Application of the Random Forest Algorithm for Accurate Bipolar Disorder Classification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Model Development
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S.N.; Boiman, E.E.; Goodwin, F.K. Diagnosing bipolar disorder and the effect of antidepressants: A naturalistic study. J. Clin. Psychiatry 2000, 61, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Chancel, R.; Lopez-Castroman, J.; Baca-Garcia, E.; Mateos Alvarez, R.; Courtet, P.; Conejero, I. Biomarkers of bipolar disorder in late life: An evidence-based systematic review. Curr. Psychiatry Rep. 2024, 26, 78–103. [Google Scholar] [CrossRef]
- Favaretto, E.; Bedani, F.; Brancati, G.E.; De Berardis, D.; Giovannini, S.; Scarcella, L.; Martiadis, V.; Martini, A.; Pampaloni, I.; Perugi, G.; et al. Synthesising 30 years of clinical experience and scientific insight on affective temperaments in psychiatric disorders: State of the art. J. Affect. Disord. 2024, 362, 406–415. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Agustini, B.; Köhler-Forsberg, O.; Cusin, C.; Katz, D.; Sylvia, L.G.; Peters, A.; Berk, M. Diagnosis and treatment of bipolar disorder: A review. JAMA 2023, 330, 1370–1380. [Google Scholar] [CrossRef]
- Bandyopadhyay Prasanta, S.; Forster Malcolm, R.; Oxford, E.; Barkow Jerome, H.; Leda, C.; John, T.; William, B.; Richardson Robert, C.; Beck Aaron, T.; John, R.A. American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders: Dsm-5, Washington, DC, American Psychiatric Publishing, 2013. Ananth Mahesh, In defense of an evolutionary concept of health nature, norms, and human biology, Aldershot, England, Ashgate. Philosophy 2014, 39, 683–724. [Google Scholar]
- de Dios, C.; Goikolea, J.M.; Colom, F.; Moreno, C.; Vieta, E. Bipolar disorders in the new DSM-5 and ICD-11 classifications. Rev. Psiquiatr. Salud Ment. (Engl. Ed.) 2014, 7, 179–185. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Oliva, V.; Fico, G.; De Prisco, M.; Gonda, X.; Rosa, A.R.; Vieta, E. Bipolar disorders: An update on critical aspects. Lancet Reg. Health–Eur. 2024, 48, 101135. [Google Scholar] [CrossRef]
- García Blanco, A.C.; Sierra, P.; Livianos, L. Nosology, epidemiology and pathogenesis of bipolar disorder: Recent approaches. Psiquiatr. Biológica 2014, 21, 89–94. [Google Scholar]
