1. Introduction
Animal models of human disease have been extremely useful for understanding cancer pathways and effective interventions, but few can match quintessential spontaneous inflammatory bowel disease (IBD) as the prelude to subsequent colorectal cancers (CRCs) contracted by the Cotton Top Tamarin (CTT),
Saguinus oedipus [
1], a resident of northwestern Columbia. While one-third of CTTs succumb to CRC, less than two percent have liver metastasis [
2], and the underlying mechanisms of avoidance are of acute relevance to humans with cancer, where liver metastasis is a harbinger of death in 70% of cancer victims, and 20–35% have liver spread at the time that the primary cancer is diagnosed [
3]. Biomarkers are currently available for the early diagnosis of CRC in humans, but CTT cancer biomarkers in lower order primates have only recently been described and their genomes registered [
4,
5]. Some researchers point to stress and the microbiome as a contributor to the development of CRC [
6,
7]. The CTT is also subject to the development of lymphomata and known to be susceptible to infections such as Epstein–Barr virus and Coronavirus [
8,
9,
10], suggesting an element of immune deficiency. Its Brazilian cousin, the common marmoset (CM),
Callithrix jacchus, also contracts IBD, but usually not CRC, providing a convenient control. The carcinoembryonic antigen was first discovered in humans in 1965 [
11] and almost a quarter of a century later in apes and chimpanzees [
12]. Seven years thereafter, a mouse carcinoembryonic antigen (CEA) analog was discovered [
13], which ushered in burgeoning interest in this model. This was the era of monoclonal antibodies, which provided the essential tools to demystify markers on CTT lymphatic cells [
14,
15]. This paper will follow attempts to characterize and detail adhesion molecules and antigens in broad detail and attempt to define their role in the avoidance of liver metastasis, possibly extending these observations to humans.
2. Methods
The animal tissues were initially procured from two main sources, the Marmoset Research Center, Oak Ridge, Tennessee, and the New England Regional Primate Center, Boston, MA, USA. When the latter center closed, the tissues were transferred to the Tulane National Primate Research Center, Covington, Louisiana, USA. Tissues were only obtained from deceased or terminally ill euthanized animals, since the CTT has been on the endangered list since 1973. Where comparisons with humans with IBD were made, colon washings and tissue biopsies were obtained from patients of the Kaiser Permanente Medical Center, Sacramento, CA, USA, and later from a longitudinal study conducted at the Detroit Veterans Administration Medical Center, Detroit, Michigan, USA, with both centers subject to institutional board review (IRB). Briefly, extracts were prepared using Dounce, homogenized with 1 gm/10 mL of saline and magnesium chloride, and centrifuged at 1000× g in Sorvall RC-5B refrigerated centrifuge. The supernatant was aspirated and subjected to a high speed of 10,000× g, the supernatants represent the membrane enriched extract, and the protein content was determined as described below.
Common methods for analysis were enzyme-linked immunosorbent assay (ELISA), Western blotting (WB), and immunohistochemistry (IHC). The ELISA utilized 96-well microtiter plates (Nunc, Copenhagen, Denmark), in which samples with identical protein content (5 µg/well) were determined via a Lowry colorimetric assay. A Vector Laboratories kit with the primary monoclonal antibodies (shown in
Table 1) or control antibody (UPC10, Sigma-Aldrich, St Louis, Mo, USA) was used to determine the background reaction, and secondary antibodies to either IgG or IgM linked to alkaline phosphatase. There were 3 consecutive washes between successive applications of 5% Tween-20 in phosphate-buffered saline (PBS). The final step was to add p-p-nitrophenyl-phosphate substrate (Millipore-Sigma, St. Louis, Mo, USA), which gives a yellow color and was measured using a spectrophotometer (Thermo-Fisher, Waltham, MA, USA) at 405 nm for 30 mins. The results were reported as optical density (OD) minus background/5 µg per well.
