Exploring Fungal Abundance and WHO Fungal Priority Pathogens in Agricultural Fields: A One Health Perspective in Northeast Thailand
Abstract
1. Introduction
2. Materials and Methods
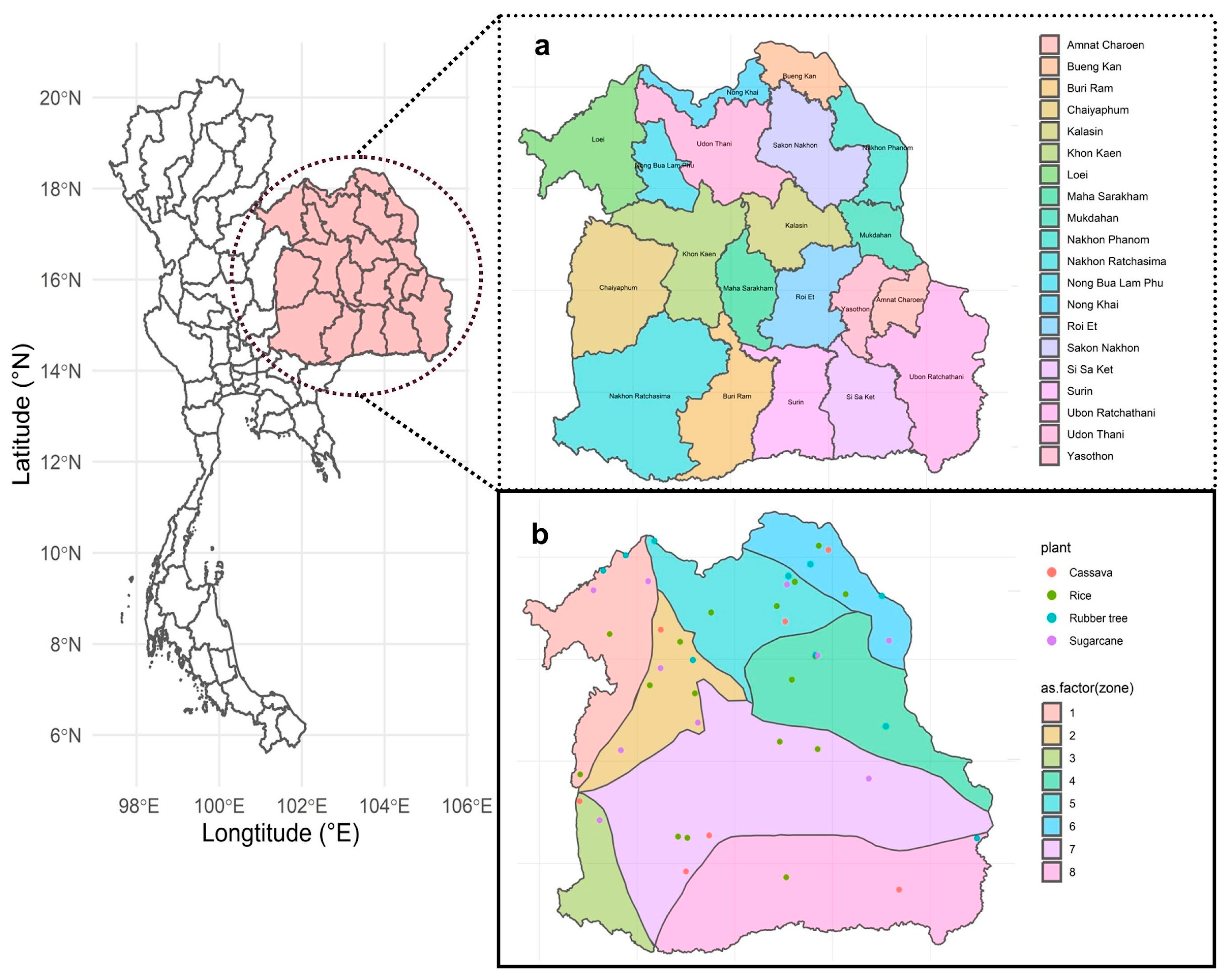
2.1. Sample Collection
2.2. Fungal Metabarcoding Sequencing
2.3. Bioinformatics and Data Analysis
3. Results
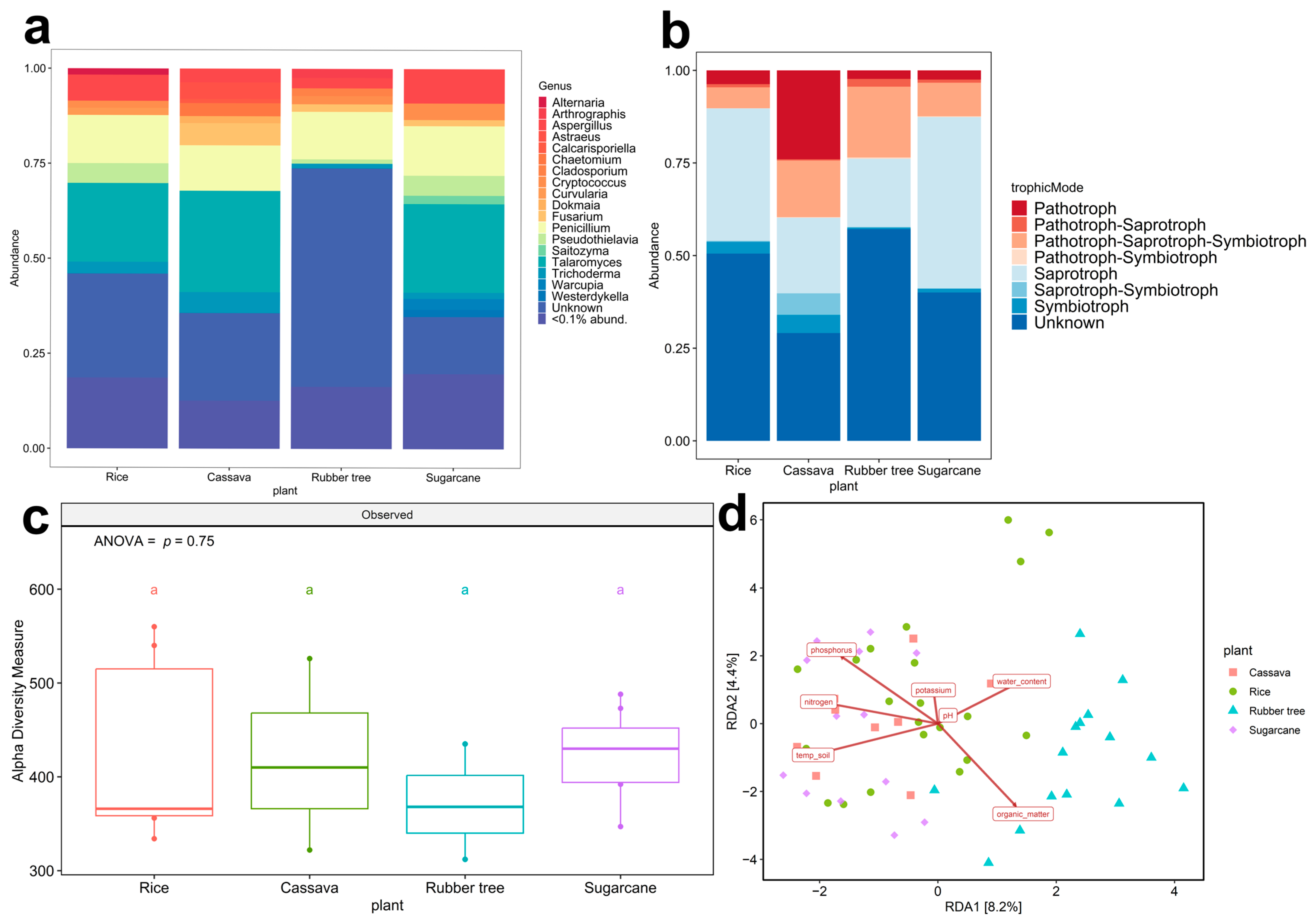
3.1. Plant Fields and Geological Features Significantly Affect Overall Fungal Richness and Communities
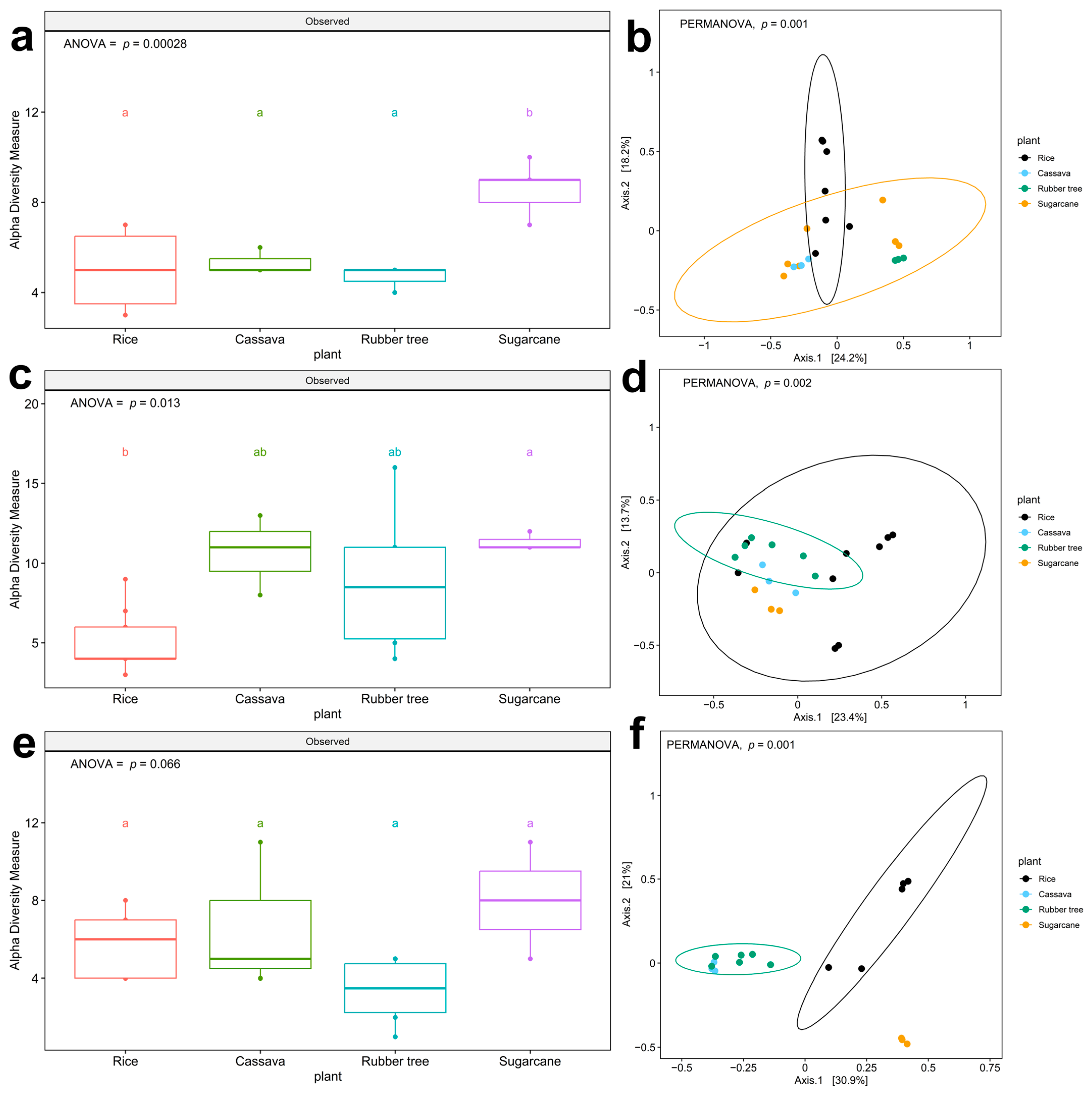
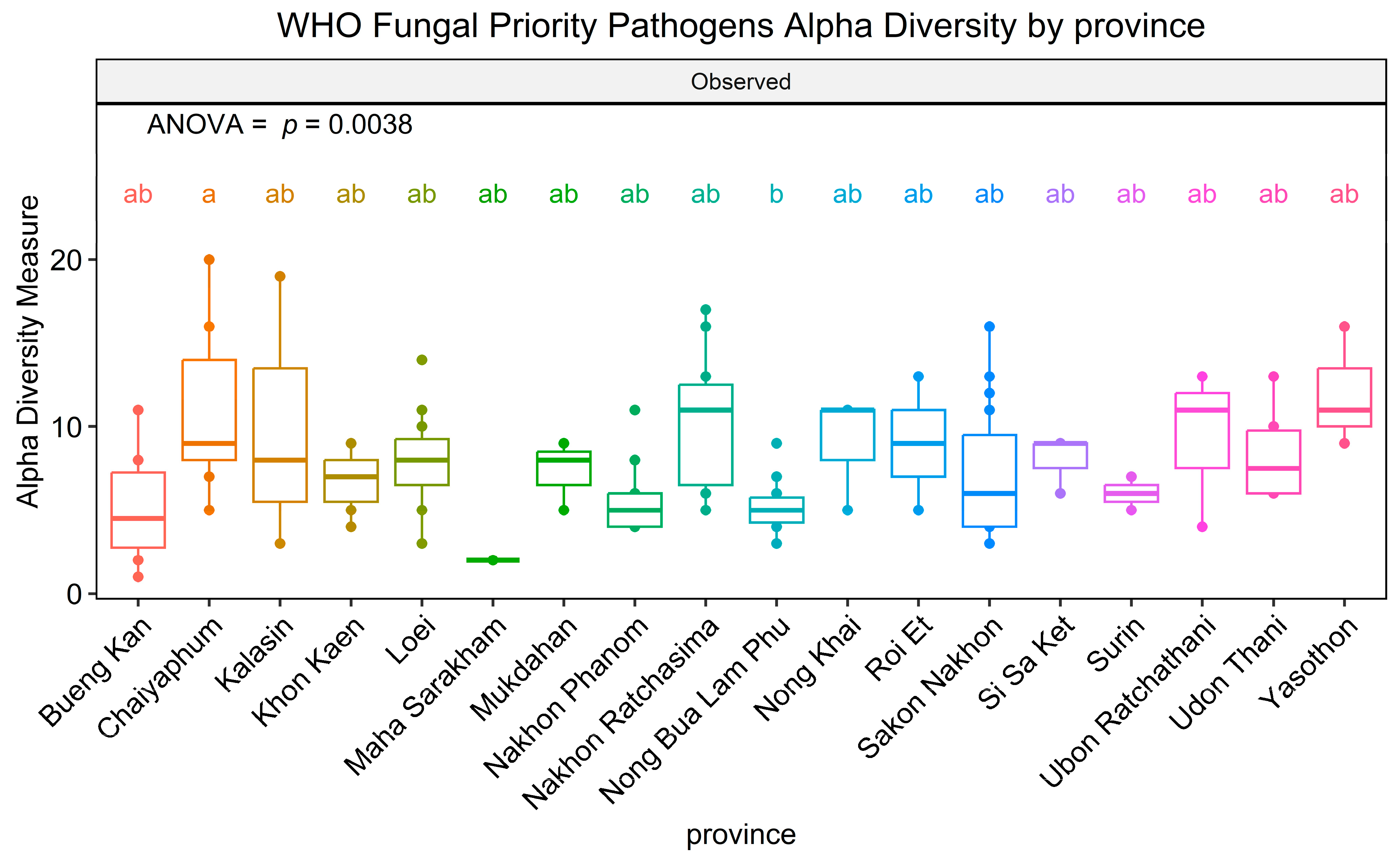
3.2. The Richness of WHO FPPL Pathogens Varied Among Different Plant Fields and Geological Zones
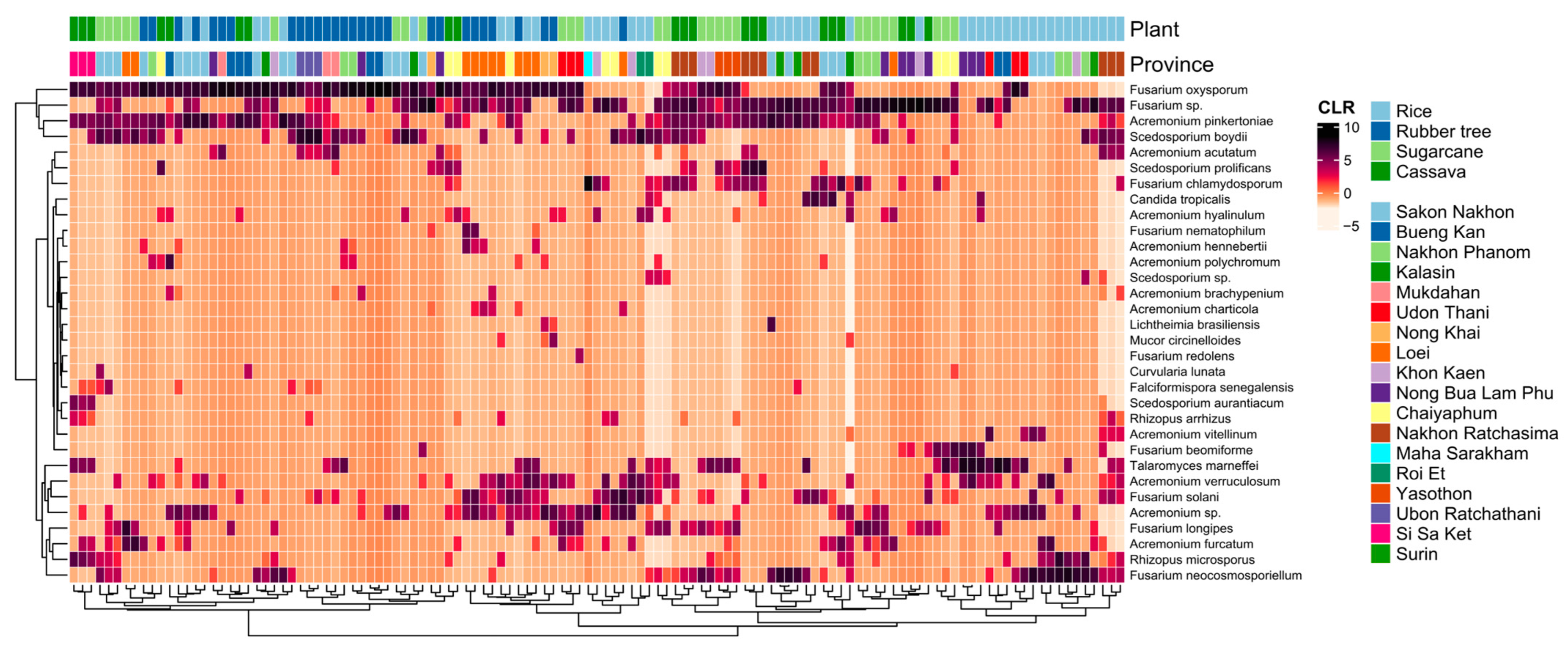
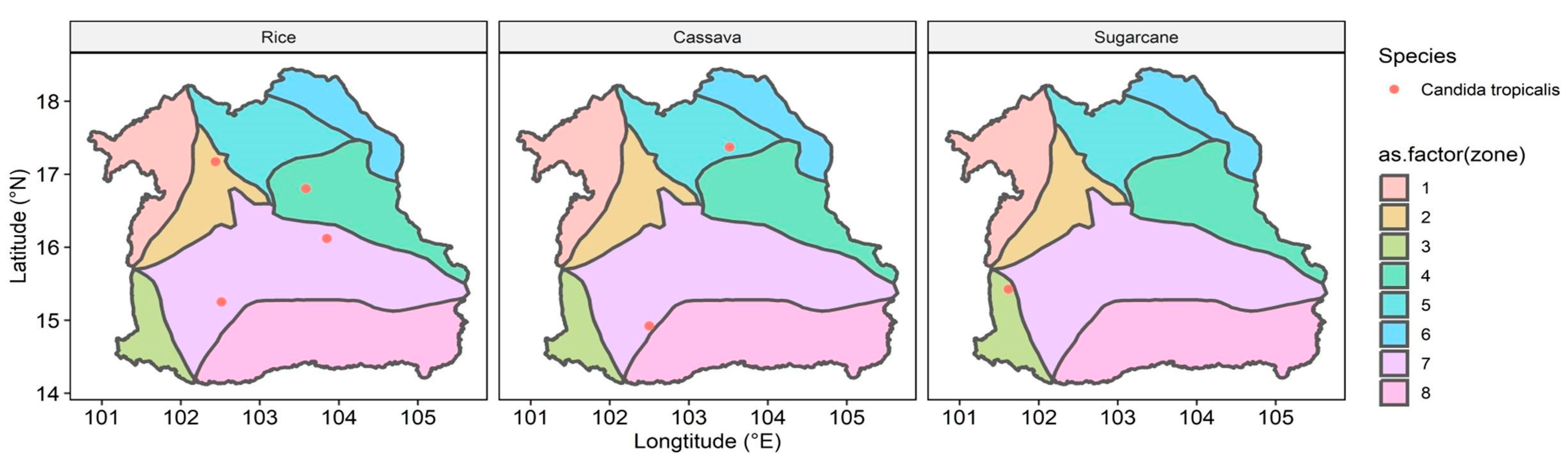
3.3. The Distribution and Documentation of WHO FPPL Pathogens Across Northeast Thailand
