Anti-Cancer Strategies Using Anaerobic Spore-Forming Bacteria Clostridium: Advances and Synergistic Approaches
Abstract
1. Introduction
2. Biological Characteristics of Clostridium
2.1. Anaerobic Nature and Tumor Targeting Through Spore Formation
2.2. Pathogenic vs. Therapeutic Strains
3. Clostridium-Based Anti-Cancer Mechanisms
3.1. Direct Tumor Lysis
3.2. Immune Modulation
3.3. Combination of Clostridium with Other Therapies
| Clostridium-Based Approach | Mechanism | Description | Applications | References |
| Spore injection | Selective germination in hypoxic tumor regions | Clostridium spores germinate specifically in low-oxygen zones, proliferating and causing tumor cell lysis. | Shown to reduce tumors in models and early human trials using Clostridium novyi-NT spores. | [41,46] |
| Clostridiumnovyi-NT (Engineered) | Attenuation to remove toxins, tumor-specific germination | Genetically modified to eliminate harmful alpha-toxins, allowing safer intratumoral proliferation. | Demonstrated tumor shrinkage in animal models and Phase 1 clinical trial in a patient with leiomyosarcoma. | [46] |
| Enzyme Prodrug Therapy (CDEPT) | Enzyme activation of prodrugs into cytotoxic agents within the tumor, (“bystander effect”) | Genetically engineered Clostridium expresses enzymes that convert non-toxic prodrugs into active chemotherapy agents only within the tumor, limiting systemic toxicity. | Studies with prodrug-converting enzymes like nitroreductase show promising local cancer cell death. | [47] |
| Proteolytic activity | Direct breakdown of tumor tissue through enzymes | Some strains like C. sporogenes and C. novyi exhibit inherent proteolytic action, which aids in breaking down the tumor extracellular matrix, increasing the efficacy of tumor lysis and immune response. | Used as a basis in research for combining proteolytic Clostridium strains with other therapeutic gene modifications. | [41,48] |