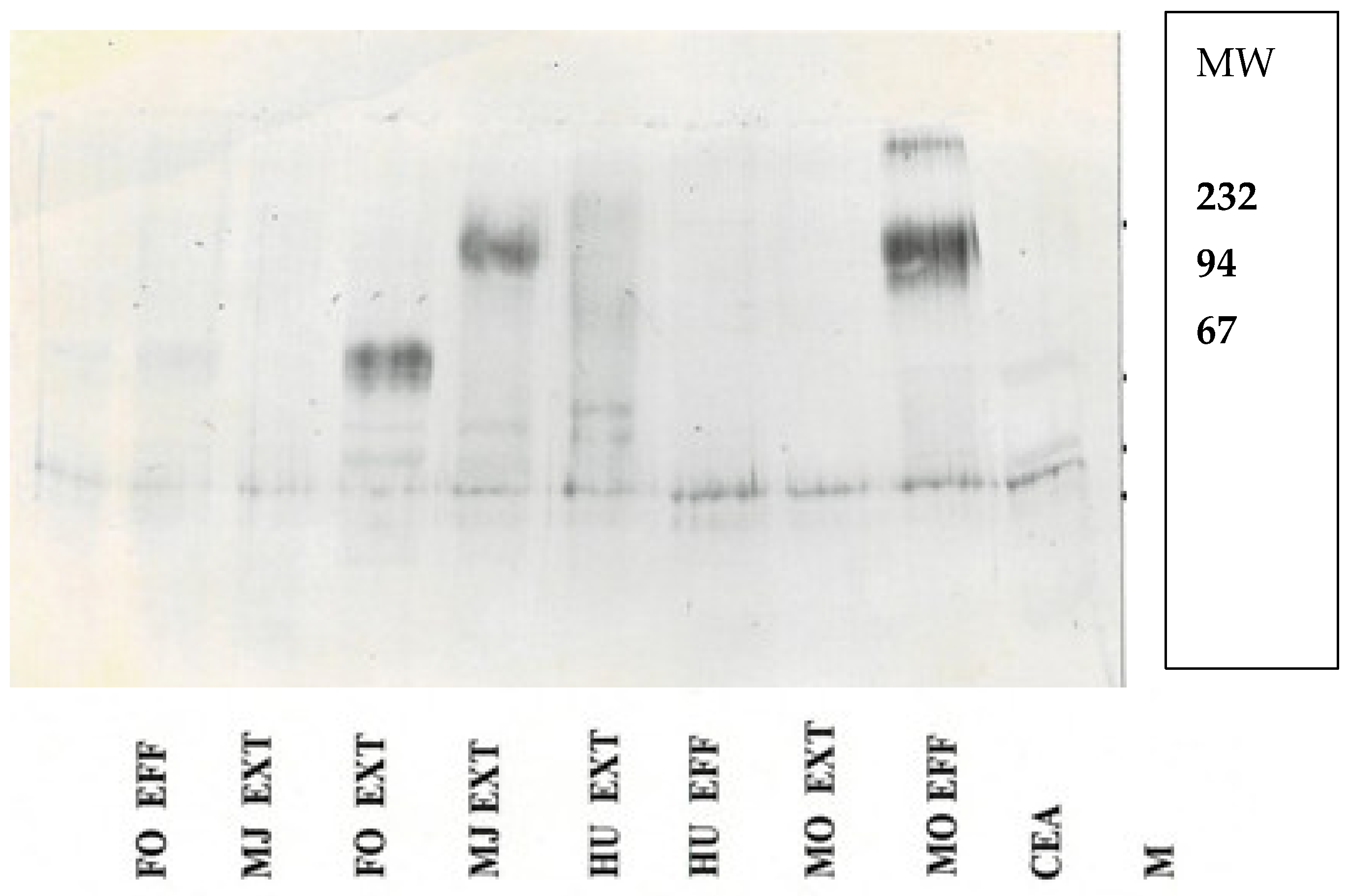
Western blotting was performed on a BioRad apparatus (Hercules, CA, USA), loading 10 µg of protein per well of extract. Then, 9.6% SDS-polyacrylamide gels (Millipore Sigma, St. Louis, MO, USA) were loaded at 10 ug/lane on SDS-gels and electrophoresed according to the manufacturer’s directions. The gel proteins were transferred to a milk powder-blocked PVDF membrane (Kirkegaard and Perry, Gaithersburg, MD, USA), which was then reacted with the primary and secondary antibodies as described above, and then with a number of substrates for alkaline phosphate or peroxide, depending on the linker attached to the secondary antibody. A lane for the molecular weight ladder (Thermo-Fisher Waltham, MA, USA) was used to determine the relative mobility of the protein in question, and its picture was documented.
Immunohistochemistry was performed on paraffin-embedded tissue fragments, properly oriented, and cut with a microtome to a thickness of 5 µm. After deparaffination and hydration steps, the slides were reacted with selected MAbs, and the Vector kits (Vector Labs., Newark, CA, USA) were used to develop a color with the available substrate, such as alpha-amino benzidine to produce a black color with added zinc chloride. The slides were then cover slipped with a clear adhesive and examined at various magnifications, and photographic documentation was duly noted. The grading system for intensity that we used is given as follows: 0—no stain; 0.5—equivocal stain; 1+—definite but faint, 2+—medium intensity; 3+—strong intensity; and 4+—very strong intensity.
