Post-COVID-19 Pandemic Sequelae in Liver Diseases
Abstract
1. The State-of-the-Art
2. The Burden of Liver Disease in the Post-COVID Era
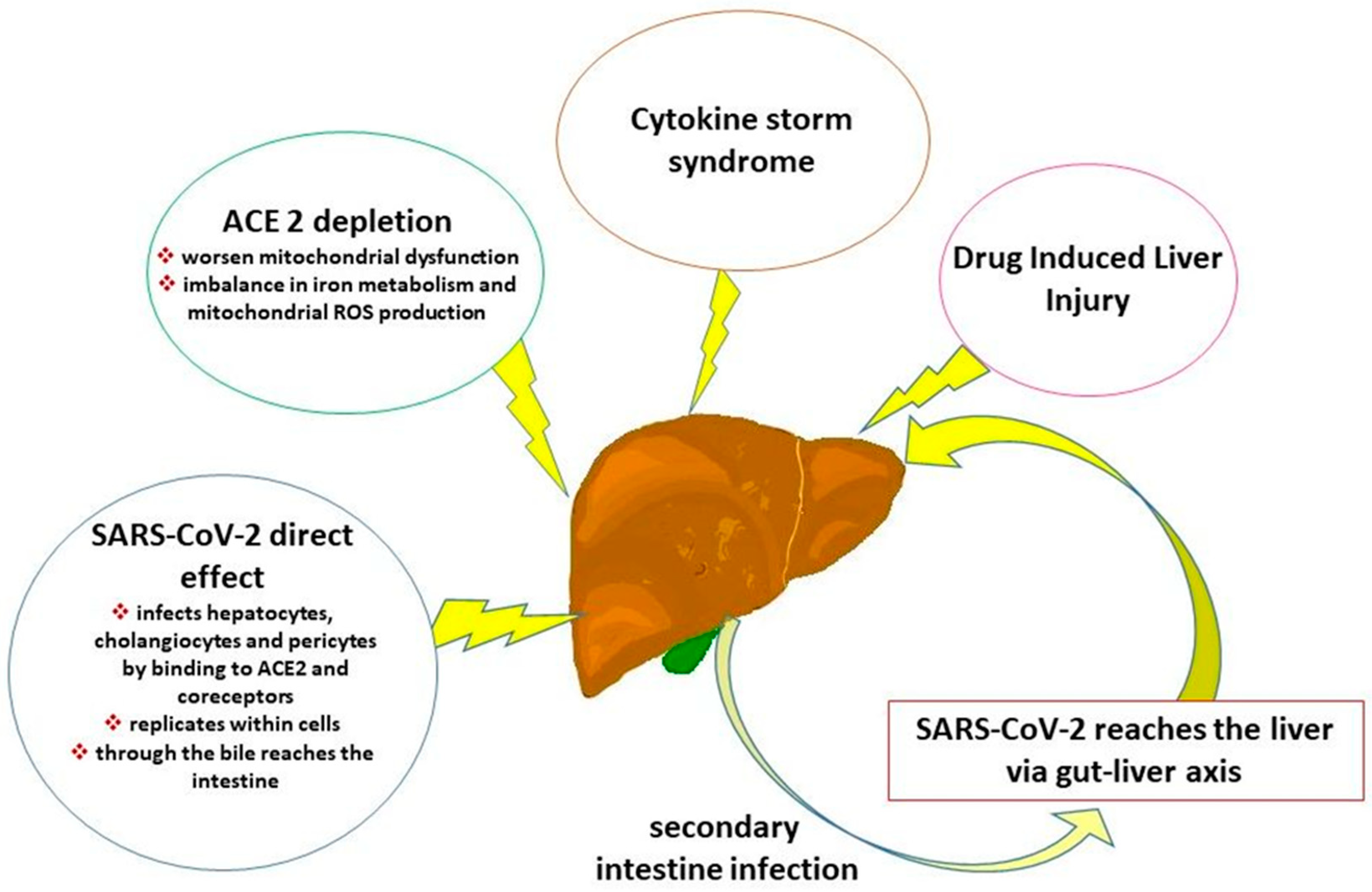
3. Pathophysiology of COVID-19-Associated Liver and Hepatobiliary Damage
4. COVID-19 Effect on Liver Inflammation and Fibrosis
5. Short- and Long-Term Consequences of COVID-19 on Liver Diseases
| Authors | Study Design | Country | Number of Patients | Study Population | Time Interval Between COVID-19 and Laboratory/Instrumental Evaluation | Short-Term (<6 Months) COVID Effects | Long-Term (>6 Months) COVID Effects | Risk Factor Associated | |
|---|---|---|---|---|---|---|---|---|---|
| Liver | de Lima et al. [5] | cross-sectional, quantitative, descriptive, and analytical observational study | Brazil | 243 patients, aged ≥18 years | Long COVID (average age approximately 50 years), without prior chronic liver disease | from 30 to 632 days | GGT, ferritin and total bilirubin in men were above the reference values | Increased ALT and AST levels, especially those hospitalized during acute phase | hospitalization, male, >5 long COVID symptoms were associated with short-term long COVID |
| Zeuzem et al. [38] | cross-sectional and longitudinal | Germany | 142 SARS-CoV-2 patients (two cohrts): 29 acute HCV, 23 chronic HCV, 31 cirrhotic | SARS-CoV-2 (mean age cohort 1 = 47.6; mean age cohort 2 = 50.2), acute HCV (median age = 45 years), non-cirrhotic Chronic HCV (median age = 56 years), cirrhotic chronic HCV (median age = 56 years), | For acute HCV patients: at baseline, at the end of DAA treatment (week 8), and at 3-month follow-up; For Chronic HCV cirrhotic and non-cirrhotic: baseline, end of treatment (week 8) and at 6 and 24 months after the start of DAA; For SARS-CoV-2 patients: 3, 6, 9, and 14 months | More rapid decline in cytokine and chemokine concentrations after SARS-CoV-2 infection (at month 3) in comparison to all HCV cohorts | Some subjects still had elevated soluble inflammatory mediators levels, e.g., IL6, TNFα, IFNγ (after 6–9 months) in comparison with healthy controls | ||
| Gupta et al. [43] | Clinical Trial (NCT05060497) | UK | 21 patients | 21 patients post-COVID-19 (median age = 54 years) and 10 controls | within 5–7 months of discharge | Liver volume 28% greater in patients than controls. Similarly, the measure of liver inflammation | |||
| Lau et al. [44] | prospective case–control study | Australia | 34 post-COVID-19 patients | 34 post COVID patients (average age of 41.1 years) without pre-existing liver conditions and 34 controls | <2 months post-infection within 2 to <4 months post-COVID-19 <6 months post-COVID-19 | COVID-19 group showed significantly higher liver stiffness values than the control group | |||
| Lucena Valera et al. [53] | retrospective multicenter study | Spain | 575 patients requiring admission | Hospitalized COVID-19 patients (mean age = 68 ± 15 years) between January and June 2020 | 6-months post-infection | AST, ALT, FIB-4 were significantly reduced at six months after the resolution of infection. 68.4% of patients had FIB-4 < 1.45 after the resolution of infection | patients with higher values of FIB-4 (at baseline or at the time of admission), are at high risk of suffering a poor prognosis COVID-19-associated | ||
