Radiation Diagnostics of the Maxillofacial Region and Skeleton Bone Density in the Case of Vitamin D Insufficiency: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- Availability of written informed consent from the patient to participate in the study.
- Age not less than 18 and not more than 50 years.
- Established diagnosis: tooth loss (K08.1-ICD 10), vitamin D3 imbalance (severe deficiency (<10 ng/mmol), and vitamin D3 deficiency (10–20 ng/mmol).
- Absence of severe somatic pathology.
- Endocrinologist approval for skeleton bone densitometry.
2.2. Exclusion Criteria of Main RCT
- Age under 18 and over 50 years.
- Pregnancy, breast feeding.
- The presence of concomitant pathology: blood diseases, decompensated diabetes mellitus, immunodeficiency states, tuberculosis, or malignant neoplasms.
2.3. Methodology for Conducting Computed Densitometry
- During the procedure, when the density of bone tissue of the femoral neck and spine is measured, the patient lies on a soft ‘table’ of the tomograph. The table moves inside the gantry of the tomograph, and X-rays pass through the patient’s body and hit the detector.
- The patient should lie as still as possible to avoid blurring the image. He or she may even be asked to hold his or her breath for a few seconds.
- The test usually lasts 5–10 min. During the examination, the person does not experience any discomfort.
2.4. Statistics Analysis
3. Results
3.1. Jaws CBCT Results
3.2. Bone Tissue Mineral Density Evaluation Results
3.2.1. Skeleton QCT Densitometry
3.2.2. DXA Results
3.3. Comparison of DXA and QCT and Their Correlation with Jawbone Optical Density According to CBCT
4. Discussion
Limitations of Our Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMD | Bone mineral density |
| CBCT | Cone beam computed tomography |
| DI | Dental implantation |
| DXA | Dual-energy X-ray absorptiometry |
| QCT | Quantitative computed tomography |
References
- Pigarova, E.A.; Glazieva, V.S.; Povaliaeva, A.A.; Dzeranova, L.K.; Belovalova, I.M.; Dedov, I.I. Features of diagnosis and treatment of patients with vitamin d deficiency in real clinical practice. Obes. Metab. 2024, 21, 151–160. [Google Scholar] [CrossRef]
- Wang, C.J.; McCauley, L.K. Osteoporosis and Periodontitis. Curr. Osteoporos. Rep. 2016, 14, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Chambrone, L.; Goldstein, A.; Rodrigues, J.A.; Zenóbio, E.; Feres, M.; Figueiredo, L.C.; Cassoni, A.; Shibli, J.A. Impact of osteoporosis in dental implants: A systematic review. World J. Orthop. 2015, 6, 311–315. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Wactawski-Wende, J. Periodontal diseases and osteoporosis: Association and mechanisms. Ann. Periodontol. 2001, 6, 197–208. [Google Scholar] [CrossRef]