2.1. Study Outcome and Data Collection
Selected monoclonal antibodies that are reactant with biomarker outcomes were obtained from a number of sources and are tubulated in
Table 1 above. They were used in enzyme-linked immunoassays (ELISA), immunohistochemistry, and Western blotting experiments.
2.2. Hierarchical Clustering and Clustering Process
Often, particularly when different primate species are compared, teleogenetic trees need to be considered and organized accordingly. Since CRC has been observed in several species, these differences need to be compared. It is safe to say that cancer is not a common disease in most non-human primate species. The CTT and, less frequently, the closely related CM, have a higher incidence of CRC and small bowel cancers, respectively, than other non-human primates (NHP), and other tumor types are common for these 2 species. The genomics that we performed with the BigBlast Dye Terminator methodology [
2] have shown significant differences, but in the CEA Ig pivot region (hinge), where the PELPK pentapeptide is located, the differences appear to be muted.
2.3. Ethical Approval
As noted above, monkey tissues were obtained from registered biorepositories at one of the three Primate Research (New England, Yerkes, and Tulane) Centers. Earlier samples were obtained from the Marmoset Colony at Oak Ridge (MARCOR), Tennessee. Human samples for comparison were obtained from patients enrolled in the NIPCON study, approved by the Wayne State University School of Medicine IRB, Wayne State University Human Investigation committees, #070700MP4F, and all patients gave informed consent.
2.4. Statistical Analysis