| Kolesova et al. [54] | cross-sectional, single-center study | Latvia | 124 COVID-19 and post-COVID patients | 66 COVID-19 patients, 58 post-COVID (mean age = 42.1 ± 13.4 years), 17 control subjects | 3–6 months after the recovery | Increased FIB-4 in 5% of patients of post-COVID, of whom 2% had FIB-4 ≥ 3.25, corresponding to advanced liver fibrosis, even with inflammatory markers in the normal range. | |||
| Hatipoğlu et al. [55] | Research conducted using Patient Data Registry | USA | 52 patients hospitalized for COVID-19 with chronic liver disease | 52 patients hospitalized for COVID-19 (mean age = 56.8 ± 13.9 years) and 92 controls with chronic liver disease. 42% cirrhotic COVID-19 patients and 26% cirrhotic in control group. NFS was calculated only in MASLD patients | 12.2 months after admission | increased 30-day mortality in cirrhotic COVID-19 patients | Hospitalized COVID-19 patients showed a significant rise in FIB-4 index and NFS (in MASLD) with normalization on follow-up; patients with chronic liver disease without cirrhosis did not reveal changes in FIB-4 and NFS at one year | ||
| Bota et al. [58] | longitudinal research | Romania | 238 participants; 117 Long COVID <65 years; 71 Long COVID ≥65 years; 50 no Long COVID | hospitalized COVID-19 patients without chronic liver diseases | six months after hospitalization | Elderly Long COVID patients showed a strong increment of liver enzymes post-discharge | FIB-4, NFS, and APRI to assess liver fibrosis were significantly higher in patients with Long COVID, particularly in the elderly group | ||
| Liu et al., [59] | comparative cohort study | China | 23,838 subjects, 6786 with examinations data during the COVID-19 pandemic | large-scale population cohort | comparison of examination indicators between November 2020 and June 2023 | T-wave alterations, particularly in subjects >45 years with chronic diseases such as hypertension, liver steatosis, and hyperglycemia | |||
| Hepatobiliary | Leonhardt et al., [29] | ambidirectional observational study | Germany | 25 SSC-CIP patients | adults (median age = 59 years) hospitalized COVID-19 pneumonia confirmed by PCR who developed SSC-CIP, compared with control group without SSC-CIP | 1 year after SSC-CIP onset | Without transplantation, only 40.0% of patients with SSC-CIP were alive 1 year after SSC-CIP occurrence | Multivariate analysis confirmed high levels of fibrinogen and LDH as independent risk factors for occurrence of SSC-CIP in ventilated COVID-19 patients | |
| Lee et al. [63] | multinational population-based cohort study | Korea UK USA | 90,399 COVID-19; 386,787 Non-COVID-19 | SARS-CoV-2 patients (45.83 ± 13.26 years) and non-infected individuals, as controls | <3, 3–6, and ≥6 months | Increased incidence of digestive and hepatobiliary diseases, and other gastrointestinal abnormalities in SARS-CoV-2 patients during the post-acute phase | -The risk for gastrointestinal and hepatobiliary diseases was pronounced according to the COVID-19 severity and during the initial 3 months -SARS-CoV-2 vaccination reduced the risk of gastrointestinal diseases but not hepatobiliary diseases and other digestive abnormalities | ||