- Dodd, D.Z.; Rowe, D.J. The relationship between postmenopausal osteoporosis and periodontal disease. Am. Dent. Hyg. Assoc. 2014, 87, 336–344. [Google Scholar]
- Blake, G.M.; Fogelman, I. The clinical role of dual energy X-ray absorptiometry. Eur. J. Radiol. 2009, 71, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, A.; Scalzo, G.; Masala, S.; Tarantino, U.; Guglielmi, G. Body composition assessment by dual-energy X-ray absorptiometry (DXA). La Radiol. Medica 2009, 114, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Strollo, R.; Paladini, A.; Briganti, S.I.; Pozzilli, P.; Epstein, S. The alliance of mesenchymal stem cells, bone, and diabetes. Int. J. Endocrinol. 2014, 2014, 690783. [Google Scholar] [CrossRef]
- Nizovtsova, L.A.; Morozov, S.P.; Petryaykin, A.V.; Bosin, V.Y.; Sergunova, K.A.; Vladzimirskiy, A.V.; Shantarevich, M.Y. On the unification of bone densitometry and interpretation of its results. J. Radiol. Nucl. Med. 2018, 99, 158–163. [Google Scholar] [CrossRef]
- Setiawati, R.; Di Chio, F.; Rahardjo, P.; Nasuto, M.; Dimpudus, F.J.; Guglielmi, G. Quantitative assessment of abdominal aortic calcifications using lateral lumbar radiograph, dual-energy X-ray absorptiometry, and quantitative computed tomography of the spine. J. Clin. Densitom. 2016, 19, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Villarraga-Gómez, H.; Herazo, E.L.; Smith, S.T. X-ray computed tomography: From medical imaging to dimensional metrology. Precis. Eng. 2019, 60, 544–569. [Google Scholar] [CrossRef]
- Amstrup, A.K.; Jakobsen NF, B.; Moser, E.; Sikjaer, T.; Mosekilde, L.; Rejnmark, L. Association between bone indices assessed by DXA, HR-pQCT and QCT scans in post-menopausal women. J. Bone Miner. Metab. 2015, 34, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Koch, V.; Hokamp, N.G.; Albrecht, M.H.; Gruenewald, L.D.; Yel, I.; Borggrefe, J.; Wesarg, S.; Eichler, K.; Burck, I.; Gruber-Rouh, T.; et al. Accuracy and precision of volumetric bone mineral density assessment using dual-source dual-energy versus quantitative CT: A phantom study. Europ. Radiol. Experim. 2021, 5, 43. [Google Scholar] [CrossRef]
- Marquezan, M.; Lau, T.C.; Mattos, C.T.; da Cunha, A.C.; Nojima, L.I.; Sant’Anna, E.F.; de Souza, M.M.G.; de Souza Araújo, M.T. Bone mineral density: Methods of measurement and its influence on primary stability of miniscrews. Angle Orthod. 2012, 82, 62–66. [Google Scholar] [CrossRef]