| Immune activation | Tumor cell lysis and immune response stimulation | As Clostridium proliferates in the tumor, it causes cell lysis that releases tumor antigens, which can then trigger immune cell infiltration and anti-tumor immune responses. | Clostridium novyi-NT therapy shows immune activation with inflammatory and tumor response in dog and rat models. | [47,49,50] |
| Combination with radiation/ chemotherapy | Increased colonization and tumor hypoxia exploitation | Radiation can increase hypoxia and necrosis, creating an even more favorable environment for Clostridium colonization and synergistic effects with immune and tumor cell targeting. | Shown to enhance the therapeutic effects in preclinical studies when used in conjunction with chemo/radiotherapy. | [27] |
| Clostridium Species | Toxicology | Mode of Action | Genetic Modifications | References |
| C. novyi-NT | Inflammation Bacterial spread Immune reactions | Targets hypoxic tumors Causes tumor cell lysis Activates immune response | α-toxin gene removed for safety | [22] |
| C. perfringens | Intestinal damage Inflammation Bacterial spread | Produces enterotoxin (CPE) | Usage of c-terminal fragment of CPE (c-CPE) | [51,52] |
| C. sporogenes | Immune clearance Bacterial spread | Anaerobic tumor colonization Activates prodrugs | Engineered for prodrug activation (e.g., overexpressing NTR enzyme) | [53] |
| C. difficile | High toxicity (Toxin A and B) Gut inflammation Bacterial spread | Potential tumor targeting but mainly produces toxins | Not suitable for therapy | [54] |
4. Synthetic Biology for Clostridium-Based Anti-Cancer Therapies
4.1. Genetic Modification
4.2. Expression of Prodrugs
4.3. Delivery of Therapeutic Proteins
5. Preclinical and Clinical Studies
5.1. Preclinical Models
5.2. Clinical Trials
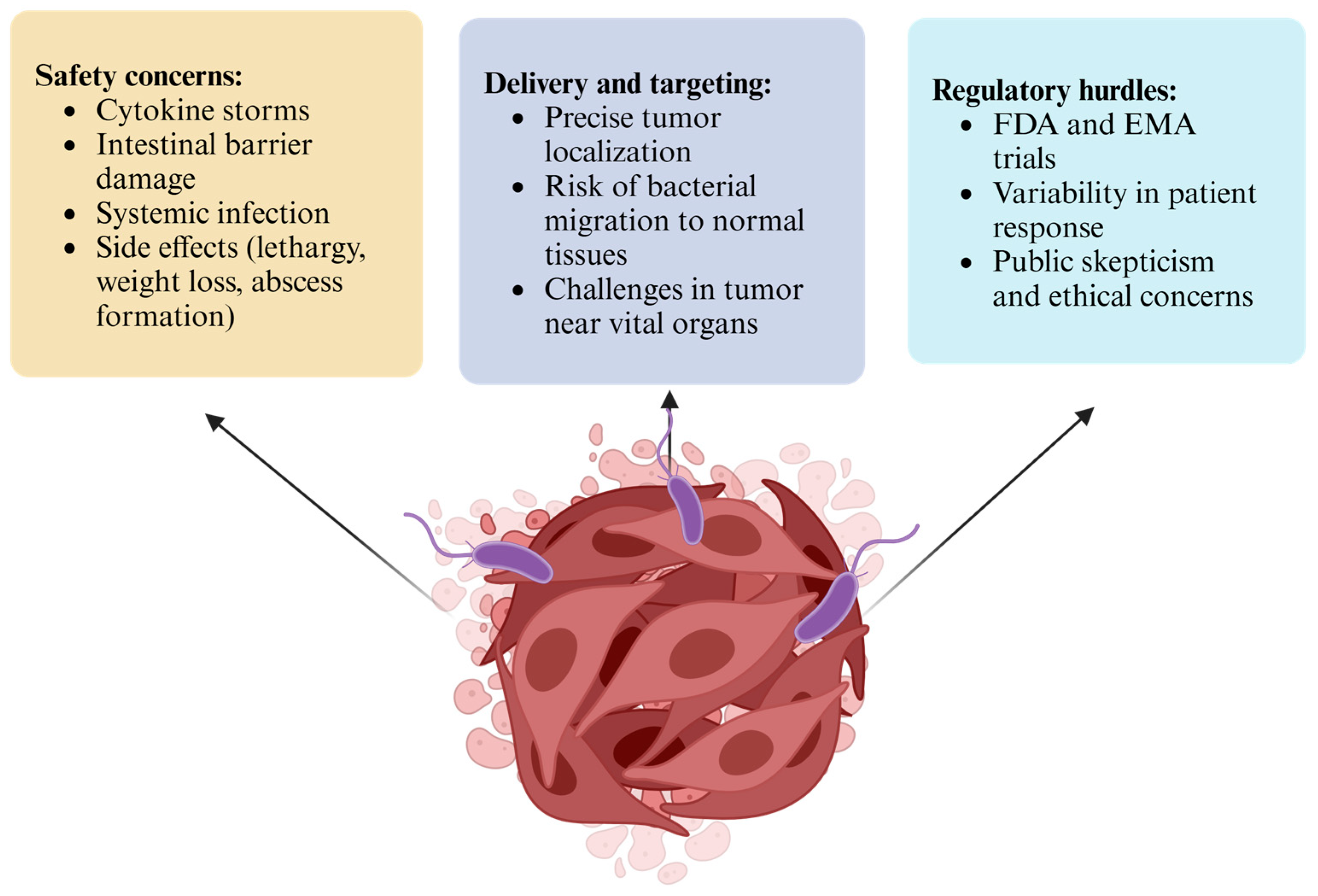
6. Challenges and Limitations
6.1. Safety Concerns
6.2. Delivery and Targeting
6.3. Regulatory Hurdles
7. Future Perspectives
7.1. Next-Generation Engineering
7.2. Personalized Medicine
7.3. Integration with Emerging Technologies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cancer; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 11 November 2024).
