Anti-Inflammatory Effects and Human Skin Safety of the Eastern Traditional Herb Mosla japonica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Measurement of Total Phenolic and Total Flavonoid Content
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Measurement of Nitric Oxide (NO) Production
2.6. Measurement of Prostaglandin E (PGE)2 and Pro-Inflammatory Cytokines
2.7. Western Blot Analysis
2.8. Primary Skin Irritation Test
2.9. Statistical Analysis
3. Results and Discussion
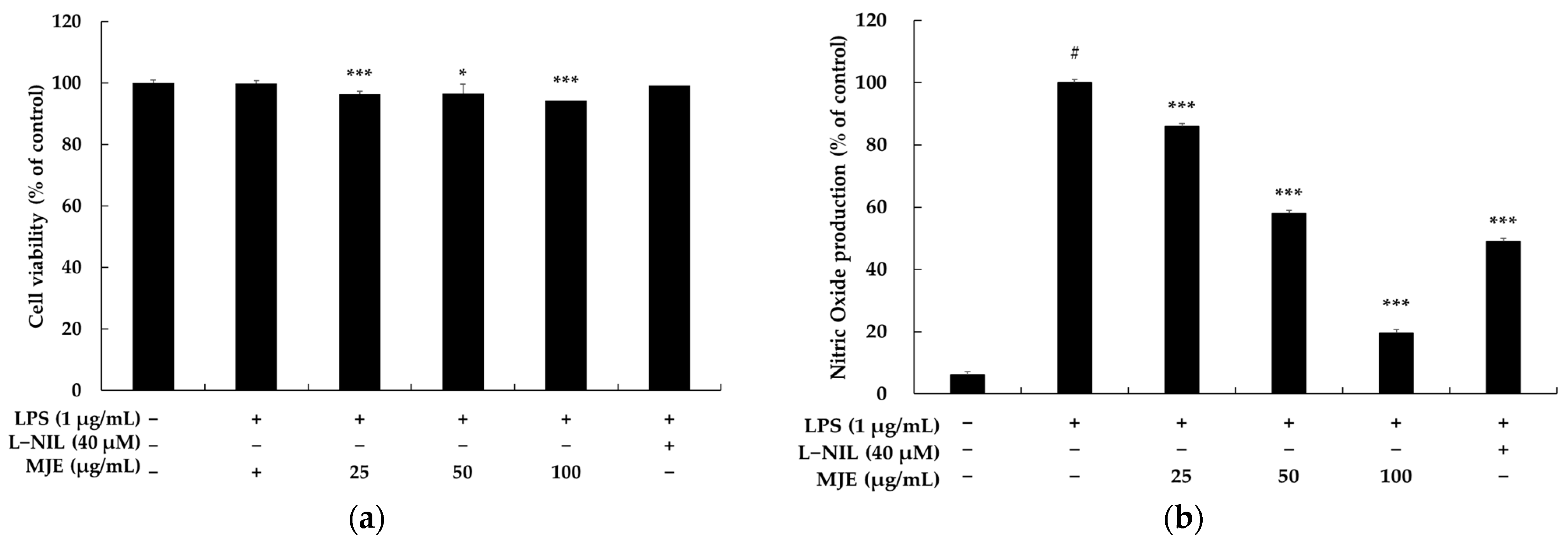
3.1. The Effect of MJE on Cell Viability in RAW 264.7 Cells
3.2. The Effect of MJE on NO Production in RAW 264.7 Cells
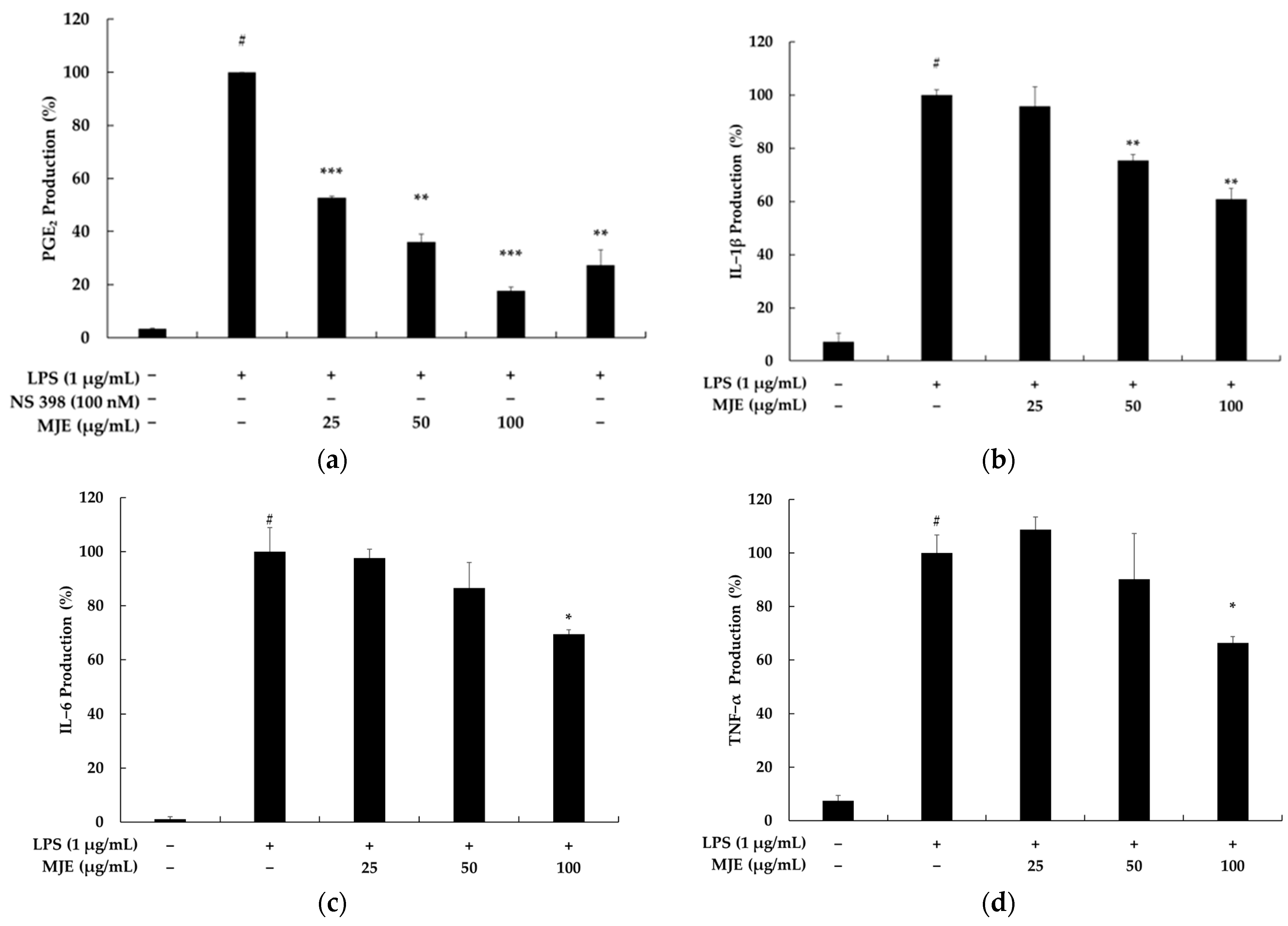
3.3. The Effect of MJE on PGE2 and Pro-Inflammatory Cytokine Production in RAW 264.7 Cells
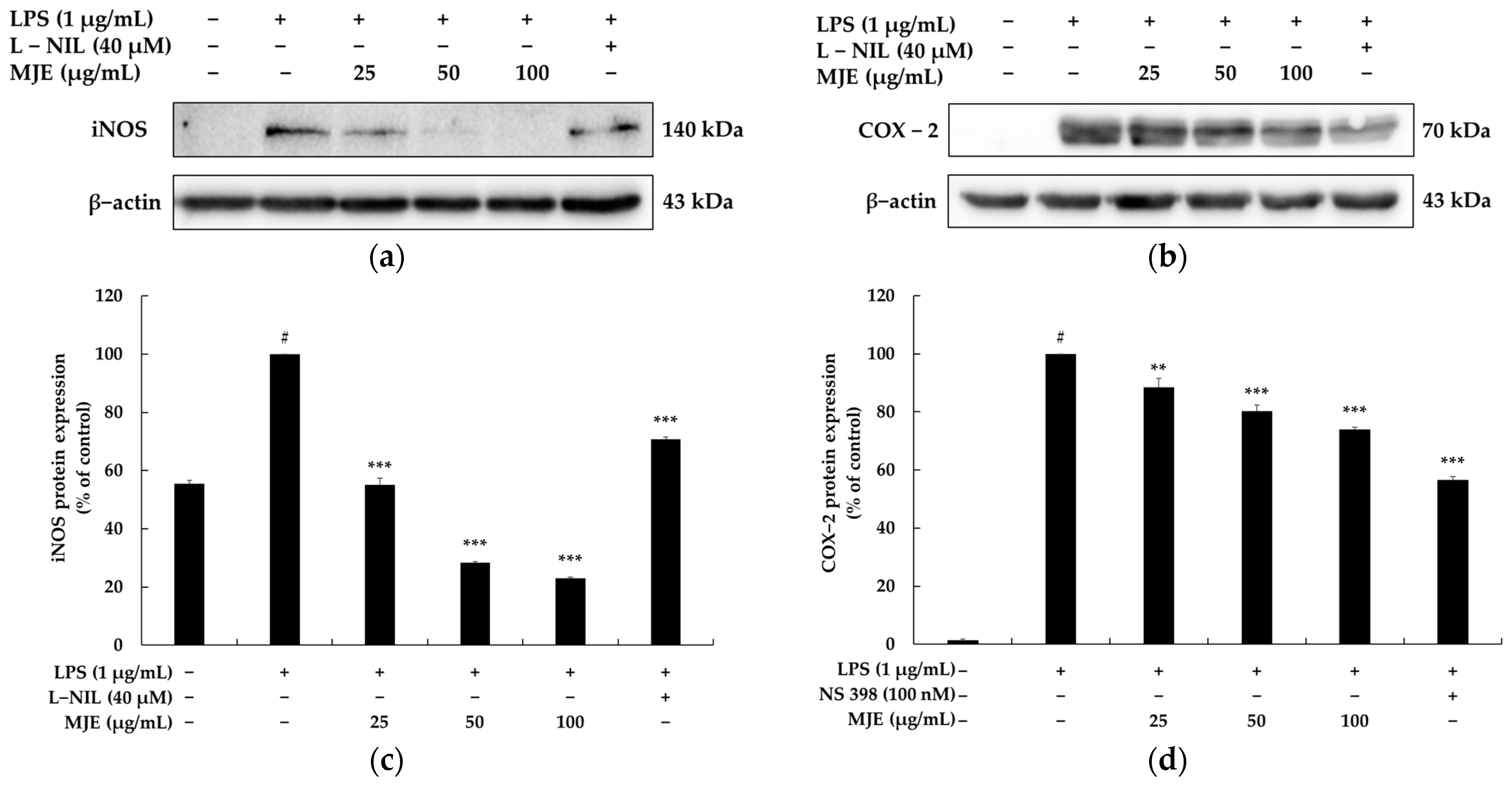
3.4. The Effect of MJE on iNOS and COX-2 Protein Expression in RAW 264.7 Cells
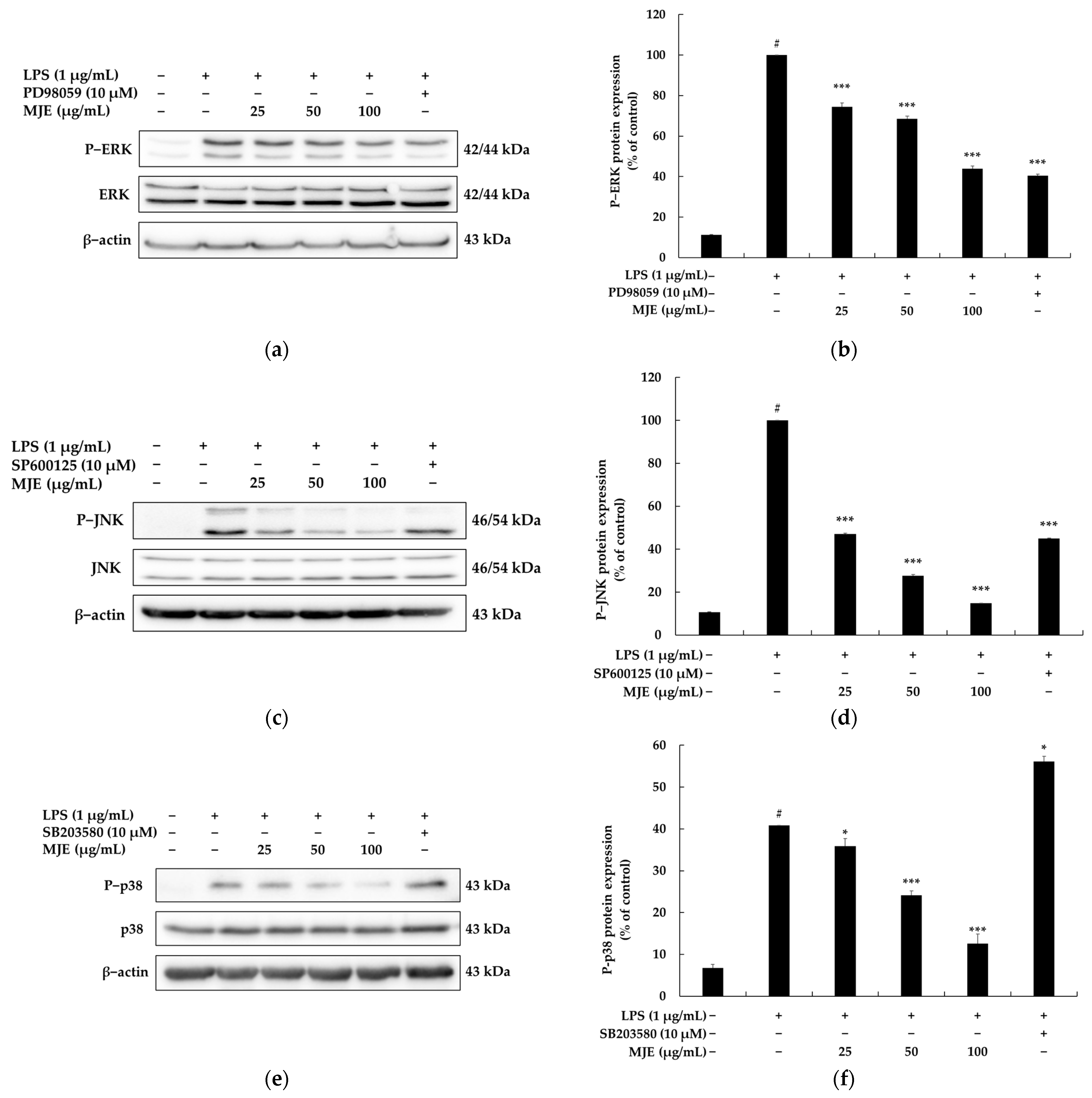
3.5. The Effect of MJE on the MAPK Signaling Pathway in RAW 264.7 Cells
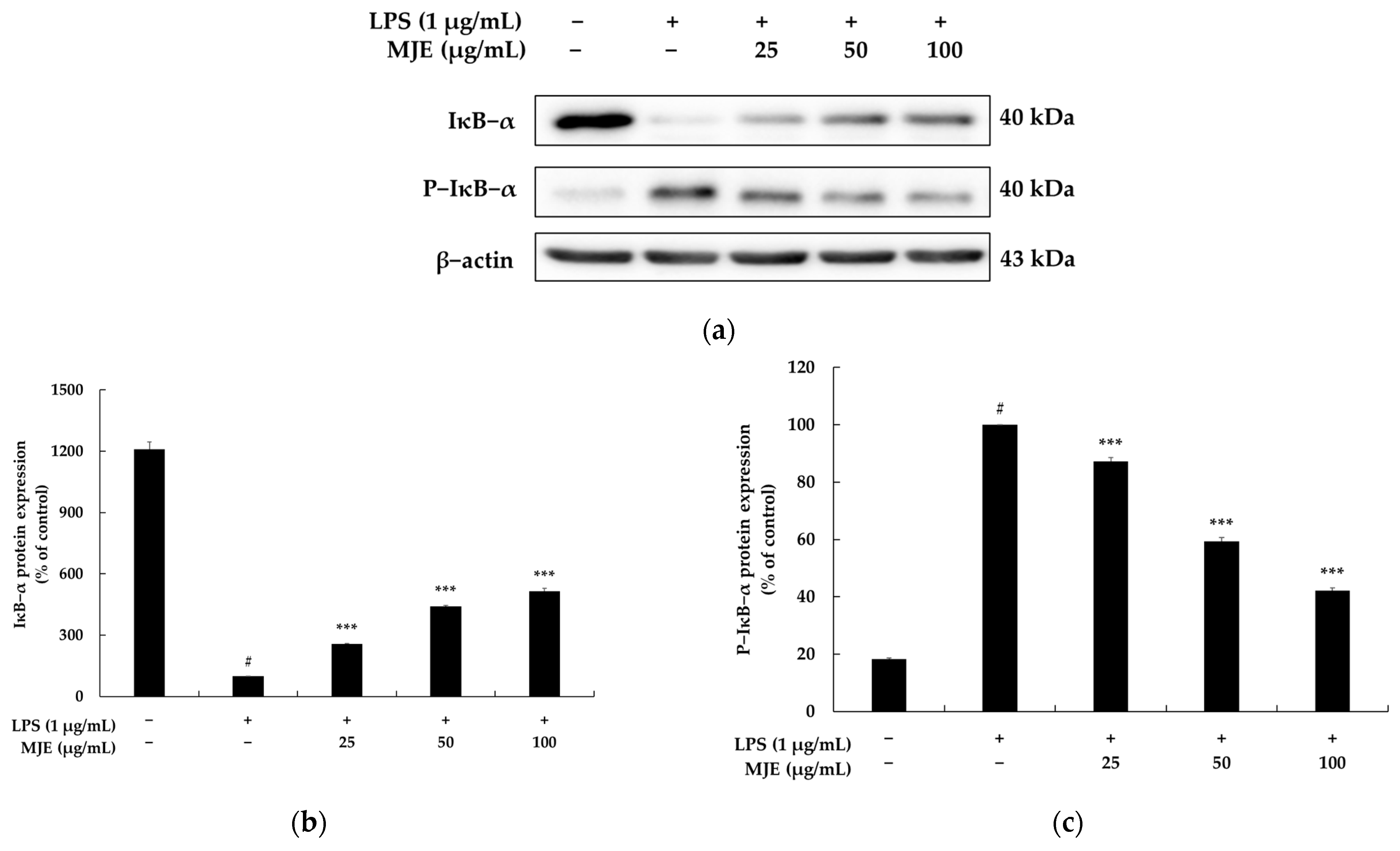
3.6. The Effect of MJE on the NF-κB Signaling Pathway in RAW 264.7 Cells
3.7. Total Phenolic and Flavonoid Content of MJE
3.8. Primary Skin Irritation Test of MJE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, W.; Ma, M.; Chen, X.; Min, J.; Li, L.; Zheng, Y.; Li, Y.; Wang, J.; Wang, Q. Traditional Chinese Medicine and Constitutional Medicine in China, Japan and Korea: A Comparative Study. Am. J. Chin. Med. 2017, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cha, W.S.; Oh, J.H.; Park, H.J.; Ahn, S.W.; Hong, S.Y.; Kim, N.I. Historical difference between traditional Korean medicine and traditional Chinese medicine. Neurol. Res. 2007, 29, S5–S9. [Google Scholar] [CrossRef]