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development, and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Faksri, K.; Kaewkes, W.; Chaicumpar, K.; Chaimanee, P.; Wongwajana, S. Epidemiology and identification of potential fungal pathogens causing invasive fungal infections in a tertiary care hospital in northeast Thailand. Med. Mycol. 2014, 52, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Ngamchokwathana, C.; Chongtrakool, P.; Waesamaae, A.; Chayakulkeeree, M. Risk factors and outcomes of non-albicans Candida bloodstream infection in patients with Candidemia at Siriraj Hospital—Thailand’s largest National Tertiary Referral Hospital. J. Fungi 2021, 7, 269. [Google Scholar] [CrossRef]
- Tan, B.H.; Chakrabarti, A.; Li, R.Y.; Patel, A.K.; Watcharananan, S.P.; Liu, Z.; Chindamporn, A.; Tan, A.L.; Sun, P.L.; Wu, U.I.; et al. Incidence and species distribution of candidaemia in Asia: A laboratory-based surveillance study. Clin. Microbiol. Infect. 2015, 21, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Větrovský, T.; Morais, D.; Kohout, P.; Lepinay, C.; Algora, C.; Hollá, S.A.; Bahnmann, B.D.; Bílohnědá, K.; Brabcová, V.; D’alò, F.; et al. GlobalFungi, a global database of fungal occurrences from high-throughput-sequencing metabarcoding studies. Sci. Data 2020, 7, 308. [Google Scholar] [CrossRef]
- Izuno, A.; Kanzaki, M.; Artchawakom, T.; Wachrinrat, C.; Isagi, Y. Vertical structure of phyllosphere fungal communities in a tropical forest in Thailand uncovered by high-throughput sequencing. PLoS ONE 2016, 11, e0166669. [Google Scholar] [CrossRef]
- Amma, S.; Toju, H.; Wachrinrat, C.; Sato, H.; Tanabe, A.S.; Artchawakom, T.; Kanzaki, M. Composition and diversity of soil fungi in Dipterocarpaceae-dominated seasonal tropical forests in Thailand. Microbes Environ. 2018, 33, 135–143. [Google Scholar] [CrossRef]