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Walk, E.E.; Yohe, S.L.; Beckman, A.; Schade, A.; Zutter, M.M.; Pfeifer, J.; Berry, A.B.; College of American Pathologists Personalized Health Care Committee. The cancer immunotherapy biomarker testing landscape. Arch. Pathol. Lab. Med. 2020, 144, 706–724. [Google Scholar] [CrossRef] [PubMed]
- Nygren, P. What is cancer chemotherapy? Acta Oncol. 2001, 40, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Dupont, A.; Belanger, A.; St-Arnaud, R.; Giguere, M.; Lacourciere, Y.; Emond, J.; Monfette, G. Treatment of prostate cancer with gonadotropin-releasing hormone agonists. Endocr. Rev. 1986, 7, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Field, S.; Bleehen, N. Hyperthermia in the treatment of cancer. Cancer Treat. Rev. 1979, 6, 63–94. [Google Scholar] [CrossRef]
- Kumar, A.R.; Devan, A.R.; Nair, B.; Vinod, B.S.; Nath, L.R. Harnessing the immune system against cancer: Current immunotherapy approaches and therapeutic targets. Mol. Biol. Rep. 2021, 48, 8075–8095. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv. Mater. 2017, 29, 1700996. [Google Scholar] [CrossRef]
- Luna, J.I.; Grossenbacher, S.K.; Murphy, W.J.; Canter, R.J. Targeting cancer stem cells with natural killer cell immunotherapy. Expert Opin. Biol. Ther. 2017, 17, 313–324. [Google Scholar] [CrossRef]
- National Cancer Institute. Types of Cancer Treatment; National Institutes of Health: Bethesda, MD, USA, 2025. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 11 November 2024).
- Hariz, M.I. Complications of deep brain stimulation surgery. Mov. Disord. Off. J. Mov. Disord. Soc. 2002, 17, S162–S166. [Google Scholar] [CrossRef]
- Amos, S.M.; Duong, C.P.; Westwood, J.A.; Ritchie, D.S.; Junghans, R.P.; Darcy, P.K.; Kershaw, M.H. Autoimmunity associated with immunotherapy of cancer. Blood J. Am. Soc. Hematol. 2011, 118, 499–509. [Google Scholar] [CrossRef]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef]
- Guallar-Garrido, S.; Julián, E. Bacillus Calmette-Guérin (BCG) therapy for bladder cancer: An update. ImmunoTargets Ther. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Puri, R. Development of a recombinant interleukin-4-Pseudomonas exotoxin for therapy of glioblastoma. Toxicol. Pathol. 1999, 27, 53–57. [Google Scholar] [CrossRef]
- Busch, W. Aus der Sitzung der medicinischen Section vom 13 November 1867. Berl. Klin. Wochenschr. 1868, 5, 137. [Google Scholar]
- Fehleisen, F. Ueber die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Übertragbarkeit auf den Menschen. Dtsch. Med. Wochenschr. 1882, 8, 553–554. [Google Scholar]
- Li, X.; Wang, H.; Du, X.; Yu, W.; Jiang, J.; Geng, Y.; Guo, X.; Fan, X.; Ma, C. Lactobacilli inhibit cervical cancer cell migration in vitro and reduce tumor burden in vivo through upregulation of E-cadherin. Oncol. Rep. 2017, 38, 1561–1568. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Awasthi, A.; Nomellini, J.; Smit, J.; Suresh, M. Anti-tumor effects of the bacterium Caulobacter crescentus in murine tumor models. Cancer Biol. Ther. 2006, 5, 485–491. [Google Scholar] [CrossRef]
- Zheng, J.H.; Min, J.-J. Targeted cancer therapy using engineered Salmonella typhimurium. Chonnam Med. J. 2016, 52, 173–184. [Google Scholar] [CrossRef]
- Staedtke, V.; Roberts, N.J.; Bai, R.-Y.; Zhou, S. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016, 3, 144–152. [Google Scholar] [CrossRef]