- Campos-Ugaz, W.A.; Garay, J.P.P.; Rivera-Lozada, O.; Diaz, M.A.A.; Fuster-Guillén, D.; Arana, A.A.T. An overview of bipolar disorder diagnosis using machine learning approaches: Clinical opportunities and challenges. Iran. J. Psychiatry 2023, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.S.; Khanom, J.; Bhuiyan, M.A.; Tumpa, Z.N.; Rabby, S.F.; Bilgaiyan, S. The Early Detection of Dementia Disease Using Machine Learning Approach. In Proceedings of the 2023 International Conference on Computer Communication and Informatics (ICCCI), Coimbatore, India, 23–25 January 2023; pp. 1–6. [Google Scholar]
- Montazeri, M.; Montazeri, M.; Bahaadinbeigy, K.; Montazeri, M.; Afraz, A. Application of machine learning methods in predicting schizophrenia and bipolar disorders: A systematic review. Health Sci. Rep. 2023, 6, e962. [Google Scholar] [CrossRef] [PubMed]
- Bzdok, D.; Meyer-Lindenberg, A. Machine learning for precision psychiatry: Opportunities and challenges. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 223–230. [Google Scholar] [CrossRef]
- Mora, D.; Nieto, J.A.; Mateo, J.; Bikdeli, B.; Barco, S.; Trujillo-Santos, J.; Soler, S.; Font, L.; Bosevski, M.; Monreal, M.; et al. Machine learning to predict outcomes in patients with acute pulmonary embolism who prematurely discontinued anticoagulant therapy. Thromb. Haemost. 2022, 122, 570–577. [Google Scholar] [CrossRef]
- Soria, C.; Arroyo, Y.; Torres, A.M.; Redondo, M.Á.; Basar, C.; Mateo, J. Method for classifying schizophrenia patients based on machine learning. J. Clin. Med. 2023, 12, 4375. [Google Scholar] [CrossRef]
- Suárez, M.; Martínez, R.; Torres, A.M.; Ramón, A.; Blasco, P.; Mateo, J. A Machine Learning-Based Method for Detecting Liver Fibrosis. Diagnostics 2023, 13, 2952. [Google Scholar] [CrossRef]
- Garrido, N.J.; González-Martínez, F.; Losada, S.; Plaza, A.; Del Olmo, E.; Mateo, J. Innovation through Artificial Intelligence in Triage Systems for Resource Optimization in Future Pandemics. Biomimetics 2024, 9, 440. [Google Scholar] [CrossRef]
- Suárez, M.; Gil-Rojas, S.; Martínez-Blanco, P.; Torres, A.M.; Ramón, A.; Blasco-Segura, P.; Torralba, M.; Mateo, J. Machine Learning-Based Assessment of Survival and Risk Factors in Non-Alcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma for Optimized Patient Management. Cancers 2024, 16, 1114. [Google Scholar] [CrossRef]