| Hartl et al. [64] | retrospective study | Austria | 46 patients without advanced chronic liver disease; 19 patients with advanced chronic liver disease | 65 Adult hospitalized COVID-19 patients (67.7 ± 19.6) with chronic liver disease | Median follow-up time = 34.5 (IQR 107.0) days | During follow-up, 47.7% of chronic liver disease patients had severe cholestasis; 15.4% of chronic liver disease patients developed SSC; 1 patient with preexisting primary sclerosing cholangitis showed disease progression; 26.3% of advanced chronic liver disease patients had a decompensation event | COVID-19–associated SSC occurred predominantly in patients with MASLD/MASH and metabolic risk factors. |
6. Management Strategies and Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Fan, C.; Tang, J.; Zhang, N. Meta-analysis of liver injury in patients with COVID-19. Medicine 2023, 102, e34320. [Google Scholar] [CrossRef]
- World Health Organization. Europe. Post COVID-19 Condition (Long COVID); World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 7 December 2022).
- NICE. NICE Guideline [NG188]. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; NICE: Manchester, UK, 2024; Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 18 December 2020).
- Ely, E.W.; Brown, L.M.; Fineberg, H.V.; National Academies of Sciences, Engineering, and Medicine Committee on Examining the Working Definition for Long COVID. Long COVID Defined. N. Engl. J. Med. 2024, 391, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- de Lima, I.C.; de Menezes, D.C.; Uesugi, J.H.E.; Bichara, C.N.C.; da Costa Vasconcelos, P.F.; Quaresma, J.A.S.; Falcão, L.F.M. Liver Function in Patients with Long-Term Coronavirus Disease 2019 of up to 20 Months: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 5281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, Y.; Zhang, C.; Wang, H.; Zhao, L.; Wang, H.; Su, Y.; Yang, M. Mechanism of SARS-CoV-2 Invasion into the Liver and Hepatic Injury in Patients with COVID-19. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022003. [Google Scholar] [CrossRef]
- De Smet, V.; Verhulst, S.; van Grunsven, L.A. Single cell RNA sequencing analysis did not predict hepatocyte infection by SARS-CoV-2. J. Hepatol. 2020, 73, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Fondevila, M.F.; Mercado-Gómez, M.; Rodríguez, A.; Gonzalez-Rellan, M.J.; Iruzubieta, P.; Valentí, V.; Escalada, J.; Schwaninger, M.; Prevot, V.; Dieguez, C.; et al. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J. Hepatol. 2021, 74, 469–471. [Google Scholar] [CrossRef]
- Eskridge, W.; Cryer, D.R.; Schattenberg, J.M.; Gastaldelli, A.; Malhi, H.; Allen, A.M.; Noureddin, M.; Sanyal, A.J. Metabolic Dysfunction-Associated Steatotic Liver Disease and Metabolic Dysfunction-Associated Steatohepatitis: The Patient and Physician Perspective. J. Clin. Med. 2023, 12, 6216. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Nour, T.Y.; Altintaş, K.H. Effect of the COVID-19 pandemic on obesity and it is risk factors: A systematic review. BMC Public Health 2023, 23, 1018. [Google Scholar] [CrossRef]
- Adenote, A.; Dumic, I.; Madrid, C.; Barusya, C.; Nordstrom, C.W.; Rueda Prada, L. NAFLD and Infection, a Nuanced Relationship. Can. J. Gastroenterol. Hepatol. 2021, 2021, 5556354. [Google Scholar] [CrossRef]