The statistical analysis was performed using a computer statistical package (instat, version 2, Graphpad Software Inc., San Diego, CA, USA). The normality of values was tested using the online Kolmogorov–Smirnov calculator,
https://www.socscistatistics.com/tests/kolmogorov/, accessed on 5 December 2023. The ordinal data were analyzed using Student’s
t-test or the Mann–Whitney test for non-normality values. The non-ordinal data were analyzed using the Chi-square test or the Odds Ratio, and the confidence interval value proportions were presented as percentages in graphs, with the
p values depicted. Linear correlations were analyzed using the least-squares method, and the r coefficient values were depicted on the relevant graph. The probability values were regarded as significant at the <0.05 level.
4. Discussion
For more than three decades, we have toiled to understand the mechanisms of disruption of liver metastasis and now believe that we stand before the final gate. While CRC is a leading cause of cancer death and is mediated via the final common pathway of liver metastasis, leading to death in nearly 70% of CRC cases, many other common cancers share this final common pathway [
32]. p38γ probably plays a role in liver metastasis, and there are at least two relevant p38γ blockers of this pathway that have been used in clinical studies, and which we included in our last study [
32]. p38γ induces
ras, which may play a role in human cancer and metastasis, but does not appear to be active in CTTs, while the emphasis on non-human primate work appears to be moving in the direction of adopting CMs as an animal model, as they are currently not an endangered species.
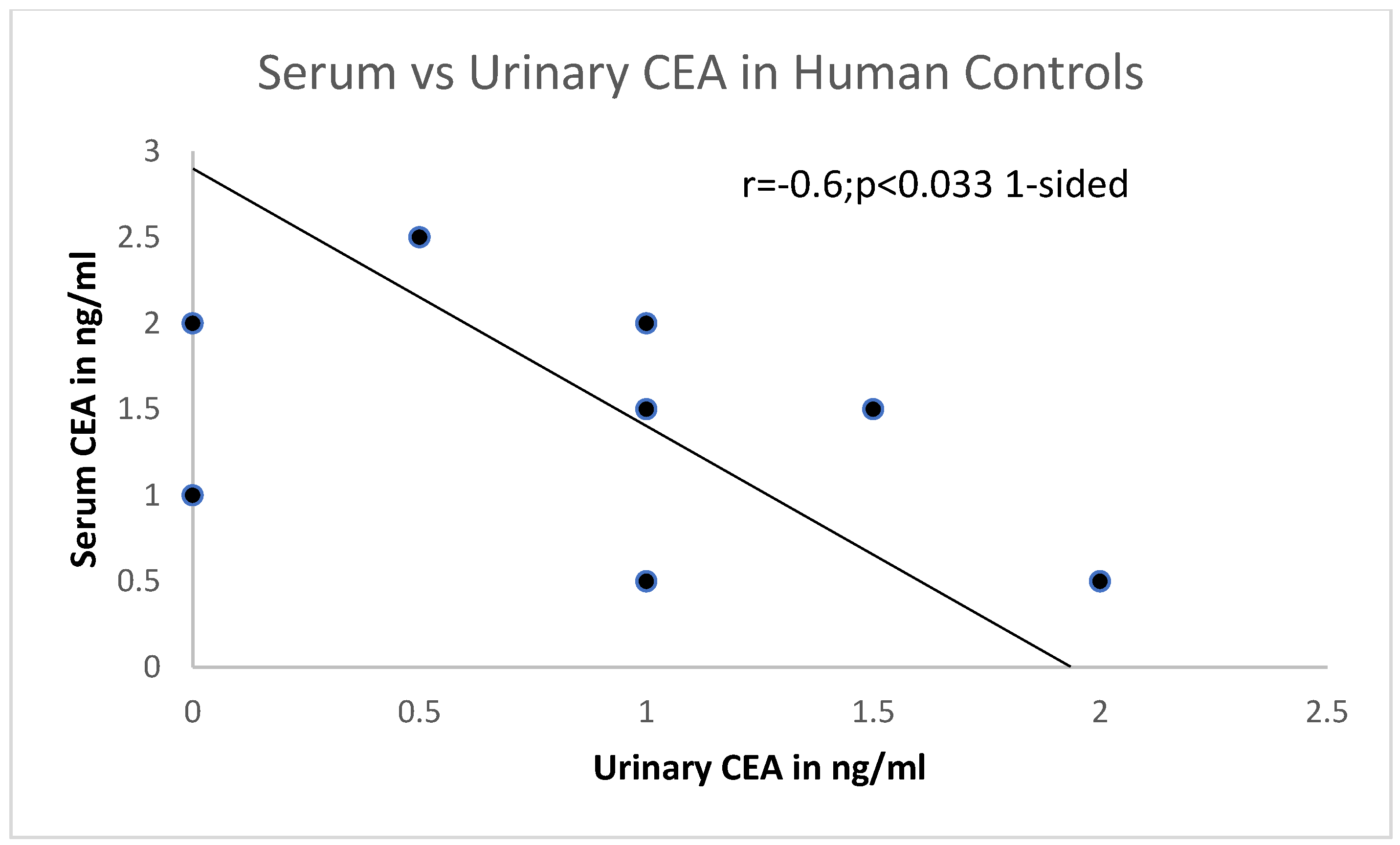