- Viaud, M.; Pasquier, A.; Brygoo, Y. Diversity of soil fungi studied by PCR–RFLP of ITS. Mycol. Res. 2000, 104, 1027–1032. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Anju, V.T.; Busi, S.; Mohan, M.S.; Salim, S.A.; Ar, S.; Imchen, M.; Kumavath, R.; Dyavaiah, M.; Prasad, R. Surveillance and mitigation of soil pollution through metagenomic approaches. Biotechnol. Genet. Eng. Rev. 2024, 40, 589–622. [Google Scholar] [CrossRef]
- Cobo-Díaz, J.F.; Baroncelli, R.; Le Floch, G.; Picot, A. Combined metabarcoding and co-occurrence network analysis to profile the bacterial, fungal, and Fusarium communities and their interactions in maize stalks. Front. Microbiol. 2019, 10, 261. [Google Scholar] [CrossRef]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Floch, P.; Molle, F. Irrigated agriculture and rural change in Northeast Thailand: Reflections on present developments. In Governing the Mekong: Engaging in the Politics of Knowledge; Strategic Information and Research Development Centre: Petaling Jaya, Malaysia, 2013; pp. 185–212. [Google Scholar]
- Choenkwan, S.; Fox, J.M.; Rambo, A.T. Agriculture in the mountains of northeastern Thailand: Current situation and prospects for development. Mt. Res. Dev. 2014, 34, 95–106. [Google Scholar] [CrossRef]
- Khamwong, C.; Praneetvatakul, S. Impact of climate change on agricultural revenue and adaptation strategies of farmers in northeastern Thailand. Kasetsart J. Soc. Sci. 2011, 32, 214–228. [Google Scholar]
- Udomthanathira, K. Nares Thailand Khon Kaen Geological Features of the Northeastern Region of Thailand. 2019. Available online: https://www.iok2u.com/variety/geology/geo-nares-019 (accessed on 26 May 2023).