- Chan, K.E.; Koh, T.J.L.; Tang, A.S.P.; Quek, J.; Yong, J.N.; Tay, P.; Tan, D.J.H.; Lim, W.H.; Lin, S.Y.; Huang, D.; et al. Global Prevalence and Clinical Characteristics of Metabolic-associated Fatty Liver Disease: A Meta-Analysis and Systematic Review of 10 739 607 Individuals. J. Clin. Endocrinol. Metab. 2022, 107, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Danpanichkul, P.; Pang, Y.; Suparan, K.; Auttapracha, T.; Sirimangklanurak, S.; Attia, A.M.; Thimphitthaya, C.; Ni Law, M.S.; Yu, Z.; Soliman, M.A.; et al. Increased MASH-associated liver cancer in younger demographics. Hepatol. Commun. 2025, 9, e0629. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Danpanichkul, P.; Wijarnpreecha, K.; Cholankeril, G.; Loomba, R.; Ahmed, A. Current Burden of Steatotic Liver Disease and Fibrosis among Adults in the United States, 2017–2023. Clin. Mol. Hepatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sgamato, C.; Rocco, A.; Compare, D.; Minieri, S.; Marchitto, S.A.; Maurea, S.; Nardone, G. Autoimmune liver diseases and SARS-CoV-2. World J. Gastroenterol. 2023, 29, 1838–1851. [Google Scholar] [CrossRef]
- Gerussi, A.; Restelli, U.; Croce, D.; Bonfanti, M.; Invernizzi, P.; Carbone, M. Cost of illness of Primary Biliary Cholangitis—A population-based study. Dig. Liver Dis. 2021, 53, 1167–1170. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Ito, T.; Nguyen, M.H. Perspectives on the Underlying Etiology of HCC and Its Effects on Treatment Outcomes. J. Hepatocell. Carcinoma 2023, 10, 413–428. [Google Scholar] [CrossRef]
- Kim, D.; Alshuwaykh, O.; Dennis, B.B.; Cholankeril, G.; Ahmed, A. Trends in Etiology-based Mortality From Chronic Liver Disease Before and During COVID-19 Pandemic in the United States. Clin. Gastroenterol. Hepatol. 2022, 20, 2307–2316.e3. [Google Scholar] [CrossRef]
- Makuza, J.D.; Wong, S.; Morrow, R.L.; Binka, M.; Darvishian, M.; Jeong, D.; Adu, P.A.; Cua, G.; Yu, A.; Velásquez García, H.A.; et al. Impact of COVID-19 pandemic on hepatocellular carcinoma surveillance in British Columbia, Canada: An interrupted time series study. J. Viral Hepat. 2024, 31, 592–600. [Google Scholar] [CrossRef]
- Silvestri, C.; Stasi, C.; Profili, F.; Bartolacci, S.; Sessa, E.; Tacconi, D.; Villari, L.; Carrozzi, L.; Dotta, F.; Bargagli, E.; et al. Retrospective Study on the Features and Outcomes of a Tuscany COVID-19 Hospitalized Patients Cohort: Preliminary Results. J. Clin. Med. 2024, 13, 4626. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Cozzolino, D.; Nevola, R.; Abitabile, M.; Carusone, C.; Cinone, F.; Cuomo, G.; Nappo, F.; Sellitto, A.; Umano, G.R.; et al. Liver Involvement during SARS-CoV-2 Infection Is Associated with a Worse Respiratory Outcome in COVID-19 Patients. Viruses 2023, 15, 1904. [Google Scholar] [CrossRef]
- Poudel, S.; Mishra, A.; Poudel, S.C.; Baskota, A.; Bhattarai, M.; Aryal, A.; Kunwar, A. Liver injury at admission and outcomes in patients with COVID-19 disease: A prospective cohort study. Ann. Med. Surg. 2023, 85, 1534–1538. [Google Scholar] [CrossRef]
- Avdonin, P.P.; Rybakova, E.Y.; Trufanov, S.K.; Avdonin, P.V. SARS-CoV-2 Receptors and Their Involvement in Cell Infection. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2023, 17, 1–11. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Ahluwalia, A. Endothelial cells and blood vessels are major targets for COVID-19-induced tissue injury and spreading to various organs. World J. Gastroenterol. 2022, 28, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, M.; Lasagni, A.; Radu, C.M.; Calistri, A.; Pilan, M.; Valle, C.; Bonaffini, P.A.; Vitiello, A.; Toffanin, S.; Venturin, C.; et al. Procoagulant phenotype of virus-infected pericytes is associated with portal thrombosis and intrapulmonary vascular dilations in fatal COVID-19. J. Hepatol. 2024, 81, 872–885. [Google Scholar] [CrossRef]