- Park, H.L.; Lee, H.S.; Shin, B.C.; Liu, J.P.; Shang, Q.; Yamashita, H.; Lim, B. Traditional medicine in china, Korea, and Japan: A brief introduction and comparison. Evid. Based. Complement. Alternat. Med. 2012, 2012, 429103. [Google Scholar] [CrossRef]
- Lim, B.; Koh, B.; Kim, Y.; Kim, Y.; Yun, Y. History, Present and Prospect of Korean Medicine. In History, Present and Prospect of World Traditional Medicine; World Scientific Publishing Co., Pte Ltd.: Singapore, 2024; pp. 447–542. [Google Scholar] [CrossRef]
- Tohda, M. Clarification of generating mechanisms of various types of depression based on the Wakan-yaku theory for development of novel antidepressants. J. Trad. Med. 2012, 29, 85–88. [Google Scholar] [CrossRef]
- Chakrabarty, S.P.; Tanoue, M.; Penteado, A. The Trouble Is, You Think You Have Time: Traditional Knowledge of Indigenous Peoples in Japan and India, the Reality of Biodiversity Exploitation. Environ. Manag. 2023, 72, 100–112. [Google Scholar] [CrossRef]
- Hayasaka, S.; Kodama, T.; Ohira, A. Traditional Japanese herbal (kampo) medicines and treatment of ocular diseases: A review. Am. J. Chin. Med. 2012, 40, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.C.; Machado, J.P.; Monteiro, F.J.; Greten, H.J. Understanding Traditional Chinese Medicine Therapeutics: An Overview of the Basics and Clinical Applications. Healthcare 2021, 9, 257. [Google Scholar] [CrossRef]
- Ma, D.; Wang, S.; Shi, Y.; Ni, S.; Tang, M.; Xu, A. The development of traditional Chinese medicine. J. Trad. Chin. Med. Sci. 2021, 8, S1–S9. [Google Scholar] [CrossRef]