The comparison of humans with IBD and cancer with the IBD therapeutic response in CTTs is interesting. The CTT is dependent on the same medicines used in IBD, and we have much to learn from CTTs with respect to cancer and IBD developments. Just after the turn of the last century, we had already noted differences in the repertoire of antigens and were interested to find and document differences in the form of IBD and incipient cancers arising from the IBD risk factor [
31]. Particularly of interest, the T84.66 MAbs directed at the N-terminal “hinge” portion of the CEA molecule did not always react, and we suspected that this may be caused by mutations in the recognized epitope [
3,
29]. This avenue of investigation had been opened by the discovery of the genetic sequence of CEA [
29] and homology between other members of the CEA–immunoglobulin gene superfamily [
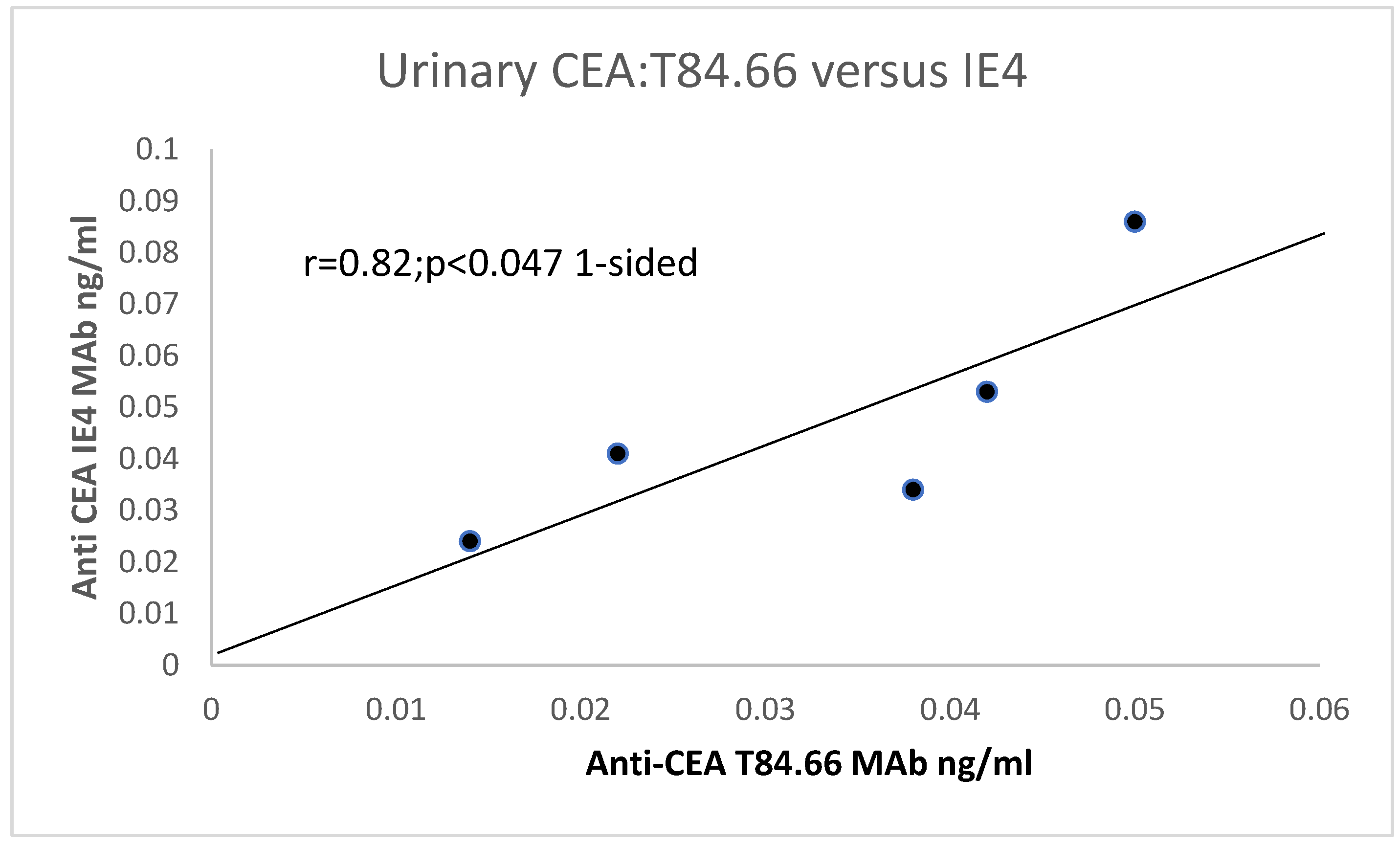
30]. We found quite a range of reactivity to CEA using the CEA AIA kit and smaller variation in CEA size clustering, as revealed by T84.66 MAb [
31].
K
ras p21, EGFR, neuroendocrinal factors (chromogranin-A), and telomerase [
33] may be operative in both human and CTT CRC as common gene mutations. This enzyme maintains the length of the TTAGG at the ends of the chromosome, and as the cell ages, telomeres are lost due to this process of replicative senescence [
34]. Some cells may escape this process via telomerase reactivation, and their increased survival may lead to genetic mutations and the possibility of irreversible carcinogenesis. Thus, increased telomerase activity may be used as an early biomarker of cancer detection. We therefore investigated these factors using MAbs and the Trapeze assay in the CCT and the human. K-
Ras p21 was absent in the CTTs, and they do not seem to express the common gene mutations (
KRAS).