- Aganja, R.P.; Sivasankar, C.; Senevirathne, A.; Lee, J.H. Salmonella as a promising curative tool against cancer. Pharmaceutics 2022, 14, 2100. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Ou, Q.; Chen, M.; Tian, S.; Tang, J.; Li, R.; Liang, Q.; Chen, Z.; Wang, C. Listeria-vectored cervical cancer vaccine candidate strains reduce MDSCs via the JAK-STAT signaling pathway. BMC Biol. 2024, 22, 88. [Google Scholar] [CrossRef]
- Ding, Y.-D.; Shu, L.-Z.; He, R.-S.; Chen, K.-Y.; Deng, Y.-J.; Zhou, Z.-B.; Xiong, Y.; Deng, H. Listeria monocytogenes: A promising vector for tumor immunotherapy. Front. Immunol. 2023, 14, 1278011. [Google Scholar] [CrossRef]
- Yazawa, K.; Fujimori, M.; Amano, J.; Kano, Y.; Taniguchi, S.i. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000, 7, 269–274. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ghazvini, K.; Khazaei, M.; Hasanian, S.M.; Avan, A.; Soleimanpour, S. The use of Clostridium in cancer therapy: A promising way. Rev. Res. Med. Microbiol. 2022, 33, 121–127. [Google Scholar] [CrossRef]
- Theys, J.; Lambin, P. Clostridium to treat cancer: Dream or reality? Ann. Transl. Med. 2015, 3, S21. [Google Scholar]
- Connell, H.C. The study and treatment of cancer by proteolytic enzymes: Preliminary report. Can. Med. Assoc. J. 1935, 33, 364. [Google Scholar]
- Parker, R.C.; Plummer, H.C.; Siebenmann, C.O.; Chapman, M.G. Effect of histolyticus infection and toxin on transplantable mouse tumors. Proc. Soc. Exp. Biol. Med. 1947, 66, 461–467. [Google Scholar] [CrossRef]
- Fox, M.; Lemmon, M.; Mauchline, M.; Davis, T.; Giaccia, A.; Minton, N.; Brown, J. Anaerobic bacteria as a delivery system for cancer gene therapy: In vitro activation of 5-fluorocytosine by genetically engineered clostridia. Gene Ther. 1996, 3, 173–178. [Google Scholar]
- Barbé, S.; Van Mellaert, L.; Theys, J.; Geukens, N.; Lammertyn, E.; Lambin, P.; Anné, J. Secretory production of biologically active rat interleukin-2 by Clostridium acetobutylicum DSM792 as a tool for anti-tumor treatment. FEMS Microbiol. Lett. 2005, 246, 67–73. [Google Scholar] [CrossRef]
- Huang, T.; Li, S.; Li, G.; Tian, Y.; Wang, H.; Shi, L.; Perez-Cordon, G.; Mao, L.; Wang, X.; Wang, J. Utility of Clostridium difficile toxin B for inducing anti-tumor immunity. PLoS ONE 2014, 9, e110826. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef]
- Gupta, K.H.; Nowicki, C.; Giurini, E.F.; Marzo, A.L.; Zloza, A. Bacterial-based cancer therapy (BBCT): Recent advances, current challenges, and future prospects for cancer immunotherapy. Vaccines 2021, 9, 1497. [Google Scholar] [CrossRef]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.-Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, ra111–ra249. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Tian, X.; Shi, X. Learning from Clostridium novyi-NT: How to defeat cancer. J. Cancer Res. Ther. 2018, 14, S1–S6. [Google Scholar] [CrossRef]
- Bettegowda, C.; Huang, X.; Lin, J.; Cheong, I.; Kohli, M.; Szabo, S.A.; Zhang, X.; Diaz, L.A., Jr.; Velculescu, V.E.; Parmigiani, G. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat. Biotechnol. 2006, 24, 1573–1580. [Google Scholar] [CrossRef]
- Staedtke, V.; Gray-Bethke, T.; Liu, G.; Liapi, E.; Riggins, G.J.; Bai, R.-Y. Neutrophil depletion enhanced the Clostridium novyi-NT therapy in mouse and rabbit tumor models. Neuro-Oncol. Adv. 2022, 4, vdab184. [Google Scholar] [CrossRef]