- Park, T.J.; Park, S.P. Legal and economic perspectives on fair and equitable benefit sharing in the Nagoya Protocol. Conserv. Biol. 2024, 22, e14410. [Google Scholar] [CrossRef]
- Smith, D.; da Silva, M.; Jackson, J.; Lyal, C. Explanation of the Nagoya Protocol on Access and Benefit Sharing and its implication for microbiology. Microbiology 2017, 163, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xu, J.; Chi, X.L.; Zang, C.X.; Que, L. Influence of Nagoya Protocol on traditional Chinese medicine. China J. Chin. Mater. Medica 2018, 43, 396–400. [Google Scholar] [CrossRef]
- Duan, Z.Y.; Sun, Y.P.; Wang, Z.B.; Kuang, H.X. Moslae Herba: Botany, Traditional Uses, Phytochemistry, and Pharmacology. Molecules 2024, 29, 1716. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Huang, P.; Zhou, J.; Tan, G.; Zeng, J.; Liu, W. Mosla chinensis Extract Enhances Growth Performance, Antioxidant Capacity, and Intestinal Health in Broilers by Modulating Gut Microbiota. Microorganisms 2024, 12, 2647. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.Y.; Jiang, N.; Yang, J.Y.; Yang, L.Y.; Du, J.C.; Chen, X.Q.; Liu, D.; Li, R.T.; Zhong, J.D. Antiviral and anti-inflammatory activities of chemical constituents from twigs of Mosla chinensis Maxim. Nat. Prod. Bioprospect. 2024, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Han, B.; Zeng, Y.; Shen, L.; Liu, X.; Wang, Q.; Liao, M.; Li, J. HPLC-DAD Analysis, SFE-CO2 Extraction, and Antibacterial Activity on Bioactive Compounds from Mosla chinensis Maxim. Molecules 2023, 28, 7724. [Google Scholar] [CrossRef]