- Côté-Allard, U.; Jakobsen, P.; Stautland, A.; Nordgreen, T.; Fasmer, O.B.; Oedegaard, K.J.; Tørresen, J. Long–short ensemble network for bipolar manic-euthymic state recognition based on wrist-worn sensors. IEEE Pervasive Comput. 2022, 21, 20–31. [Google Scholar] [CrossRef]
- Perez Arribas, I.; Goodwin, G.M.; Geddes, J.R.; Lyons, T.; Saunders, K.E. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl. Psychiatry 2018, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Metin, B.; Uyulan, Ç.; Ergüzel, T.T.; Farhad, S.; Çifçi, E.; Türk, Ö.; Tarhan, N. The deep learning method differentiates patients with bipolar disorder from controls with high accuracy using EEG data. Clin. EEG Neurosci. 2024, 55, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Pettorruso, M.; Guidotti, R.; d’Andrea, G.; De Risio, L.; D’Andrea, A.; Chiappini, S.; Carullo, R.; Barlati, S.; Zanardi, R.; Rosso, G.; et al. Predicting outcome with Intranasal Esketamine treatment: A machine-learning, three-month study in Treatment-Resistant Depression (ESK-LEARNING). Psychiatry Res. 2023, 327, 115378. [Google Scholar] [CrossRef]
- Di Stefano, V.; D’Angelo, M.; Monaco, F.; Vignapiano, A.; Martiadis, V.; Barone, E.; Fornaro, M.; Steardo, L.; Solmi, M.; Manchia, M.; et al. Decoding Schizophrenia: How AI-Enhanced fMRI Unlocks New Pathways for Precision Psychiatry. Brain Sci. 2024, 14, 1196. [Google Scholar] [CrossRef] [PubMed]
- Janeva, D.; Krsteski, S.; Tashkovska, M.; Jovanovski, N.; Kartalov, T.; Taskovski, D.; Ivanovski, Z.; Gerazov, B. A System for Differentiation of Schizophrenia and Bipolar Disorder based on rsfMRI. In Proceedings of the 2023 30th International Conference on Systems, Signals and Image Processing (IWSSIP), Ohrid, North Macedonia, 27–29 June 2023; pp. 1–5. [Google Scholar]
- Bader, M.; Abdelwanis, M.; Maalouf, M.; Jelinek, H.F. Detecting depression severity using weighted random forest and oxidative stress biomarkers. Sci. Rep. 2024, 14, 16328. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Gong, J.; Wang, T.; Gong, L.; Li, K.; Wang, Y. Identifying the risk of depression in a large sample of adolescents: An artificial neural network based on random forest. J. Adolesc. 2024, 96, 1485–1497. [Google Scholar] [CrossRef]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narrandes, S.; Wang, Y.; Xu, W. Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genom. Proteom. 2018, 15, 41–51. [Google Scholar]