- Shokri, A.; Ramezani, L.; Bidgoli, M.; Akbarzadeh, M.; Ghazikhanlu-Sani, K.; Fallahi-Sichani, H. Effect of field-of-view size on gray values derived from cone-beam computed tomography compared with the Hounsfield unit values from multidetector computed tomography scans. Imaging Sci. Dent. 2018, 48, 31–39. [Google Scholar] [CrossRef]
- In, J. Introduction of a pilot study. Korean J. Anesthesiol. 2017, 70, 601–605. [Google Scholar] [CrossRef]
- Boehm, E.; Kraft, E.; Biebl, J.T.; Wegener, B.; Stahl, R.; Feist-Pagenstert, I. Quantitative computed tomography has higher sensitivity detecting critical bone mineral density compared to dual-energy X-ray absorptiometry in postmenopausal women and elderly men with osteoporotic fractures: A real-life study. Arch. Orthop. Trauma Surg. 2024, 144, 179–188. [Google Scholar] [CrossRef]
- Siddiqee, M.H.; Bhattacharjee, B.; Siddiqi, U.R.; MeshbahurRahman, M. High prevalence of vitamin D deficiency among the South Asian adults: A systematic review and meta-analysis. BMC Public Health 2021, 21, 1823. [Google Scholar] [CrossRef]
- Rathish, N.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef]
- Yeum, K.-J.; Song, B.C.; Joo, N.-S. Impact of Geographic Location on Vitamin D Status and Bone Mineral Density. Int. J. Environ. Res. Public Health 2016, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, W.Z.; Hegazy, R.A. Vitamin D and the skin: Focus on a complex relationship: A review. J. Adv. Res. 2015, 6, 793–804. [Google Scholar] [CrossRef]
- Dimai, H.P. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T-and Z-score, and reference databases. Bone 2017, 104, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Mongkornkarn, S.; Suthasinekul, R.; Sritara, C.; Lertpimonchai, A.; Tamsailom, S.; Udomsak, A. Significant association between skeletal bone mineral density and moderate to severe periodontitis in fair oral hygiene individuals. J. Investig. Clin. Dent. 2019, 10, e12441. [Google Scholar] [CrossRef]
- Bartl, R.; Bartl, C. Oral bone loss due to periodontitis and systemic osteoporosis. Osteoporos. Man. Prev. Diagn. Manag. 2019, 399–401. [Google Scholar]
- Aghaloo, T.; Pi-Anfruns, J.; Moshaverinia, A.; Slim, D.; Grogan, T.; Hadaya, D. The Effects of Systemic Diseases and Medications on Implant Osseointegration: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2019, 34, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Khoo BC, C.; Brown, K.; Zhu, K.; Price, R.I.; Prince, R.L. Effects of the Assessment of 4 Determinants of Structural Geometry on QCT- and DXA-Derived Hip Structural Analysis Measurements in Elderly Women. J. Clin. Densitom. 2014, 17, 38–46. [Google Scholar] [CrossRef]