- Liu, H.N.; Jiang, X.X.; Naeem, A.; Chen, F.C.; Wang, L.; Liu, Y.X.; Li, Z.; Ming, L.S. Fabrication and Characterization of β-Cyclodextrin/Mosla chinensis Essential Oil Inclusion Complexes: Experimental Design and Molecular Modeling. Molecules 2022, 28, 37. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, K.; Liu, Z.; Sun, Y.; Zhou, L.; Xu, M.; Dai, X.; Xiong, Y.; Zhang, H. Bioactive constituents of Mosla chinensis-cv. Jiangxiangru ameliorate inflammation through MAPK signaling pathways and modify intestinal microbiota in DSS-induced colitis mice. Phytomedicine 2021, 93, 153804. [Google Scholar] [CrossRef]
- Lei, D.; Li, L.; Huang, S.; Liu, L.; Cai, P.; Gu, Z.; Shu, K.; Li, S.; Hong, T.; Liu, Z. The Subchronic Toxic Effects of Mosla chinensis Maxim in Normal Rats. Biomed. Res. Int. 2020, 2020, 4521586. [Google Scholar] [CrossRef]
- Cai, W.; Wu, L.R.; Zhang, S.L. Lignans from Mosla scabra Ameliorated Influenza A Virus-Induced Pneumonia via Inhibiting Macrophage Activation. Evid. Based. Complement. Alternat. Med. 2022, 2022, 1688826. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue Assays to Estimate The Total Phenolic Content of Juices and Teas Using 96-Well Microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and they scavenging effects on super-oxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Dax, C.I.; Lottspeich, F.; Müllner, S. In vitro model system for the identification and characterization of proteins involved in inflammatory processes. Electrophoresis 1998, 19, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Q.; He, X.; Wang, Y.; Zhu, G.; Yu, L. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of Cordyceps militaris spent substrate. PLoS ONE 2023, 18, e0291363. [Google Scholar] [CrossRef]