Chromogranin-A did not differentiate between cancer CTT tissue or normal resident neuroendocrine cells. However, Adnab-9 MAb binding (adenoma biomarker 9) and now the likely innate immune system indicator, was higher in normal humans (
p < 0.05) than IBD washings, and also positive in IBD human extracts. K-
Ras p21 was elevated in humans with IBD (
p < 0.05). EGFR was maximal in CTT extracts (
p < 0.005), known to occur in 80–90% of human CRC [
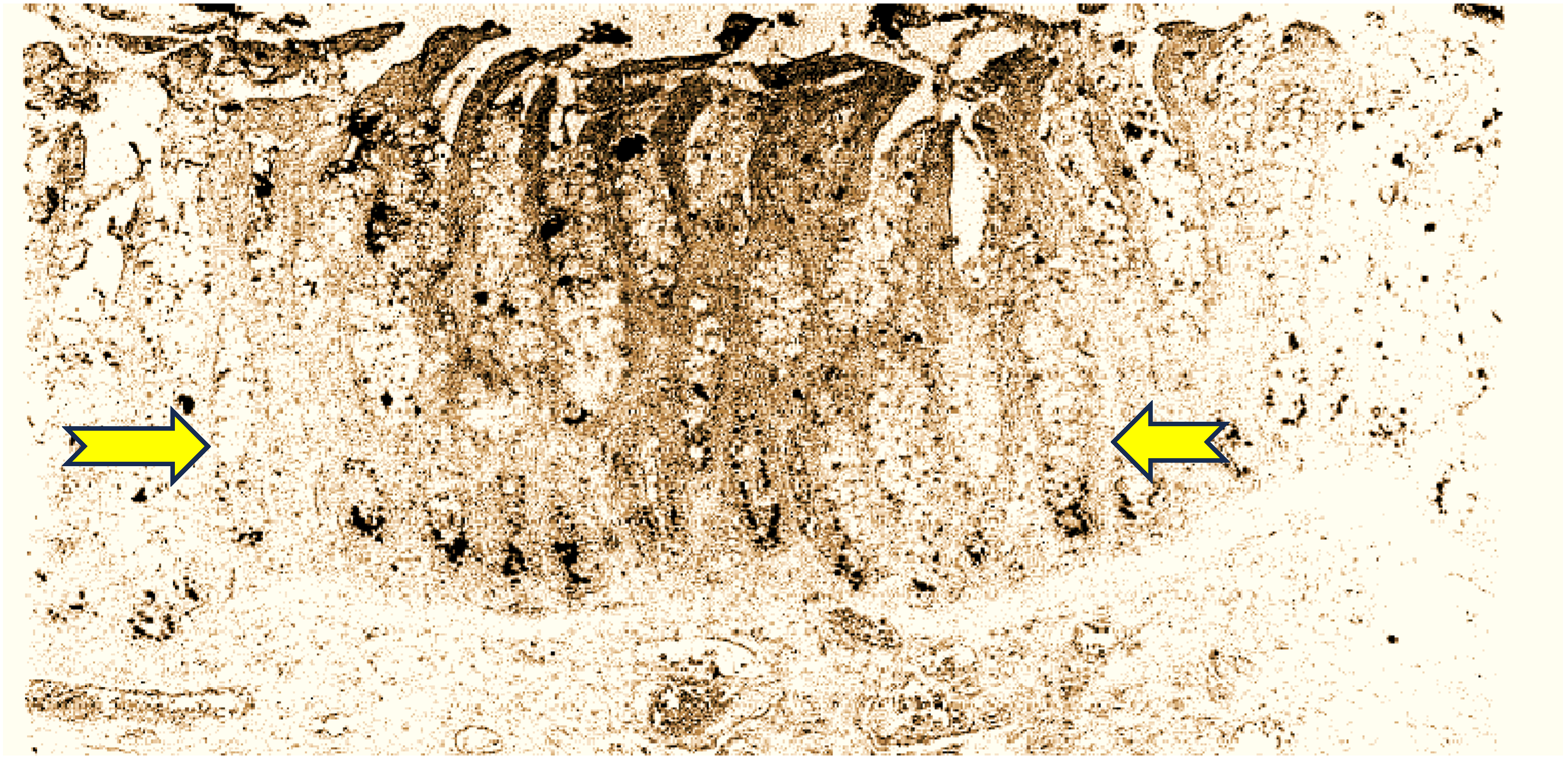
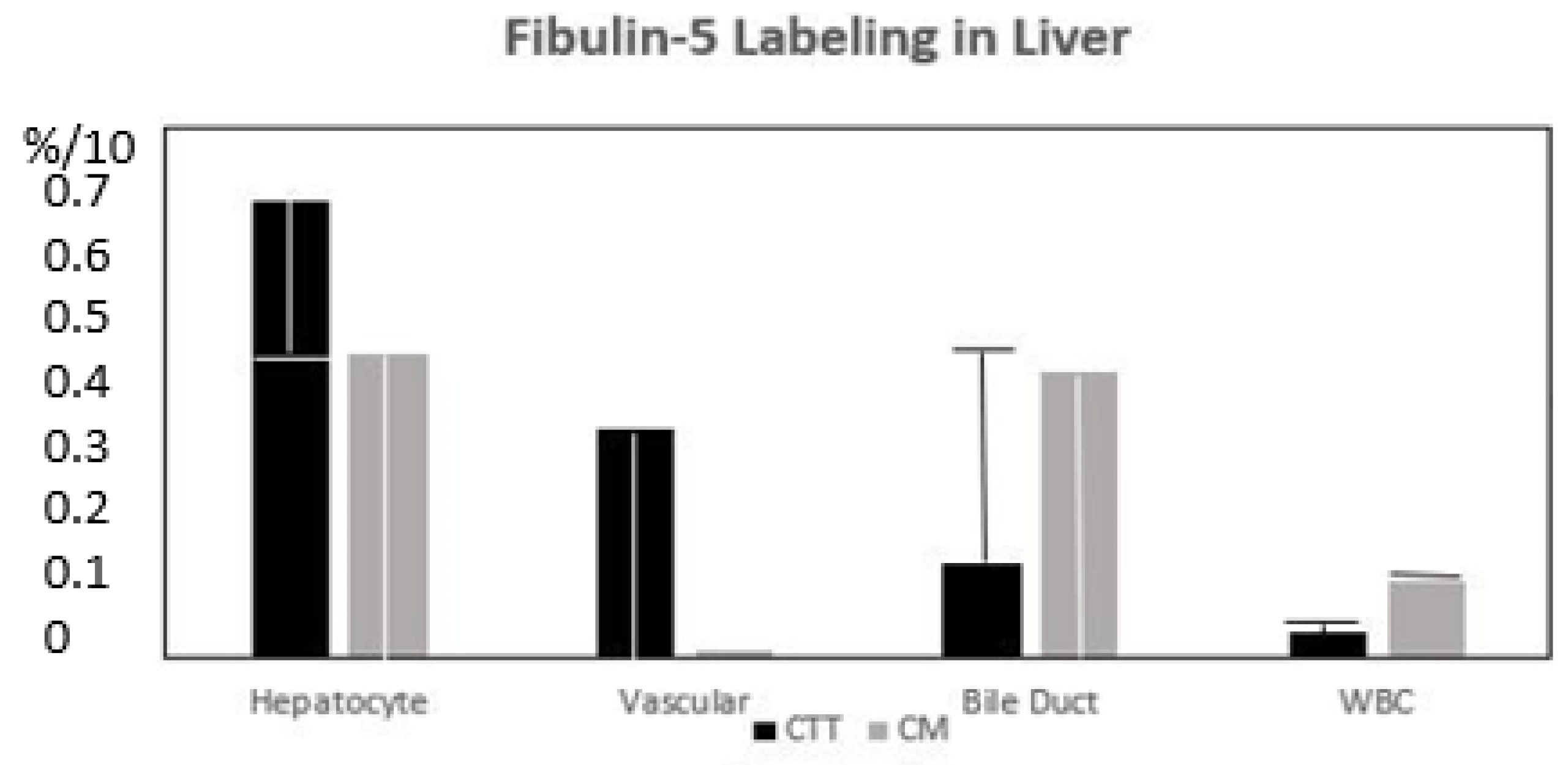
27]. Aminoproteoglycans (APGs) were also maximal in the CTT extracts, and given their widespread distribution in CTT (see
Figure 1), this is not surprising. Telomerase is known to be low in human washings or tissue [
33], and 86% of our human IBD samples were negative, but in contrast, over half of the CTT washings were positive, supporting their role in the early detection of cancer [
24].
In terms of research interest, there were 5187 Pubmed entries since 1930 for CMs compared to 1149 for CTTs since 1965, the date of the outset of largescale exportation to pharmaceutical companies, research facilities, and the like. Only 18% of the above publications center on CTTs, although there will be overlap due to certain articles featuring both CTTs and CMs [
10,
25,
31,
32,
33,
34,
35,
36,
37,
38]. The peak for CTTs was in the year 2000, with 43 articles, and the curve conforms to a Gaussian distribution, with only 8 publications for 2024 thus far. If we are able to complete our genome work, we do anticipate an uptick in interest.