- Elkersh, N.M.; Talaab, M.R.; Ahmed, W.M.; Gaweesh, Y.S. Utility of cone beam computed tomography of the mandible in detection of osteoporosis in postmenopausal women. Alex. Dent. J. 2019, 44, 46–51. [Google Scholar] [CrossRef]
- Sghaireen, M.G.; Ganji, K.K.; Alam, M.K.; Srivastava, K.C.; Shrivastava, D.; Ab Rahman, S.; Patil, S.R.; Al Habib, S. Comparing the Diagnostic Accuracy of CBCT Grayscale Values with DXA Values for the Detection of Osteoporosis. Appl. Sci. 2020, 10, 4584. [Google Scholar] [CrossRef]
- Yanushevich, O.O.; Kozlova, M.V.; Mkrtumyan, A.M.; Belyakova, A.S.; Kozlova, L.S. The characteristics of dental implantation in patients with osteoporosis. Treat. Profilaxys 2014, 3, 85–90. [Google Scholar]
- Shokri, A.; Ghanbari, M.; Maleki, F.H.; Ramezani, L.; Amini, P.; Tapak, L. Relationship of Gray Values in Cone Beam Computed Tomography and Bone Mineral Density Obtained by Dual Energy X-ray Absorptiometry. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Barngkgei, I.; Al Haffar, I.; Khattab, R. Osteoporosis prediction from the mandible using cone-beam computed tomography. Imaging Sci. Dent. 2014, 44, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Zurl, B.; Tiefling, R.; Winkler, P.; Kindl, P.; Kapp, K.S. Hounsfield units variations: Impact on CT-density based conversion tables and their effects on dose distribution. Strahlenther. Onkol. 2014, 190, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Tarasenko, S.V.; Dydykina, I.S.; Nikolaeva, E.N.; Tsarev, V.N.; Makarevich, A.A. The importance of additional methods for examining patients with chronic generalized periodontitis in combination with rheumatoid arthritis. Clin. Stomatol. 2019, 3, 36–39. [Google Scholar] [CrossRef]
- Ostrovskaya, L.U.; Khanina, A.I. Treatment of chronic generalized periodontitis at women in the period of postmenopause. Saratov J. Med. Sci. Res. 2015, 11, 69–73. [Google Scholar]
- Ufimtseva, M.A.; Bochkarev, Y.M.; Gurkovskaya, E.P.; Puhtinskaya, P.S.; Nikolaeva, K.I.; Lesnaya, O.D. Osteoporosis as a result of the long-term administration of glucocorticoids in patients suffering from acantholytic pemphigus. Vestn. Dermatol. I Venerol. 2016, 3, 56–61. [Google Scholar] [CrossRef]
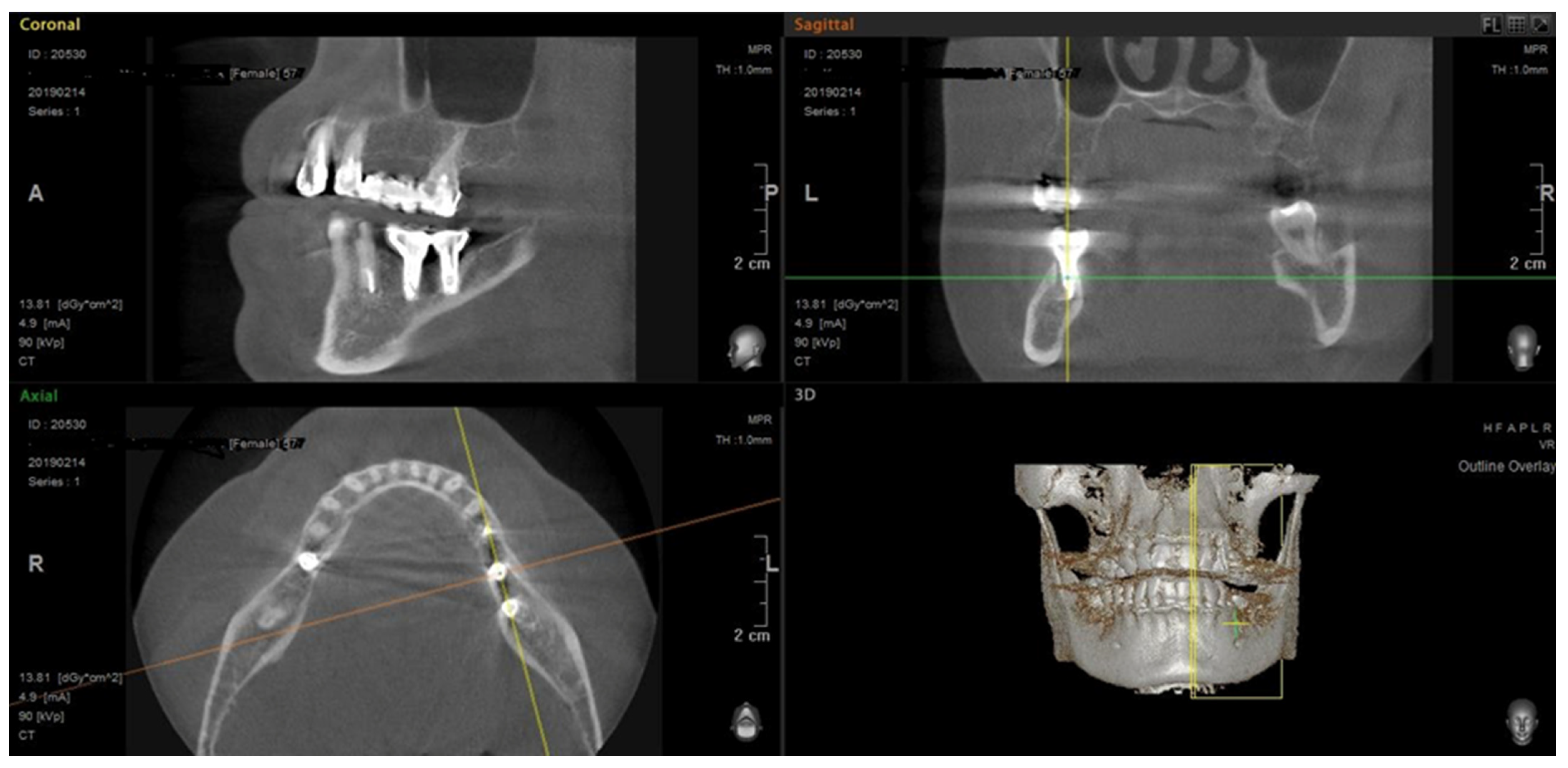

| Jawbone Density Type | Maxilla (n = 12) | Mandible (n = 12) | p (Fisher’s Exact Test) |
|---|---|---|---|
| D1 (>1350 Hu) | 1 (8.4%) | 2 (16.7%) | >0.05 (F = 1) |
| D2 (850–1350 Hu) | 5 (41.6%) | 6 (50%) | >0.05 (F = 1) |
| D3 (350–850 Hu) | 6 (50%) | 4 (33.3%) | >0.05 (F = 0.68) |
| D4 (<350 Hu) | 0 | 0 | NA * |
| Jawbone Density Type | Maxilla (n = 12) | Mandible (n = 12) | p (Fisher’s Exact Test) |
|---|---|---|---|
| D1 (>1350 Hu) | 1 (8.4%) | 2 (16.7%) | >0.05 (F = 1) |