- Arnhold, J. Host-Derived Cytotoxic Agents in Chronic Inflammation and Disease Progression. Int. J. Mol. Sci. 2023, 24, 3016. [Google Scholar] [CrossRef]
- Khalef, L.; Lydia, R.; Filicia, K.; Moussa, B. Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments. Cell Biochem. Funct. 2024, 42, e4007. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P.; Parveen, A.; Kim, S.Y. Nitric Oxide as a Target for Phytochemicals in Anti-Neuroinflammatory Prevention Therapy. Int. J. Mol. Sci. 2021, 22, 4771. [Google Scholar] [CrossRef]
- Inkanuwat, A.; Sukaboon, R.; Reamtong, O.; Asawanonda, P.; Pattaratanakun, A.; Saisavoey, T.; Sangtanoo, P.; Karnchanatat, A. Nitric Oxide Synthesis Inhibition and Anti-Inflammatory Effect of Polypeptide Isolated from Chicken Feather Meal in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Food Technol. Biotechnol. 2019, 57, 200–212. [Google Scholar] [CrossRef]
- Tsikas, D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005, 39, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Csonka, C.; Páli, T.; Bencsik, P.; Görbe, A.; Ferdinandy, P.; Csont, T. Measurement of NO in biological samples. Br. J. Pharmacol. 2015, 172, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Martínez-Colón, G.J.; Moore, B.B. Prostaglandin E2 as a Regulator of Immunity to Pathogens. Pharmacol. Ther. 2018, 185, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Sreeramkumar, V.; Fresno, M.; Cuesta, N. Prostaglandin E2 and T cells: Friends or foes? Immunol. Cell Biol. 2012, 90, 579–586. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: Obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Hnasko, T.S.; Hnasko, R.M. The Western Blot. Methods Mol. Biol. 2015, 1318, 87–96. [Google Scholar] [CrossRef]