For the interested reader, below we provide a description of meetings and workshops to show that it was these goodwill meetings that have advanced the field into this epoch of discovery. At this juncture, the following is the expected trajectory of what we hope to achieve within this year, until the time of the next CEA workshop in Wurzberg, FRG.
If the CTT is the bulwark against distant metastasis, we recognized that only a thorough evaluation and mapping of all extant pathways in the livers of the Callitrichidea clades would need to be completed. A team was put together to further this end. The Tulane and Yerkes Primate Centers kindly offered to provide the necessary livers. Work to purify mRNA from the livers began at the University of Michigan in the Laboratory of the Kinesiology Department. This resulted in the passable quality of the mRNA liver transcriptomes and these were sequenced. The reads that were obtained were subjected to bioinformatics scrutiny, with a number of pathways obtained. The major impediment to obtaining full bioinformatics was relying on genomic sequence data that could be compared and contrasted between the CTTs, CMs, and humans. Genomic data were available for the CM and the human, but not for the CTT, and substantial funds would have been needed to achieve a full genomic CTT sequence. Providence provided us with the full sequence published by Rasmussen et al. in a masterful international collaboration, for which they are deserving full accolades. Funds were generously provided, and we are currently probing the resultant transcriptomes with a spinoff bioinformatics company affiliated with Wayne State University. The authors of this paper wish to make it clear that without participation and international collaboration over many decades, we would not be standing on the threshold of what may very well be a major breakthrough for us primates.