- Leonhardt, S.; Jürgensen, C.; Frohme, J.; Grajecki, D.; Adler, A.; Sigal, M.; Leonhardt, J.; Voll, J.M.; Kruse, J.M.; Körner, R.; et al. Hepatobiliary long-term consequences of COVID-19: Dramatically increased rate of secondary sclerosing cholangitis in critically ill COVID-19 patients. Hepatol. Int. 2023, 17, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Hsu, M.T.; Lee, M.Y.; Chou, C.K. Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses 2022, 14, 1188. [Google Scholar] [CrossRef]
- Cano, L.; Desquilles, L.; Ghukasyan, G.; Angenard, G.; Landreau, C.; Corlu, A.; Clément, B.; Turlin, B.; Le Ferrec, E.; Aninat, C.; et al. SARS-CoV-2 receptor ACE2 is upregulated by fatty acids in human MASH. JHEP Rep. 2023, 6, 100936. [Google Scholar] [CrossRef]
- Warner, F.J.; Rajapaksha, H.; Shackel, N.; Herath, C.B. ACE2: From protection of liver disease to propagation of COVID-19. Clin. Sci. 2020, 134, 3137–3158. [Google Scholar] [CrossRef]
- de Miranda, A.S.; Simões e Silva, A.C. Liver. In Angiotensin-(1-7); Springer: Cham, Switzerland, 2019; pp. 191–199. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Wu, L.; Li, X.; Liao, Z. COVID-19 associated liver injury: An updated review on the mechanisms and management of risk groups. Liver Res. 2023, 5, 207–215. [Google Scholar] [CrossRef]
- Mercado-Gómez, M.; Prieto-Fernández, E.; Goikoetxea-Usandizaga, N.; Vila-Vecilla, L.; Azkargorta, M.; Bravo, M.; Serrano-Maciá, M.; Egia-Mendikute, L.; Rodríguez-Agudo, R.; Lachiondo-Ortega, S.; et al. The spike of SARS-CoV-2 promotes metabolic rewiring in hepatocytes. Commun. Biol. 2022, 5, 827. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.; Cheng, C.C.; Mistretta, D.; Ambike, S.; Sacherl, J.; Velkov, S.; Liao, B.H.; Bester, R.; Gültan, M.; Polezhaeva, O.; et al. SARS-CoV-2 Productively Infects Human Hepatocytes and Induces Cell Death. J. Med. Virol. 2025, 97, e70156. [Google Scholar] [CrossRef]
- Napodano, C.; Pocino, K.; Stefanile, A.; Marino, M.; Miele, L.; Gulli, F.; Basile, V.; Pandolfi, F.; Gasbarrini, A.; Rapaccini, G.L.; et al. COVID-19 and hepatic involvement: The liver as a main actor of the pandemic novel. Scand. J. Immunol. 2021, 93, e12977. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, A.; Kumar, S.D.; Oltmanns, C.; Witte, M.; Mischke, J.; Drick, N.; Fuge, J.; Pink, I.; Tauwaldt, J.; Debarry, J.; et al. Different dynamics of soluble inflammatory mediators after clearance of respiratory SARS-CoV-2 versus blood-borne hepatitis C virus infections. Sci. Rep. 2024, 14, 29013. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Espada, A.; Salgado-de la Mora, M.; Rodriguez-Paniagua, B.M.; Limon-de la Rosa, N.; Martinez-Gutierrez, M.I.; Pastrana-Brandes, S.; Navarro-Alvarez, N. Histopathological impact of SARS-CoV-2 on the liver: Cellular damage and long-term complications. World J. Gastroenterol. 2024, 30, 2866–2880. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