- Ghosh, R.; Gilda, J.E.; Gomes, A.V. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert. Rev. Proteom. 2014, 11, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Mahmood, T.; Yang, P.C. Western blot: Technique, theory and trouble shooting. N. Am. J. Med. Sci. 2014, 6, 160. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef]
- Nailwal, N.P.; Doshi, G.M. Role of intracellular signaling pathways and their inhibitors in the treatment of inflammation. Inflammopharmacology 2021, 29, 617–640. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian, MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef]
- Liao, H.; Zheng, J.; Lu, J.; Shen, H.L. NF-κB Signaling Pathway in Rheumatoid Arthritis: Mechanisms and Therapeutic Potential. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Jin, X.; Song, S.; Wang, J.; Zhang, Q.; Qiu, F.; Zhao, F. Tiliroside, the major component of Agrimonia pilosa Ledeb ethanol extract, inhibits MAPK/JNK/p38-mediated inflammation in lipopolysaccharide-activated RAW 264.7 macrophages. Exp. Ther. Med. 2016, 12, 499–505. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Shin, E.M.; Guo, L.Y.; Youn, U.J.; Bae, K.; Kang, S.S.; Zou, L.B.; Kim, Y.S. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation. Eur. J. Pharmacol. 2008, 586, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Shen, S.S.; Deng, X.; Shiu, H.T.; Siu, W.S.; Leung, P.C.; Ko, C.H.; Cheng, B.H. Dihydrofisetin exerts its anti-inflammatory effects associated with suppressing ERK/p38 MAPK and Heme Oxygenase-1 activation in lipopolysaccharide-stimulated RAW 264.7 macrophages and carrageenan-induced mice paw edema. Int. Immunopharmacol. 2018, 54, 366–374. [Google Scholar] [CrossRef]