It is therefore perhaps timely to reflect on what we have learned from these animals. The first peak of CTT-related publications was in 1985. The impetus for this effort was the workshop entitled as follows: Is the Marmoset an experimental model for the study of gastrointestinal disease? It was held under the auspices of Oak Ridge Associated Universities from 18 to 20 April 1984, and sponsored by the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) and the National Cancer Institute (NCI), Oak Ridge Associated Universities (ORAU), and the National Foundation for Ileitis and Colitis. The planning committee members were as follows: Dr. W Brown (Colorado); Dr. N. Clapp (ORAU); Dr. D. Podolsky (Boston); Dr. R. Riddell (Chicago); Dr. A. Rogers (Cambridge, MA); Dr. K, Vener (NIDDK), and Dr. S. Wolman (NYU). The outcome was a special supplementary edition with 32 published articles [
20,
39] and only 4 other years have surpassed this number. Researchers who contributed were among the top in this area of endeavor and many are well known to this day [
40]. Many previously published articles were incorporated into a volume on CTTs in 1993 with 30 titles, and where many of the previously published findings were recapitulated, so there is bound to be some duplication. However, there are published conference transcripts in the 1985 supplementary edition, and the same debates are ongoing today, originating from two discussion sessions and a concluding session. There were other supplementary special journal editions devoted to the CTT, such as that of 1994, published in Agents and Actions [
1], arising from a workshop arranged by Dr. Neal Clapp at the World Congress Inflammation event in the Austria Center Vienna, 10–15 October 1993, hosted by the International Association of Inflammation Societies; and a 1996 conference edition in Alimentary Pharmacology and Therapeutics [
40].
Out of the many discussions the principal author had with Dr. Neal Clapp, director of MARCOR (Marmoset Colony at Oak Ridge) and a primary mover of this entire edifice of research, one was especially of note. It was postulated that the reason liver metastasis had been absent was that the blood supply differed from that of humans. This appeared to partially emanate from Discussion 1, with a comment from a participant answering a question to the effect that he had said that the lesions resemble ischemic mucosal disease (page 33S of the supplement). In his reply, he said that he had not looked at blood flow, but at necropsy, which appeared to be normal. Dr. Clapp confirmed that the portal blood system in CTTs and humans were not dissimilar (personal communication), and that this did not explain the paucity of liver metastasis in CTTs. He and his colleagues suggested that this phenomenon was perhaps due to the inability of the cancer to invade the portal circulation for anatomic, immunologic, or physiologic reasons, in contrast to the ease with which it spreads to other distant organs and lymph nodes [
41]. Over the years, the CEA/PSG (pregnancy specific β-1 glycoproteins) part of the Ig superfamily of proteins has included Callitrichid presentations at the 4th international workshop 22–25 August 1993 at Harvard Medical School in Boston; the 6th from 5 to 8 September 1995 at Hotel Este’rel, Montreal, QC, under the auspices of McGill University; the 13th from 11 to 14 August 2002 at The Millcroft Inn, Alton, Ontario, Canada, under the aegis of The University of Toronto, where CEA gene changes were implicated in the lack of liver metastasis; the 16th on 8–11 September 2019 at the Banff Centre for Arts and Creativity, Alberta, CA; and the 31st CEA/PSG Symposium, 3–4 October 2023 at @Ease, 1345 Avenue of the Americas, New York City, hosted by the Feinstein Institutes for Medical Research and the Cold Spring Harbor Laboratory.
It would be remiss of us to avoid mentioning the viral interactions with the CTT, given the many challenges we face today from viral vectors. The most common viruses affecting Callitrichidae, in common with other non-human primates, are coronaviruses [
42,
43]. Outbreaks of colitis and diarrhea at MARCOR implicated
Campylobacter fetus subsp.
jejuni in 20% and coronavirus-like particles in 24% of cases [
44]. The latter organism has also been reported in monkeys [
44].
Limitations
In general, our studies and most others dealing with Callitrichidae species have small numbers. Specifically, tissue samples are collected at necropsy because of the endangered species provision, and the tissues are not fresh, adversely affecting the integrity of the sample.