- Lebbe, A.; Aboulwafa, A.; Bayraktar, N.; Mushannen, B.; Ayoub, S.; Sarker, S.; Abdalla, M.N.; Mohammed, I.; Mushannen, M.; Yagan, L.; et al. New Onset of Acute and Chronic Hepatic Diseases Post-COVID-19 Infection: A Systematic Review. Biomedicines 2024, 12, 2065. [Google Scholar] [CrossRef]
- Bruzzone, C.; Bizkarguenaga, M.; Gil-Redondo, R.; Diercks, T.; Arana, E.; García de Vicuña, A.; Seco, M.; Bosch, A.; Palazón, A.; San Juan, I.; et al. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience 2020, 23, 101645. [Google Scholar] [CrossRef]
- Gupta, A.; Nicholas, R.; McGing, J.J.; Nixon, A.V.; Mallinson, J.E.; McKeever, T.M.; Bradley, C.R.; Piasecki, M.; Cox, E.F.; Bonnington, J.; et al. DYNamic Assessment of Multi-Organ level dysfunction in patients recovering from COVID-19: DYNAMO COVID-19. Exp. Physiol. 2024, 109, 1274–1291. [Google Scholar] [CrossRef]
- Lau, J.Y.S.; O’Hara, S.; Lombardo, P.; Goodyear, M. Assessment of the liver with two-dimensional shear wave elastography following COVID-19 infection: A pilot study. Australas. J. Ultrasound Med. 2024, 27, 167–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stasi, C.; Milani, S. Evolving strategies for liver fibrosis staging: Non-invasive assessment. World J. Gastroenterol. 2017, 23, 191–196. [Google Scholar] [CrossRef]
- Żmudka, K.; Jaroszewicz, J.; Zarębska-Michaluk, D.; Rogalska, M.; Czupryna, P.; Rorat, M.; Kozielewicz, D.; Maciukajć, J.; Kiciak, S.; Krępa, M.; et al. Association between Liver Damage and Disease Progression Markers with Mortality Risk and Mechanical Ventilation in Hospitalized COVID-19 Patients: A Nationwide Retrospective SARSTer Study. Viruses 2024, 16, 1530. [Google Scholar] [CrossRef]
- Kamiya, Y.; Shinoda, M.; Ishii, N.; Yamamoto, S.; Sekine, T.; Morikawa, M.; Ota, S.; Toyama-Kousaka, M.; Takahashi, H.; Takei, H.; et al. Comparison of liver fibrosis scores and fatty liver on computed tomography as risk factors for severity of COVID-19. JGH Open 2024, 8, e70004. [Google Scholar] [CrossRef] [PubMed]
- Galiero, R.; Loffredo, G.; Simeon, V.; Caturano, A.; Vetrano, E.; Medicamento, G.; Alfano, M.; Beccia, D.; Brin, C.; Colantuoni, S.; et al. Impact of liver fibrosis on COVID-19 in-hospital mortality in Southern Italy. PLoS ONE 2024, 19, e0296495. [Google Scholar] [CrossRef] [PubMed]
- Brozat, J.F.; Ntanios, F.; Malhotra, D.; Dagenais, S.; Katchiuri, N.; Emir, B.; Tacke, F. NAFLD and NASH are obesity-independent risk factors in COVID-19: Matched real-world results from the large PINC AI™ Healthcare Database. Liver Int. 2024, 44, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.J.; Feng, I.C.; Lai, C.C.; Ho, C.H.; Kan, W.C.; Sheu, M.J.; Kuo, H.T. The mortality of hospitalized patients with COVID-19 and non-cirrhotic chronic liver disease: A retrospective multi-center study. PeerJ 2023, 11, e16582. [Google Scholar] [CrossRef]
- Parajuli, P.; Sabo, R.; Alsaadawi, R.; Robinson, A.; French, E.; Sterling, R.K. Fibrosis-4 (FIB-4) index as a predictor for mechanical ventilation and 30-day mortality across COVID-19 variants. J. Clin. Transl. Sci. 2023, 7, e213. [Google Scholar] [CrossRef]
- Ao, G.; Li, A.; Li, J.; Du, X.; Wang, Y.; Tran, C.; Qi, X. The Association between the Fibrosis-4 Index and COVID-19: A Systematic Review and Meta-Analysis. Ann. Clin. Lab. Sci. 2022, 52, 781–787. [Google Scholar]
- Lucena Valera, A.; Aller de la Fuente, R.; Sánchez Torrijos, Y.; Romero Gómez, M.; Ampuero Herrojo, J. FIB-4 score as a predictor of COVID-19-related severity in hospitalized patients. Rev. Esp. Enferm. Dig. 2024, 116, 465–471. [Google Scholar] [CrossRef]
