatus) in a Biosphere Reserve on the Central Mexican Plateau. Life 2025, 15, 508. https://doi.org/10.3390/life15040508
Durán-Rodríguez OY, García-Ávila DA, Valencia-Espinosa JA, Arroyo-Reséndiz E, Torres-Olvera MJ, Ramírez-Herrejón JP. Environmental Factors Influencing the Establishment of the Invasive Australian Redclaw Crayfish (Cherax quadricarinatus) in a Biosphere Reserve on the Central Mexican Plateau. Life. 2025; 15(4):508. https://doi.org/10.3390/life15040508
Chicago/Turabian StyleDurán-Rodríguez, Omar Y., Daniel A. García-Ávila, J. Andrés Valencia-Espinosa, Eugenio Arroyo-Reséndiz, Martín J. Torres-Olvera, and Juan P. Ramírez-Herrejón. 2025. "Environmental Factors Influencing the Establishment of the Invasive Australian Redclaw Crayfish (Cherax quadricarinatus) in a Biosphere Reserve on the Central Mexican Plateau" Life 15, no. 4: 508. https://doi.org/10.3390/life15040508
APA StyleDurán-Rodríguez, O. Y., García-Ávila, D. A., Valencia-Espinosa, J. A., Arroyo-Reséndiz, E., Torres-Olvera, M. J., & Ramírez-Herrejón, J. P. (2025). Environmental Factors Influencing the Establishment of the Invasive Australian Redclaw Crayfish (Cherax quadricarinatus) in a Biosphere Reserve on the Central Mexican Plateau. Life, 15(4), 508. https://doi.org/10.3390/life15040508