- Mankan, A.K.; Lawless, M.W.; Gray, S.G.; Kelleher, D.; McManus, R. NF-kappaB regulation: The nuclear response. J. Cell. Mol. Med. 2009, 13, 631–643. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Ricci, F.; Di Credico, A.; Gaggi, G.; Iannetti, G.; Ghinassi, B.; Gallina, S.; Olshansky, B.; Di Baldassarre, A. Metoprolol disrupts inflammatory response of human cardiomyocytes via β-arrestin2 biased agonism and NF-κB signaling modulation. Biomed. Pharmacother. 2023, 168, 115804. [Google Scholar] [CrossRef]
- He, F.; Cheng, Q.; Li, N.; Shang, Y. Carbenoxolone Ameliorates Allergic Airway Inflammation through NF-κB/NLRP3 Pathway in Mice. Biol. Pharm. Bull. 2022, 45, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huo, L.; Deng, B.; Jiang, S.; Zhao, Y.; Mo, Y.; Bai, H.; Xu, L.; Hu, C.; Mu, X. Tetramethylpyrazine inhibits the inflammatory response by downregulating the TNFR1/IκB-α/NF-κB p65 pathway after spinal cord injury. Toxicol. Appl. Pharmacol. 2024, 484, 116872. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.H.; Lin, L.W.; Lin, T.I.; Liu, C.W.; Chang, L.C.; Lin, I.C.; Wu, M.S.; Tsai, C.C. Exploring the relaxation effects of Coptis chinensis and berberine on the lower esophageal sphincter: Potential strategies for LES motility disorders. BMC Complement. Med. Ther. 2024, 24, 417. [Google Scholar] [CrossRef] [PubMed]