- Kolesova, O.; Vanaga, I.; Laivacuma, S.; Derovs, A.; Kolesovs, A.; Radzina, M.; Platkajis, A.; Eglite, J.; Hagina, E.; Arutjunana, S.; et al. Intriguing findings of liver fibrosis following COVID-19. BMC Gastroenterol. 2021, 21, 370. [Google Scholar] [CrossRef] [PubMed]
- Hatipoğlu, D.; Mulligan, C.; Wang, J.; Peticco, J.; Grinspoon, R.; Gadi, S.; Mills, C.; Luther, J.; Chung, R.T. Differential Effects of COVID-19 Hospitalization on the Trajectory of Liver Disease Progression. Gastro Hep Adv. 2023, 2, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Tsochatzis, E.A.; Hall, A.; Rosenberg, W.; Milani, S.; Dhillon, A.P.; Pinzani, M. Comparison and correlation of fibrosis stage assessment by collagen proportionate area (CPA) and the ELF panel in patients with chronic liver disease. Dig. Liver Dis. 2019, 51, 1001–1007. [Google Scholar] [CrossRef]
- Núñez, A.; Aljama, C.; Esquinas, C.; Orriols, G.; Gabriel-Medina, P.; Farago, G.; Granados, G.; Rodriguez-Frias, F.; Pons, M.; Miravitlles, M.; et al. Utility of the Enhanced Liver Fibrosis score as a blood biomarker of pulmonary fibrosis secondary to SARS-CoV-2 pneumonia. Respir. Med. 2023, 218, 107394. [Google Scholar] [CrossRef]
- Bota, A.V.; Bratosin, F.; Bandi, S.S.S.; Bogdan, I.; Razvan, D.V.; Toma, A.O.; Indries, M.F.; Csep, A.N.; Cotoraci, C.; Prodan, M.; et al. A Comparative Analysis of Liver Injury Markers in Post-COVID Syndrome among Elderly Patients: A Prospective Study. J. Clin. Med. 2024, 13, 1149. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, B.; Zeng, T.; You, C.; Li, N.; Liu, Y.; Zhang, J.; Liu, C.; Jin, P.; Feng, X.; et al. A comparative cohort study of post-COVID-19 conditions based on physical examination records in China. EBioMedicine 2025, 112, 105549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- İnal, N.; Kurumanastırlı, B.; Taşkınoğlu, T.; Duran, A.Ç.; Togay, A.; Sarıgüzel, F.M.; Kaşifoğlu, N.; Soylu, M.; Doğan, Y.; Us, E.; et al. Retrospective evaluation of “Rods and Rings” pattern detected in the anti-nuclear antibody (ANA) indirect immunofluorescence (IIF) test. Front. Immunol. 2024, 15, 1359030. [Google Scholar] [CrossRef]
- Pan, B.; Wang, X.; Lai, H.; Vernooij, R.W.M.; Deng, X.; Ma, N.; Li, D.; Huang, J.; Zhao, W.; Ning, J.; et al. Risk of kidney and liver diseases after COVID-19 infection: A systematic review and meta-analysis. Rev. Med. Virol. 2024, 34, e2523. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.A.; Ballotin, V.R.; Bigarella, L.G.; Soldera, J. Post-COVID-19 cholangiopathy: Systematic review. World J. Methodol. 2023, 13, 296–322. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Lee, J.; Lee, M.; Kim, H.J.; Son, Y.; Rhee, S.Y.; Smith, L.; Rahmati, M.; Kang, J.; et al. Long-term gastrointestinal and hepatobiliary outcomes of COVID-19: A multinational population-based cohort study from South Korea, Japan, and the UK. Clin. Mol. Hepatol. 2024, 30, 943–958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hartl, L.; Haslinger, K.; Angerer, M.; Semmler, G.; Schneeweiss-Gleixner, M.; Jachs, M.; Simbrunner, B.; Bauer, D.J.M.; Eigenbauer, E.; Strassl, R.; et al. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology 2022, 76, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Meoni, B.; Voller, F.; Silvestri, C. SARS-CoV-2 Vaccination and the Bridge between First and Fourth Dose: Where Are We? Vaccines 2022, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Buti, M.; Eberhardt, C.S.; Grossi, P.A.; Shouval, D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J. Hepatol. 2021, 74, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.M.; Pose, E.; Wittner, M.; Londoño, M.C.; Schaub, G.; Cook, J.; Dimitriadis, S.; Meacham, G.; Irwin, S.; Lim, Z.; et al. Immune responses and clinical outcomes after COVID-19 vaccination in patients with liver disease and liver transplant recipients. J. Hepatol. 2024, 80, 109–123. [Google Scholar] [CrossRef]
- Airola, C.; Andaloro, S.; Gasbarrini, A.; Ponziani, F.R. Vaccine Responses in Patients with Liver Cirrhosis: From the Immune System to the Gut Microbiota. Vaccines 2024, 12, 349. [Google Scholar] [CrossRef]
- Cui, J.; Wang, L.; Ghavamian, A.; Li, X.; Wang, G.; Wang, T.; Huang, M.; Ru, Q.; Zhao, X. Long-term antibody response after the third dose of inactivated SARS-CoV-2 vaccine in MASLD patients. BMC Gastroenterol. 2024, 24, 329. [Google Scholar] [CrossRef]
- Stasi, C.; Fallani, S.; Voller, F.; Silvestri, C. Treatment for COVID-19: An overview. Eur. J. Pharmacol. 2020, 889, 173644. [Google Scholar] [CrossRef]
- Boettler, T.; Newsome, P.N.; Mondelli, M.U.; Mondelli, M.U.; Maticic, M.; Cordero, E.; Jalan, R.; Moreau, R.; Cornberg, M.; Berg, T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020, 2, 100113. [Google Scholar] [CrossRef]
- Boettler, T.; Marjot, T.; Newsome, P.N.; Mondelli, M.U.; Maticic, M.; Cordero, E.; Jalan, R.; Moreau, R.; Cornberg, M.; Berg, T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020, 2, 100169. [Google Scholar] [CrossRef]
- Long COVID-19 Syndrome Lifestyle Intervention Study. ClinicalTrials.gov ID NCT05836402. Available online: https://clinicaltrials.gov/study/NCT05836402?cond=Long%20COVID-19%20Syndrome&term=Liver%20Diseases&limit=25&page=1&rank=1 (accessed on 29 April 2024).
- Lau, R.I.; Su, Q.; Lau, I.S.F.; Ching, J.Y.L.; Wong, M.C.S.; Lau, L.H.S.; Tun, H.M.; Mok, C.K.P.; Chau, S.W.H.; Tse, Y.K.; et al. A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2024, 24, 256–265. [Google Scholar] [CrossRef]
- Rezaei, A.; Neshat, S.; Heshmat-Ghahdarijani, K. Alterations of Lipid Profile in COVID-19: A Narrative Review. Curr. Probl. Cardiol. 2022, 47, 100907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grootemaat, A.E.; van der Niet, S.; Scholl, E.R.; Roos, E.; Schurink, B.; Bugiani, M.; Miller, S.E.; Larsen, P.; Pankras, J.; Reits, E.A.; et al. Lipid and Nucleocapsid N-Protein Accumulation in COVID-19 Patient Lung and Infected Cells. Microbiol. Spectr. 2022, 10, e0127121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasi, C. Post-COVID-19 Pandemic Sequelae in Liver Diseases. Life 2025, 15, 403. https://doi.org/10.3390/life15030403
Stasi C. Post-COVID-19 Pandemic Sequelae in Liver Diseases. Life. 2025; 15(3):403. https://doi.org/10.3390/life15030403
Chicago/Turabian StyleStasi, Cristina. 2025. "Post-COVID-19 Pandemic Sequelae in Liver Diseases" Life 15, no. 3: 403. https://doi.org/10.3390/life15030403
APA StyleStasi, C. (2025). Post-COVID-19 Pandemic Sequelae in Liver Diseases. Life, 15(3), 403. https://doi.org/10.3390/life15030403