- Sipsas, N.; Kontoyiannis, D. Occupation, lifestyle, diet, and invasive fungal infections. Infection 2008, 36, 515–525. [Google Scholar] [CrossRef]
- Srivoramas, T.; Chaiwong, T.; Sanford, M.R. Isolation of fungi from adult house fly, Musca domestica and the blow fly Chrysomya megacephala in Ubon Ratchathani province, northeastern Thailand. Int. J. Parasitol. Res. 2012, 4, 53–56. [Google Scholar] [CrossRef]
- Simwami, S.P.; Khayhan, K.; Henk, D.A.; Aanensen, D.M.; Boekhout, T.; Hagen, F.; Brouwer, A.E.; Harrison, T.S.; Donnelly, C.A.; Fisher, M.C. Low diversity Cryptococcus neoformans variety grubii multilocus sequence types from Thailand are consistent with an ancestral African origin. PLoS Pathog. 2011, 7, e1001343. [Google Scholar] [CrossRef]
- Pham, L.T.T.; Pharkjaksu, S.; Chongtrakool, P.; Suwannakarn, K.; Ngamskulrungroj, P. A predominance of clade 17 Candida albicans isolated from hemocultures in a tertiary care hospital in Thailand. Front. Microbiol. 2019, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Tangwattanachuleeporn, M.; Minarin, N.; Saichan, S.; Sermsri, P.; Mitkornburee, R.; Groß, U.; Chindamporn, A.; Bader, O. Prevalence of azole-resistant Aspergillus fumigatus in the environment of Thailand. Med. Mycol. 2017, 55, 429–435. [Google Scholar]
- Naranong, C.; Anunnatsiri, S.; Srigulbutr, S. Epidemiology and Antifungal Susceptibility in Patients with Candidemia in a University Hospital, Thailand. J. Med. Assoc. Thail. 2020, 103, 1048–1056. [Google Scholar]
- Mootsikapun, P.; Srikulbutr, S. Histoplasmosis and penicilliosis: Comparison of clinical features, laboratory findings and outcome. Int. J. Infect. Dis. 2006, 10, 66–71. [Google Scholar] [CrossRef]
- Chumpangern, W.; So-Ngern, A.; Reechaipichitkul, W.; Meesing, A.; Ratanawatkul, P.; Arunsurat, I.; Chaisuriya, N. Presentations of chronic cavitary pulmonary histoplasmosis mimic infected cystic bronchiectasis in an immunocompetent host: A case report. Respir. Med. Case Rep. 2021, 34, 101555. [Google Scholar] [PubMed]
- Harris, J.R.; Lindsley, M.D.; Henchaichon, S.; Poonwan, N.; Naorat, S.; Prapasiri, P.; Chantra, S.; Ruamcharoen, F.; Chang, L.S.; Chittaganpitch, M.; et al. High prevalence of cryptococcal infection among HIV-infected patients hospitalized with pneumonia in Thailand. Clin. Infect. Dis. 2012, 54, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Latinne, A.; Chen, H.-W.; Kuo, C.-C.; Lorica, R.; Singleton, G.; Stuart, A.; Malbas, F.F.; Demanche, C.; Chabé, M.; Michaux, J.; et al. Revisiting the Pneumocystis host specificity paradigm and transmission ecology in wild Southeast Asian rodents. Infect. Genet. Evol. 2021, 93, 104978. [Google Scholar] [CrossRef] [PubMed]
- Khayhan, K.; Hagen, F.; Norkaew, T.; Puengchan, T.; Boekhout, T.; Sriburee, P. Isolation of Cryptococcus gattii from a Castanopsis argyrophylla tree hollow (Mai-Kaw), Chiang Mai, Thailand. Mycopathologia 2017, 182, 365–370. [Google Scholar] [CrossRef]
- Chayakulkeeree, M.; Denning, D. Serious fungal infections in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 931–935. [Google Scholar] [CrossRef]
- Yiallouris, A.; Pana, Z.D.; Marangos, G.; Tzyrka, I.; Karanasios, S.; Georgiou, I.; Kontopyrgia, K.; Triantafyllou, E.; Seidel, D.; Cornely, O.A.; et al. Fungal diversity in the soil Mycobiome: Implications for ONE health. One Health 2024, 18, 100720. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Bruns, T.D. Fungal community ecology: A hybrid beast with a molecular master. Bioscience 2008, 58, 799–810. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Palmer, J.M.; Jusino, M.A.; Banik, M.T.; Lindner, D.L. Non-biological synthetic spike-in controls and the AMPtk software pipeline improve mycobiome data. PeerJ 2018, 6, 2–27. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, 2–22. [Google Scholar] [CrossRef]
- Edgar, R. Taxonomy Benchmark Tests, USEARCH Manual v8. 1. 2014. Available online: https://www.drive5.com/usearch (accessed on 5 March 2025).
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Team, R. Integrated Development for R. 2019. Available online: http://www.rstudio.com (accessed on 26 May 2023).
- Team R Core. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org (accessed on 26 May 2023).