- Feng, X.; He, P.; Zeng, C.; Li, Y.-H.; Das, S.K.; Li, B.; Yang, H.-F.; Du, Y. Novel insights into the role of Clostridium novyi-NT related combination bacteriolytic therapy in solid tumors. Oncol. Lett. 2021, 21, 110. [Google Scholar] [CrossRef]
- Dang, L.H.; Bettegowda, C.; Huso, D.L.; Kinzler, K.W.; Vogelstein, B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 15155–15160. [Google Scholar] [CrossRef]
- Cheong, I.; Huang, X.; Bettegowda, C.; Diaz, L.A., Jr.; Kinzler, K.W.; Zhou, S.; Vogelstein, B. A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science 2006, 314, 1308–1311. [Google Scholar] [CrossRef]
- Umer, B.; Good, D.; Anné, J.; Duan, W.; Wei, M.Q. Clostridial spores for cancer therapy: Targeting solid tumour microenvironment. J. Toxicol. 2012, 2012, 862764. [Google Scholar] [CrossRef]
- Nuyts, S.; Van Mellaert, L.; Theys, J.; Landuyt, W.; Lambin, P.; Anné, J. Clostridium spores for tumor-specific drug delivery. Anti-Cancer Drugs 2002, 13, 115–125. [Google Scholar] [CrossRef]
- Janku, F.; Zhang, H.H.; Pezeshki, A.; Goel, S.; Murthy, R.; Wang-Gillam, A.; Shepard, D.R.; Helgason, T.; Masters, T.; Hong, D.S. Intratumoral injection of Clostridium novyi-NT spores in patients with treatment-refractory advanced solid tumors. Clin. Cancer Res. 2021, 27, 96–106. [Google Scholar] [CrossRef]
- Theys, J.; Patterson, A.V.; Mowday, A.M. Clostridium bacteria: Harnessing Tumour necrosis for targeted gene delivery. Mol. Diagn. Ther. 2024, 28, 141–151. [Google Scholar] [CrossRef]
- Kubiak, A.M.; Claessen, L.; Zhang, Y.; Khazaie, K.; Bailey, T.S. Refined control of CRISPR-Cas9 gene editing in Clostridium sporogenes: The creation of recombinant strains for therapeutic applications. Front. Immunol. 2023, 14, 1241632. [Google Scholar] [CrossRef]
- Staedtke, V.; Sun, N.; Bai, R. Hypoxia-Targeting Bacteria in Cancer Therapy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2024. [Google Scholar]
- Varshney, G.; Malviya, R.; Sundram, S.; Meenakshi, D.U. Utilization of Microbial Genes as a Vaccine Against Cancer. In Cancer Vaccination and Challenges; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 177–196. [Google Scholar]
- Pahle, J.; Menzel, L.; Niesler, N.; Kobelt, D.; Aumann, J.; Rivera, M.; Walther, W. Rapid eradication of colon carcinoma by Clostridium perfringens Enterotoxin suicidal gene therapy. BMC Cancer 2017, 17, 129. [Google Scholar] [CrossRef]
- Banga, A.R.; Odiase, P.; Rachakonda, K.; Garg, A.P.; Adunyah, S.E.; Rachakonda, G. Application of C-terminal Clostridium perfringens enterotoxin in treatment of brain metastasis from breast cancer. Cancers 2022, 14, 4309. [Google Scholar] [CrossRef]
- Theys, J.; Pennington, O.; Dubois, L.; Anlezark, G.; Vaughan, T.; Mengesha, A.; Landuyt, W.; Anné, J.; Burke, P.; Dûrre, P. Repeated cycles of Clostridium-directed enzyme prodrug therapy result in sustained antitumour effects in vivo. Br. J. Cancer 2006, 95, 1212–1219. [Google Scholar] [CrossRef]
- Di Bella, S.; Ascenzi, P.; Siarakas, S.; Petrosillo, N.; Di Masi, A. Clostridium difficile toxins A and B: Insights into pathogenic properties and extraintestinal effects. Toxins 2016, 8, 134. [Google Scholar] [CrossRef]
- Liu, S.-C.; Ahn, G.-O.; Kioi, M.; Dorie, M.-J.; Patterson, A.V.; Brown, J.M. Optimized clostridium-directed enzyme prodrug therapy improves the antitumor activity of the novel DNA cross-linking agent PR-104. Cancer Res. 2008, 68, 7995–8003. [Google Scholar] [CrossRef] [PubMed]