| D2 (850–1350 Hu) | 11 | 10 | >0.05 (F = 1) |
| D3 (350–850 Hu) | 0 | 0 | NA * |
| D4 (<350 Hu) | 0 | 0 | NA * |
| Region | Me ± m Median Min–Max |
|---|---|
| Vertebral bone mineral density (L1, L2, L3) (Mean mineral density mg/cm3) | 149.7 ± 39.3 153.6 101.1–198.9 |
| T-criterion of left femur neck | 0.05 ± 0.71 0.46 −0.95–0.64 |
| Z-criterion of left femur neck | 0.42 ± 0.97 0.7 −0.88–1.46 |
| Region | Me ± m Median Min–Max |
|---|---|
| Vertebral bone mineral density (L1, L2, L3) Mean mineral density mg/cm3 | 108.3 ± 5.0 110.6 101.2–113.2 |
| T-criterion of left femur neck | 0 ± 1.4 0 −1.5–1.5 |
| Z-criterion of left femur neck | 0.12 ± 0.1 0.15 0.1–0.2 |
| Criterion | Group 1 QCT (n = 12) | Group 2 DXA (n = 12) |
|---|---|---|
| Vertebral bone mineral density (L1, L2, L3) (mg/cm3) | p (Mann–Whitney test) (0.068) > 0.05 | |
| T-criterion of left femur neck | p (Mann–Whitney test) (1) > 0.05 | |
| Z-criterion of left femur neck | p (Mann–Whitney test) (0.17) > 0.05 | |
| Method of Bone Density Assessment | Skeletal QCT (n = 12) | Skeletal DXA (n = 12) |
|---|---|---|
| CBCT of jaws (n = 24) | Pearson Correlation Coefficient R = −0.16 p > 0.05 | Pearson Correlation Coefficient R = 0.15 p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diachkova, E.; Tarasenko, S.; Skachkova, M.; Zhilkov, Y.; Serova, N.; Babkova, A.; Volel, B.; Blinova, E.; Kytko, E.; Meylanova, R.; et al. Radiation Diagnostics of the Maxillofacial Region and Skeleton Bone Density in the Case of Vitamin D Insufficiency: A Pilot Study. Life 2025, 15, 480. https://doi.org/10.3390/life15030480
Diachkova E, Tarasenko S, Skachkova M, Zhilkov Y, Serova N, Babkova A, Volel B, Blinova E, Kytko E, Meylanova R, et al. Radiation Diagnostics of the Maxillofacial Region and Skeleton Bone Density in the Case of Vitamin D Insufficiency: A Pilot Study. Life. 2025; 15(3):480. https://doi.org/10.3390/life15030480
Chicago/Turabian StyleDiachkova, Ekaterina, Svetlana Tarasenko, Marina Skachkova, Yury Zhilkov, Natalia Serova, Anna Babkova, Beatrice Volel, Ekaterina Blinova, Elizaveta Kytko, Renata Meylanova, and et al. 2025. "Radiation Diagnostics of the Maxillofacial Region and Skeleton Bone Density in the Case of Vitamin D Insufficiency: A Pilot Study" Life 15, no. 3: 480. https://doi.org/10.3390/life15030480
APA StyleDiachkova, E., Tarasenko, S., Skachkova, M., Zhilkov, Y., Serova, N., Babkova, A., Volel, B., Blinova, E., Kytko, E., Meylanova, R., Zaborova, V., & Kytko, O. (2025). Radiation Diagnostics of the Maxillofacial Region and Skeleton Bone Density in the Case of Vitamin D Insufficiency: A Pilot Study. Life, 15(3), 480. https://doi.org/10.3390/life15030480