- Pisner, D.A.; Schnyer, D.M. Support vector machine. In Machine Learning; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–121. [Google Scholar]
- Shia, W.-C.; Chen, D.-R. Classification of malignant tumors in breast ultrasound using a pretrained deep residual network model and support vector machine. Comput. Med. Imaging Graph. 2021, 87, 101829. [Google Scholar] [CrossRef]
- Javeed, A.; Dallora, A.L.; Berglund, J.S.; Idrisoglu, A.; Ali, L.; Rauf, H.T.; Anderberg, P. Early prediction of dementia using feature extraction battery (feb) and optimized support vector machine (svm) for classification. Biomedicines 2023, 11, 439. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Zong, M.; Zhu, X.; Wang, R. Efficient kNN classification with different numbers of nearest neighbors. IEEE Trans. Neural Netw. Learn. Syst. 2017, 29, 1774–1785. [Google Scholar] [CrossRef]
- Arian, R.; Hariri, A.; Mehridehnavi, A.; Fassihi, A.; Ghasemi, F. Protein kinase inhibitors’ classification using K-Nearest neighbor algorithm. Comput. Biol. Chem. 2020, 86, 107269. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, R.; Drabløs, F. Robust distance measures for k NN classification of cancer data. Cancer Inform. 2020, 19, 1176935120965542. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.M.; Zendehboudi, S. Application of decision tree-based ensemble learning in the classification of breast cancer. Comput. Biol. Med. 2021, 128, 104089. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, T.; Bunimovich-Mendrazitsky, S. Decision tree post-pruning without loss of accuracy using the SAT-PP algorithm with an empirical evaluation on clinical data. Data Knowl. Eng. 2023, 145, 102173. [Google Scholar] [CrossRef]
- Manzella, F.; Pagliarini, G.; Sciavicco, G.; Stan, I.E. The voice of COVID-19: Breath and cough recording classification with temporal decision trees and random forests. Artif. Intell. Med. 2023, 137, 102486. [Google Scholar] [CrossRef]
- Jayachitra, S.; Prasanth, A. Multi-feature analysis for automated brain stroke classification using weighted Gaussian naïve Bayes classifier. J. Circuits Syst. Comput. 2021, 30, 2150178. [Google Scholar] [CrossRef]