- Bansal, Y.; Chander, J.; Kaistha, N.; Singla, N.; Sood, S.; van Diepeningen, A.D. Fusarium sacchari, a cause of mycotic keratitis among sugarcane farmers–a series of four cases from North India. Mycoses 2016, 59, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- Gómez-Silva, B.; Vilo-Muñoz, C.; Galetović, A.; Dong, Q.; Castelán-Sánchez, H.G.; Pérez-Llano, Y.; Sánchez-Carbente, M.d.R.; Dávila-Ramos, S.; Cortés-López, N.G.; Martínez-Ávila, L.; et al. Metagenomics of Atacama lithobiontic extremophile life unveils highlights on fungal communities, biogeochemical cycles and carbohydrate-active enzymes. Microorganisms 2019, 7, 619. [Google Scholar] [CrossRef]
- Leeyaphan, C.; Hau, C.; Takeoka, S.; Tada, Y.; Bunyaratavej, S.; Pattanaprichakul, P.; Sitthinamsuwan, P.; Chaiprasert, A.; Sasajima, Y.; Makimura, K.; et al. Immune response in human chromoblastomycosis and eumycetoma–focusing on human interleukin-17A, interferon-gamma, tumour necrosis factor-alpha, interleukin-1 beta and human beta-defensin-2. Mycoses 2016, 59, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Sati, H.; Alastruey-Izquierdo, A.; Perfect, J.; Govender, N.P.; Harrison, T.S.; Chiller, T.; Sorrell, T.C.; Bongomin, F.; Oladele, R.; Chakrabarti, A.; et al. HIV and fungal priority pathogens. Lancet HIV 2023, 10, e750–e754. [Google Scholar] [CrossRef]
- Wang, F.; Han, R.; Chen, S. An overlooked and underrated endemic mycosis—Talaromycosis and the pathogenic fungus Talaromyces marneffei. Clin. Microbiol. Rev. 2023, 36, e0005122. [Google Scholar] [CrossRef]
- Chariyalertsak, S.; Sirisanthana, T.; Supparatpinyo, K.; Praparattanapan, J.; Nelson, K.E. Case-control study of risk factors for Penicillium marneffei infection in human immunodeficiency virus-infected patients in northern Thailand. Clin. Infect. Dis. 1997, 24, 1080–1086. [Google Scholar] [CrossRef]
- López-Fernández, L.; Sanchis, M.; Navarro-Rodríguez, P.; Nicolás, F.E.; Silva-Franco, F.; Guarro, J.; Garre, V.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Capilla, J. Understanding Mucor circinelloides pathogenesis by comparative genomics and phenotypical studies. Virulence 2018, 9, 707–720. [Google Scholar] [CrossRef]
- Yao, L.; Wang, H.; Wan, Z.; Li, R.; Yu, J. The high diversity and variable susceptibility of clinically relevant Acremonium-like species in China. Mycopathologia 2019, 184, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Z.; Li, M.; Zhang, X.; Yan, Y.; Cui, L. Quantitative assays of two soil-borne pathogens of Aconitum carmichaelii Debx., Sclerotium rolfsii and Mucor circinelloides, in the main cultivation areas of China. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100343. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Ding, C.; Jia, Z.; He, Z.; Zhang, T.; Wang, X. Declined soil suppressiveness to Fusarium oxysporum by rhizosphere microflora of cotton in soil sickness. Biol. Fertil. Soils 2015, 51, 935–946. [Google Scholar] [CrossRef]
- Zajc, J.; Zalar, P.; Gunde-Cimerman, N. Yeasts in hypersaline habitats. In Yeasts in Natural Ecosystems: Diversity; Springer: Berlin/Heidelberg, Germany, 2017; pp. 293–329. [Google Scholar]
- Boonsilp, S.; Homkaew, A.; Phumisantiphong, U.; Nutalai, D.; Wongsuk, T. Species distribution, antifungal susceptibility, and molecular epidemiology of Candida species causing candidemia in a tertiary care hospital in Bangkok, Thailand. J. Fungi 2021, 7, 577. [Google Scholar] [CrossRef] [PubMed]
- Saiprom, N.; Wongsuk, T.; Oonanant, W.; Sukphopetch, P.; Chantratita, N.; Boonsilp, S. Characterization of virulence factors in Candida species causing Candidemia in a tertiary care hospital in Bangkok, Thailand. J. Fungi 2023, 9, 353. [Google Scholar] [CrossRef]
- Leelastwattanagul, O.; Sutheeworapong, S.; Khoiri, A.N.; Dulsawat, S.; Wattanachaisaereekul, S.; Tachaleat, A.; Duangfoo, T.; Paenkaew, P.; Prommeenate, P.; Cheevadhanarak, S.; et al. Soil microbiome analysis reveals effects of periodic waterlogging stress on sugarcane growth. PLoS ONE 2023, 18, e0293834. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef]
- Bilal, L.; Asaf, S.; Hamayun, M.; Gul, H.; Iqbal, A.; Ullah, I.; Lee, I.-J.; Hussain, A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Monkai, J.; Hyde, K.D.; Xu, J.; Mortimer, P.E. Diversity and ecology of soil fungal communities in rubber plantations. Fungal Biol. Rev. 2017, 31, 1–11. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D.; Bräu, L.; Davison, J.; Jairus, T.; Robain, H.; Robin, A.; Vasar, M.; Wiriyakitnateekul, W.; Öpik, M. Diversity of root-associated arbuscular mycorrhizal fungal communities in a rubber tree plantation chronosequence in Northeast Thailand. Mycorrhiza 2016, 26, 863–877. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D.; Robin, A.; Robain, H.; Wiriyakitnateekul, W.; Bräu, L. Impact of biochar application dose on soil microbial communities associated with rubber trees in North East Thailand. Sci. Total Environ. 2019, 689, 970–979. [Google Scholar] [CrossRef]