- Evrard, A.; Cuq, P.; Ciccolini, J.; Vian, L.; Cano, J. Increased cytotoxicity and bystander effect of 5-fluorouracil and 5′-deoxy-5-fluorouridine in human colorectal cancer cells transfected with thymidine phosphorylase. Br. J. Cancer 1999, 80, 1726–1733. [Google Scholar] [CrossRef]
- Mowday, A.M.; Guise, C.P.; Ackerley, D.F.; Minton, N.P.; Lambin, P.; Dubois, L.J.; Theys, J.; Smaill, J.B.; Patterson, A.V. Advancing clostridia to clinical trial: Past lessons and recent progress. Cancers 2016, 8, 63. [Google Scholar] [CrossRef]
- Liu, S.; Minton, N.; Giaccia, A.; Brown, J. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002, 9, 291–296. [Google Scholar] [CrossRef]
- Kubiak, A.M.; Minton, N.P. The potential of clostridial spores as therapeutic delivery vehicles in tumour therapy. Res. Microbiol. 2015, 166, 244–254. [Google Scholar] [CrossRef]
- Mowday, A.M.; Dubois, L.J.; Kubiak, A.M.; Chan-Hyams, J.V.; Guise, C.P.; Ashoorzadeh, A.; Lambin, P.; Ackerley, D.F.; Smaill, J.B.; Minton, N.P. Use of an optimised enzyme/prodrug combination for Clostridia directed enzyme prodrug therapy induces a significant growth delay in necrotic tumours. Cancer Gene Ther. 2022, 29, 178–188. [Google Scholar] [CrossRef]
- Dargatz, H.; Diefenthal, T.; Witte, V.; Reipen, G.; von Wettstein, D. The heterodimeric protease clostripain from Clostridium histolyticum is encoded by a single gene. Mol. Gen. Genet. MGG 1993, 240, 140–145. [Google Scholar] [CrossRef]
- Nuyts, S.; Van Mellaert, L.; Barbe, S.; Lammertyn, E.; Theys, J.; Landuyt, W.; Bosmans, E.; Lambin, P.; Anne, J. Insertion or deletion of the Cheo box modifies radiation inducibility of Clostridium promoters. Appl. Environ. Microbiol. 2001, 67, 4464–4470. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H. Immune microenvironment and immunotherapy for chordoma. Front. Oncol. 2024, 14, 1374249. [Google Scholar] [CrossRef]
- Staedtke, V.; Bai, R.-Y.; Sun, W.; Huang, J.; Kibler, K.K.; Tyler, B.M.; Gallia, G.L.; Kinzler, K.; Vogelstein, B.; Zhou, S. Clostridium novyi-NT can cause regression of orthotopically implanted glioblastomas in rats. Oncotarget 2015, 6, 5536. [Google Scholar] [CrossRef]
- Chyuan, I.-T.; Chu, C.-L.; Hsu, P.-N. Targeting the tumor microenvironment for improving therapeutic effectiveness in cancer immunotherapy: Focusing on immune checkpoint inhibitors and combination therapies. Cancers 2021, 13, 1188. [Google Scholar] [CrossRef]
- Nelson, B.E.; Janku, F.; Fu, S.; Dumbrava, E.I.; Hong, D.S.; Karp, D.; Naing, A.; Rodon, J.; Tsimberidou, A.; Amaria, R.N.; et al. Abstract CT107: Phase Ib Study of Pembrolizumab in Combination with Intratumoral Injection of Clostridium novyi-NT in Patients with Advanced Solid Tumors. Cancer Res. 2023, 83, CT107. [Google Scholar] [CrossRef]
- Malmgren, R.A.; Flanigan, C.C. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955, 15, 473–478. [Google Scholar]
- Diaz, L.A., Jr.; Cheong, I.; Foss, C.A.; Zhang, X.; Peters, B.A.; Agrawal, N.; Bettegowda, C.; Karim, B.; Liu, G.; Khan, K. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol. Sci. 2005, 88, 562–575. [Google Scholar] [CrossRef]
- Lemmon, M.; Van Zijl, P.; Fox, M.; Mauchline, M.; Giaccia, A.; Minton, N.; Brown, J. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther. 1997, 4, 791–796. [Google Scholar] [CrossRef]