- Gohari, K.; Kazemnejad, A.; Mohammadi, M.; Eskandari, F.; Saberi, S.; Esmaieli, M.; Sheidaei, A. A Bayesian latent class extension of naive Bayesian classifier and its application to the classification of gastric cancer patients. BMC Med. Res. Methodol. 2023, 23, 190. [Google Scholar] [CrossRef]
- Queipo, M.; Barbado, J.; Torres, A.M.; Mateo, J. Approaching personalized medicine: The use of machine learning to determine predictors of mortality in a population with SARS-CoV-2 infection. Biomedicines 2024, 12, 409. [Google Scholar] [CrossRef]
- Usategui, I.; Arroyo, Y.; Torres, A.M.; Barbado, J.; Mateo, J. Systemic Lupus Erythematosus: How Machine Learning Can Help Distinguish between Infections and Flares. Bioengineering 2024, 11, 90. [Google Scholar] [CrossRef]
- Hosseinifard, B.; Moradi, M.H.; Rostami, R. Classifying depression patients and normal subjects using machine learning techniques and nonlinear features from EEG signal. Comput. Methods Programs Biomed. 2013, 109, 339–345. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.G.; Lee, S.-I. Consistent individualized feature attribution for tree ensembles. arXiv 2018, arXiv:1802.03888. [Google Scholar]
- Sun, Z.; Wang, G.; Li, P.; Wang, H.; Zhang, M.; Liang, X. An improved random forest based on the classification accuracy and correlation measurement of decision trees. Expert. Syst. Appl. 2024, 237, 121549. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Accardo, A.; Affinito, M.; Carrozzi, M.; Bouquet, F. Use of the fractal dimension for the analysis of electroencephalographic time series. Biol. Cybern. 1997, 77, 339–350. [Google Scholar]
- Nguyen-Ky, T.; Wen, P.; Li, Y. Monitoring the depth of anaesthesia using Hurst exponent and Bayesian methods. IET Signal Process. 2014, 8, 907–917. [Google Scholar] [CrossRef]
- Birnbaum, H.G.; Shi, L.; Dial, E.; Oster, E.F.; Greenberg, P.E.; Mallett, D.A. Economic consequences of not recognizing bipolar disorder patients: A cross-sectional descriptive analysis. J. Clin. Psychiatry 2003, 64, 1201–1209. [Google Scholar] [CrossRef]
- McCombs, J.S.; Ahn, J.; Tencer, T.; Shi, L. The impact of unrecognized bipolar disorders among patients treated for depression with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program: A 6-year retrospective analysis. J. Affect. Disord. 2007, 97, 171–179. [Google Scholar] [CrossRef]