- Wangkarn, S.; Grudpan, K.; Khanongnuch, C.; Pattananandecha, T.; Apichai, S.; Saenjum, C. Development of HPLC Method for Catechins and Related Compounds Determination and Standardization in Miang (Traditional Lanna Fermented Tea Leaf in Northern Thailand). Molecules 2021, 26, 6052. [Google Scholar] [CrossRef] [PubMed]
- Curtasu, M.V.; Nørskov, N.P. Comprehensive quantification of flavonoids and salicylic acid representative of Salix spp. using microLiquid Chromatography-Triple Quadrupole Mass Spectrometry: The importance of drying procedures and extraction solvent when performing classical solid-liquid extraction. J. Chromatogr. A 2023, 1705, 464139. [Google Scholar] [CrossRef]
| No. | Samples | Responders | 1st Assessment | 2nd Assessment | Reaction Grade (R) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| +1 | +2 | +3 | +4 | +1 | +2 | +3 | +4 | ||||
| 1 | MJE (100 μg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | Squalene (Control) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.-J.; Hyun, C.-G. Anti-Inflammatory Effects and Human Skin Safety of the Eastern Traditional Herb Mosla japonica. Life 2025, 15, 418. https://doi.org/10.3390/life15030418
Han H-J, Hyun C-G. Anti-Inflammatory Effects and Human Skin Safety of the Eastern Traditional Herb Mosla japonica. Life. 2025; 15(3):418. https://doi.org/10.3390/life15030418
Chicago/Turabian StyleHan, Hyun-Ju, and Chang-Gu Hyun. 2025. "Anti-Inflammatory Effects and Human Skin Safety of the Eastern Traditional Herb Mosla japonica" Life 15, no. 3: 418. https://doi.org/10.3390/life15030418
APA StyleHan, H.-J., & Hyun, C.-G. (2025). Anti-Inflammatory Effects and Human Skin Safety of the Eastern Traditional Herb Mosla japonica. Life, 15(3), 418. https://doi.org/10.3390/life15030418