- Schoen, C.; Stritzker, J.; Goebel, W.; Pilgrim, S. Bacteria as DNA vaccine carriers for genetic immunization. Int. J. Med. Microbiol. 2004, 294, 319–335. [Google Scholar] [CrossRef]
- Kuehne, S.A.; Minton, N.P. ClosTron-mediated engineering of Clostridium. Bioengineered 2012, 3, 247–254. [Google Scholar] [CrossRef]
- Staedtke, V.; Bai, R.-Y.; Kim, K.; Darvas, M.; Davila, M.L.; Riggins, G.J.; Rothman, P.B.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature 2018, 564, 273–277. [Google Scholar] [CrossRef]
- Kubiak, A.M.; de Laak, J.v.; Zhang, Y.; Bailey, T.S.; Claessen, L.; Hittmeyer, P.; Vlaswinkel, C.; Mowday, A.; Dubois, L.J.; Theys, J. Clostridia as live biotherapeutics: Can modified Clostridium species enhance disease treatments? Future Microbiol. 2023, 18, 385–388. [Google Scholar] [CrossRef]
- Pandurangan, P.; Dinesh, R.A.; MohanaSundaram, A.; Samrat, A.V.; Meenambika, S.; Vedanarayanan, V.; Meena, R.; Namasivayam, S.K.R.; Moovendhan, M. Integrating cutting-edge technologies: AI, IoT, blockchain and nanotechnology for enhanced diagnosis and treatment of colorectal cancer-A review. J. Drug Deliv. Sci. Technol. 2023, 91, 105197. [Google Scholar] [CrossRef]
- Dailey, K.M.; Small, J.M.; Pullan, J.E.; Winfree, S.; Vance, K.E.; Orr, M.; Mallik, S.; Bayles, K.W.; Hollingsworth, M.A.; Brooks, A.E. An intravenous pancreatic cancer therapeutic: Characterization of CRISPR/Cas9n-modified Clostridium novyi-Non Toxic. PLoS ONE 2023, 18, e0289183. [Google Scholar] [CrossRef] [PubMed]

| Prodrug | Prodrug-Converting Enzyme (PCE) | Bacterial Strain | Active Metabolite | Mechanism | References |
|---|---|---|---|---|---|
| 5-Fluorocytosine (5-FC) | Cytosine deaminase (CD) | C. beijerinckii, C. acetobutylicum, C. sporogenes | 5-Fluorouracil (5-FU) | Inhibits DNA/RNA synthesis and sensitizes tumors to radiotherapy. | [31,57,58] |
| CB1954 (Nitrobenzamide) | Nitroreductase (NfsB, NmeNTR) | C. beijerinckii, C. sporogenes | 4-Hydroxylamine (4HX) | Alkylates DNA in cancer cells, effective particularly in hypoxic conditions. | [53,59,60] |
| PR-104 | Nitroreductase (sNTR, HinNTR) | C. sporogenes | PR-104H | Crosslinks DNA in hypoxic cells, causing cytotoxicity specific to cancer cells. | [47,55,59] |
| Glutamated Benzoyl Nitrogen Mustard | Carboxypeptidase G2 (CPG2) | C. sporogenes | Cytotoxic nitrogen mustard derivatives | Causes DNA damage, leading to cancer cell death. | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Kim, G.-H.; Baek, K.-R.; Seo, S.-O. Anti-Cancer Strategies Using Anaerobic Spore-Forming Bacteria Clostridium: Advances and Synergistic Approaches. Life 2025, 15, 465. https://doi.org/10.3390/life15030465
Singh S, Kim G-H, Baek K-R, Seo S-O. Anti-Cancer Strategies Using Anaerobic Spore-Forming Bacteria Clostridium: Advances and Synergistic Approaches. Life. 2025; 15(3):465. https://doi.org/10.3390/life15030465
Chicago/Turabian StyleSingh, Saloni, Geun-Hyung Kim, Kwang-Rim Baek, and Seung-Oh Seo. 2025. "Anti-Cancer Strategies Using Anaerobic Spore-Forming Bacteria Clostridium: Advances and Synergistic Approaches" Life 15, no. 3: 465. https://doi.org/10.3390/life15030465
APA StyleSingh, S., Kim, G.-H., Baek, K.-R., & Seo, S.-O. (2025). Anti-Cancer Strategies Using Anaerobic Spore-Forming Bacteria Clostridium: Advances and Synergistic Approaches. Life, 15(3), 465. https://doi.org/10.3390/life15030465