- Angst, J. Bipolar disorders in DSM-5: Strengths, problems and perspectives. Int. J. Bipolar Disord. 2013, 1, 12. [Google Scholar] [CrossRef]
- van der Voort, T.Y.; van Meijel, B.; Goossens, P.J.; Hoogendoorn, A.W.; Draisma, S.; Beekman, A.; Kupka, R.W. Collaborative care for patients with bipolar disorder: Randomised controlled trial. Br. J. Psychiatry 2015, 206, 393–400. [Google Scholar] [CrossRef]
- Severus, E.; Bauer, M. Diagnosing bipolar disorders in DSM-5. Int. J. Bipolar Disord. 2013, 1, 14. [Google Scholar] [CrossRef]
- van der Voort, T.Y.; van Meijel, B.; Hoogendoorn, A.W.; Goossens, P.J.; Beekman, A.T.; Kupka, R.W. Collaborative care for patients with bipolar disorder: Effects on functioning and quality of life. J. Affect. Disord. 2015, 179, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Jie, N.-F.; Osuch, E.A.; Zhu, M.-H.; Wammes, M.; Ma, X.-Y.; Jiang, T.-Z.; Sui, J.; Calhoun, V.D. Discriminating bipolar disorder from major depression using whole-brain functional connectivity: A feature selection analysis with SVM-FoBa algorithm. J. Signal Process. Syst. 2018, 90, 259–271. [Google Scholar] [CrossRef]
- AbaeiKoupaei, N.; Al Osman, H. A multi-modal stacked ensemble model for bipolar disorder classification. IEEE Trans. Affect. Comput. 2020, 14, 236–244. [Google Scholar] [CrossRef]
- Dev, A.; Roy, N.; Islam, M.K.; Biswas, C.; Ahmed, H.U.; Amin, M.A.; Sarker, F.; Vaidyanathan, R.; Mamun, K.A. Exploration of EEG-based depression biomarkers identification techniques and their applications: A systematic review. IEEE Access 2022, 10, 16756–16781. [Google Scholar] [CrossRef]
- Yamunarani, T.; Ponniran, A.B.; Zaki, W.S.B.W.; Ali, R.A.B.M.; Sivaranjani, S. EEG–Based Bipolar Disorder Deduction Using Machine Learning. In Proceedings of the 2023 First International Conference on Advances in Electrical, Electronics and Computational Intelligence (ICAEECI), Tiruchengode, India, 19–20 October 2023; pp. 1–8. [Google Scholar]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H.; Subha, D.P. Automated EEG-based screening of depression using deep convolutional neural network. Comput. Methods Programs Biomed. 2018, 161, 103–113. [Google Scholar] [CrossRef]
- Seal, A.; Bajpai, R.; Agnihotri, J.; Yazidi, A.; Herrera-Viedma, E.; Krejcar, O. DeprNet: A deep convolution neural network framework for detecting depression using EEG. IEEE Trans. Instrum. Meas. 2021, 70, 1–13. [Google Scholar] [CrossRef]