- Nabhadalung, N. Arbuscular mycorrhizal fungi related to soil phosphorous, organic matter and pH in cassava field. Int. J. Agric. Technol. 2020, 16, 1145–1152. [Google Scholar]
- Juntahum, S.; Kuyper, T.; Boonlue, S. Distribution of arbuscular mycorrhizal fungi in sugarcane rhizosphere from various agricultural management practices in Northeast, Thailand. Curr. Res. Environ. Appl. Mycol. 2022, 12, 44–55. [Google Scholar] [CrossRef]
- Watanarojanaporn, N.; Boonkerd, N.; Tittabutr, P.; Longtonglang, A.; Young, J.P.W.; Teaumroong, N. Effect of rice cultivation systems on indigenous arbuscular mycorrhizal fungal community structure. Microbes Environ. 2013, 28, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeon, J.; Lee, K.K.; Lee, Y.-H. Compositional shift of bacterial, archaeal, and fungal communities is dependent on trophic lifestyles in rice paddy soil. Front. Microbiol. 2021, 12, 719486. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Pastor, S.; Coissac, E.; Lavergne, S.; Schwörer, C.; Theurillat, J.-P.; Heintzman, P.D.; Wangensteen, O.S.; Tinner, W.; Rey, F.; Heer, M.; et al. High resolution ancient sedimentary DNA shows that alpine plant diversity is associated with human land use and climate change. Nat. Commun. 2022, 13, 6559. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, C.; Lin, L.; Keyhani, N.O.; Zhu, M.; Zhao, Z.; Zhang, W.; Yang, C.; Su, H.; Liu, P.; et al. Assessing the structure and diversity of fungal community in plant soil under different climatic and vegetation conditions. Front. Microbiol. 2023, 14, 1288066. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef]
- Laohaudomchok, W.; Nankongnab, N.; Siriruttanapruk, S.; Klaimala, P.; Lianchamroon, W.; Ousap, P.; Jatiket, M.; Kajitvichyanukul, P.; Kitana, N.; Siriwong, W.; et al. Pesticide use in Thailand: Current situation, health risks, and gaps in research and policy. Hum. Ecol. Risk Assess. 2020, 27, 1147–1169. [Google Scholar] [CrossRef]
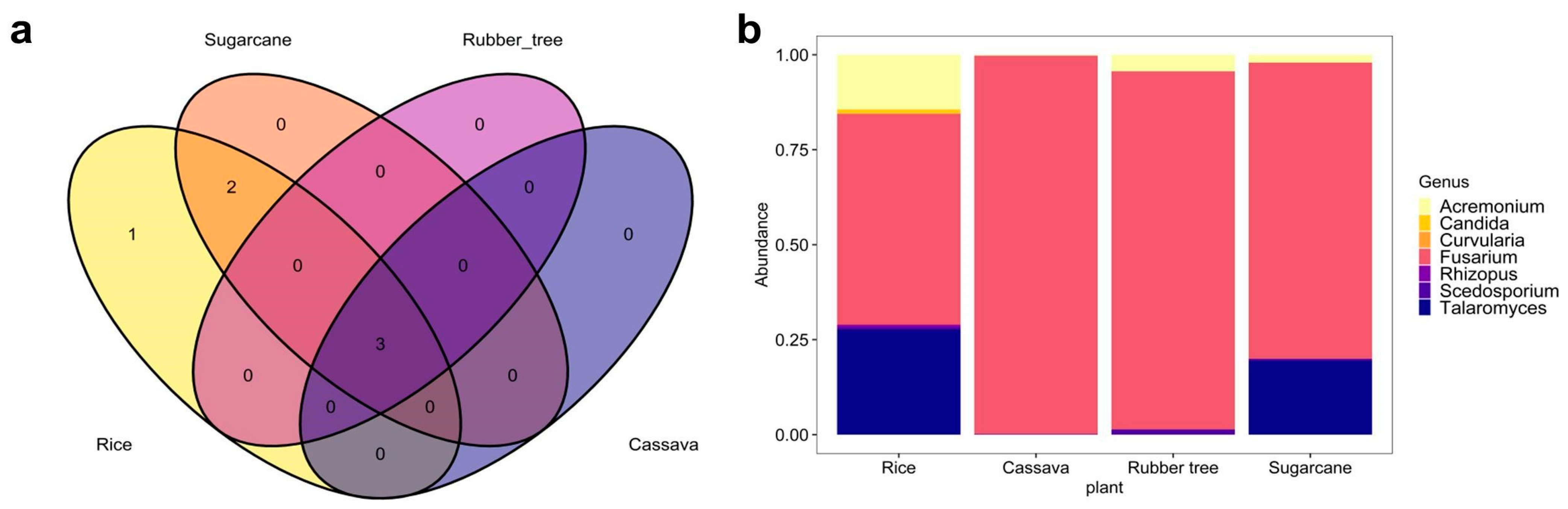
| WHO FPPL | Cassava | Rice | Rubber Tree | Sugarcane |
|---|---|---|---|---|
| Acremonium charticola a | - | - | Present | - |
| A. hennebertii a | - | Present | Present | - |
| A. vitellinum a | - | Present | - | - |
| Curvularia lunata a | Present | - | - | Present |
| Fusarium nematophilum a | - | Present | Present | - |
| F. redolens a | - | - | - | Present |
| Lichtheimia brasiliensis a | - | Present | Present | - |
| Mucor circinelloides a | - | Present | Present | - |
| Scedosporium sp. b | - | Present | - | Present |
| S. aurantiacum b | Present | Present | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakmuang, C.; Ibrahim, S.N.M.M.; Kaewjon, T.; Kraisitudomsook, N.; Somboonna, N.; Chanabun, R.; Chindamporn, A.; Pombubpa, N. Exploring Fungal Abundance and WHO Fungal Priority Pathogens in Agricultural Fields: A One Health Perspective in Northeast Thailand. Life 2025, 15, 488. https://doi.org/10.3390/life15030488
Lakmuang C, Ibrahim SNMM, Kaewjon T, Kraisitudomsook N, Somboonna N, Chanabun R, Chindamporn A, Pombubpa N. Exploring Fungal Abundance and WHO Fungal Priority Pathogens in Agricultural Fields: A One Health Perspective in Northeast Thailand. Life. 2025; 15(3):488. https://doi.org/10.3390/life15030488
Chicago/Turabian StyleLakmuang, Chayaporn, Syahriar Nur Maulana Malik Ibrahim, Teeratat Kaewjon, Nattapol Kraisitudomsook, Naraporn Somboonna, Ratmanee Chanabun, Ariya Chindamporn, and Nuttapon Pombubpa. 2025. "Exploring Fungal Abundance and WHO Fungal Priority Pathogens in Agricultural Fields: A One Health Perspective in Northeast Thailand" Life 15, no. 3: 488. https://doi.org/10.3390/life15030488
APA StyleLakmuang, C., Ibrahim, S. N. M. M., Kaewjon, T., Kraisitudomsook, N., Somboonna, N., Chanabun, R., Chindamporn, A., & Pombubpa, N. (2025). Exploring Fungal Abundance and WHO Fungal Priority Pathogens in Agricultural Fields: A One Health Perspective in Northeast Thailand. Life, 15(3), 488. https://doi.org/10.3390/life15030488