- Yasin, S.; Hussain, S.A.; Aslan, S.; Raza, I.; Muzammel, M.; Othmani, A. EEG based Major Depressive disorder and Bipolar disorder detection using Neural Networks: A review. Comput. Methods Programs Biomed. 2021, 202, 106007. [Google Scholar] [CrossRef]
- Nazari, M.-J.; Shalbafan, M.; Eissazade, N.; Khalilian, E.; Vahabi, Z.; Masjedi, N.; Ghidary, S.S.; Saadat, M.; Sadegh-Zadeh, S.-A. A machine learning approach for differentiating bipolar disorder type II and borderline personality disorder using electroencephalography and cognitive abnormalities. PLoS ONE 2024, 19, e0303699. [Google Scholar] [CrossRef]

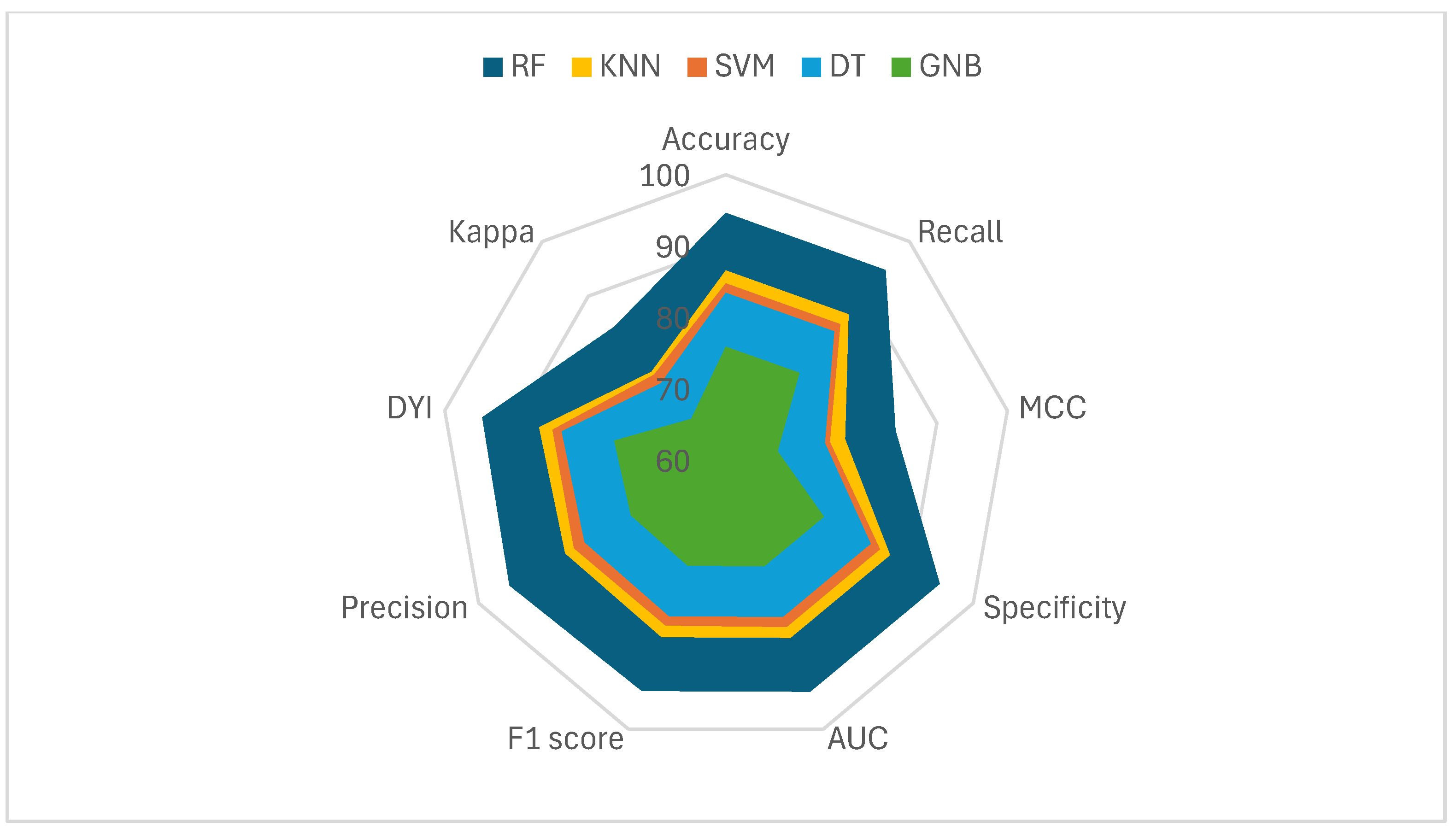
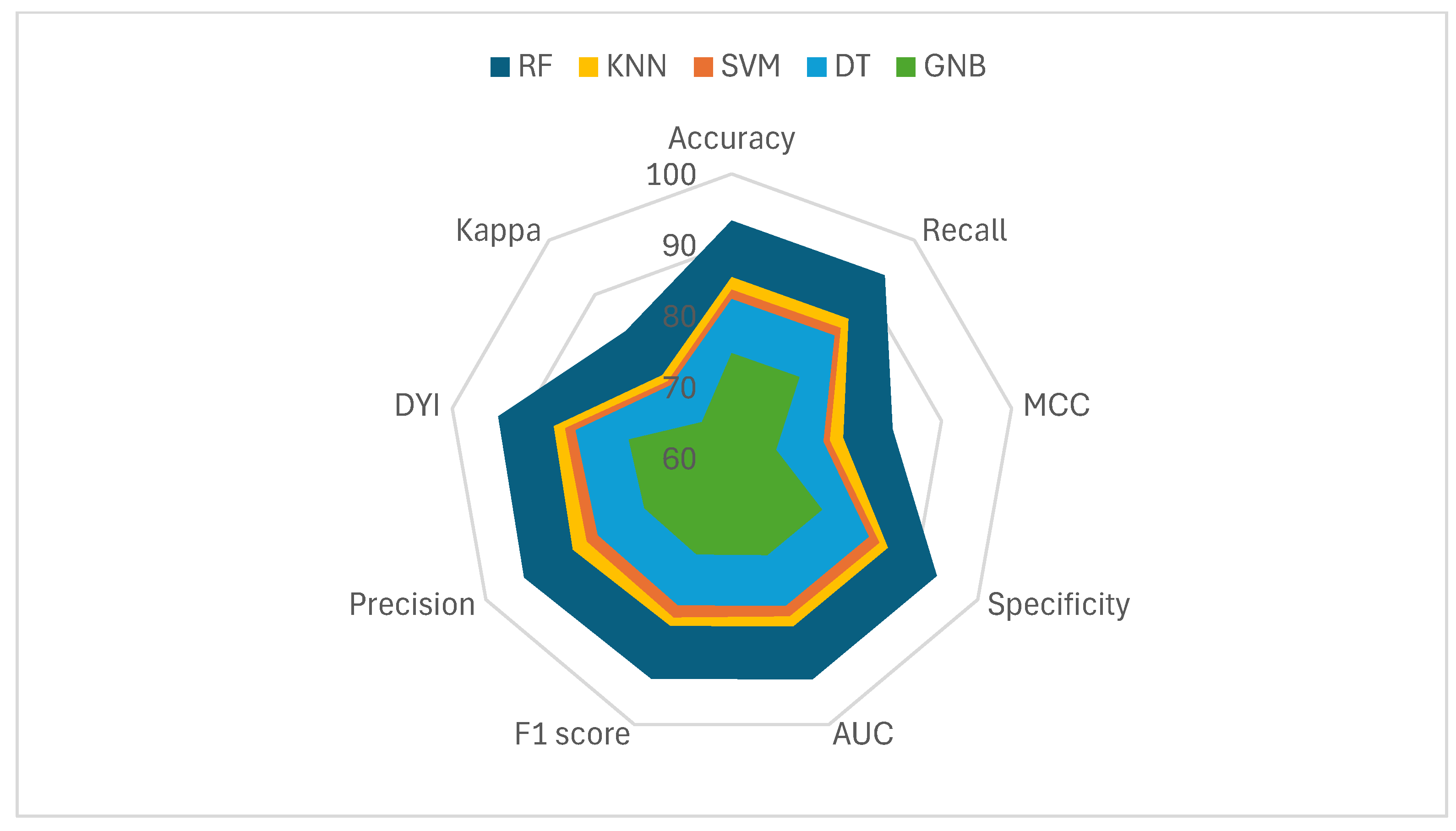
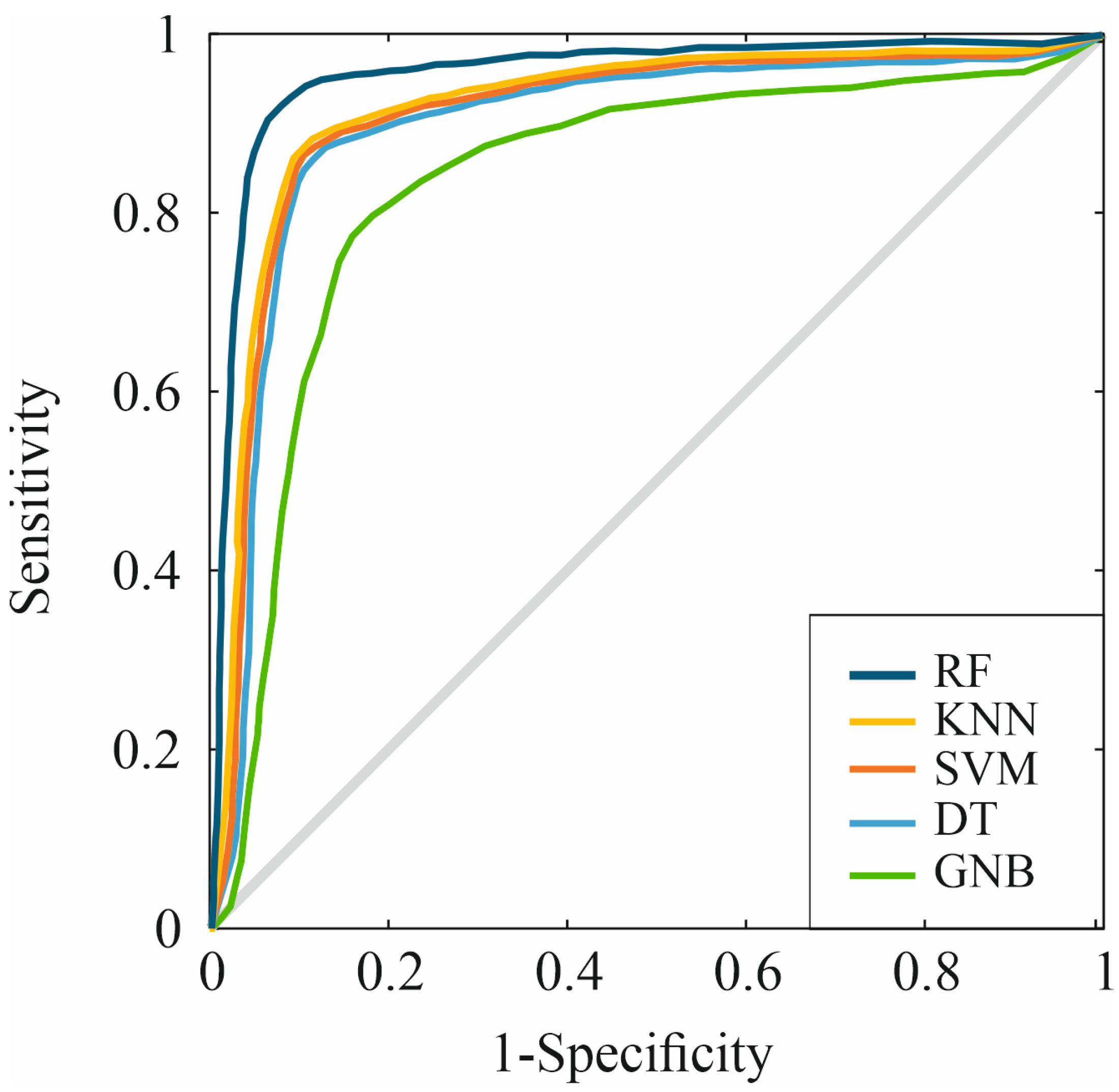
| Accuracy | MCC | F1 Score | Precision | DYI | |
|---|---|---|---|---|---|
| SVM | 83.65 | 73.98 | 83.90 | 83.56 | 83.85 |
| DT | 82.35 | 72.95 | 82.11 | 81.77 | 82.31 |
| GNB | 74.72 | 66.30 | 74.50 | 74.19 | 74.69 |
| KNN | 85.44 | 75.93 | 85.19 | 85.84 | 85.34 |
| RF | 93.41 | 82.99 | 93.13 | 93.75 | 93.37 |
| Recall | Specificity | Kappa | AUC | |
|---|---|---|---|---|
| SVM | 83.85 | 83.95 | 74.03 | 0.84 |
| DT | 82.45 | 82.26 | 73.32 | 0.82 |
| GNB | 74.81 | 74.63 | 66.52 | 0.75 |
| KNN | 85.54 | 85.34 | 75.18 | 0.85 |
| RF | 93.51 | 93.30 | 83.27 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, M.; Torres, A.M.; Blasco-Segura, P.; Mateo, J. Application of the Random Forest Algorithm for Accurate Bipolar Disorder Classification. Life 2025, 15, 394. https://doi.org/10.3390/life15030394
Suárez M, Torres AM, Blasco-Segura P, Mateo J. Application of the Random Forest Algorithm for Accurate Bipolar Disorder Classification. Life. 2025; 15(3):394. https://doi.org/10.3390/life15030394
Chicago/Turabian StyleSuárez, Miguel, Ana M. Torres, Pilar Blasco-Segura, and Jorge Mateo. 2025. "Application of the Random Forest Algorithm for Accurate Bipolar Disorder Classification" Life 15, no. 3: 394. https://doi.org/10.3390/life15030394
APA StyleSuárez, M., Torres, A. M., Blasco-Segura, P., & Mateo, J. (2025). Application of the Random Forest Algorithm for Accurate Bipolar Disorder Classification. Life, 15(3), 394. https://doi.org/10.3390/life15030394









