Abstract
The genus Ruspolia refers to a small group of plants in the Acanthaceae family, with two dominant species R. decurrens and R. hypocrateriformis essentially distributed in tropical parts of Africa. Decoctions from these plants are used in folk medicine for the treatment of a few human pathologies but the active ingredients at the origin of the bioactivities have been little studied. Here, we give an insight into the main phytochemicals of the Ruspolia species published in the literature so far and their pharmacological properties. The flavone glycosides justicialosides A-B likely serve as antioxidant agents and free radical scavengers. Several pyrrolidine alkaloids have been isolated from these Ruspolia species, notably (nor)ruspolinone and a few related products. These molecules have attracted the interest of medicinal chemists, with different synthetic routes leading to ruspolinone and analogues. There are versatile operating procedures to synthesize (nor)ruspolinone isomers. Despite these chemical efforts, the pharmacology of ruspolinone remains largely unknown. A few other Ruspolia alkaloids have been isolated, notably the rare bispyrrolidine benzodioxin alkaloid hypercratine, possibly acting as a ligand of β2-adrenergic receptors. A phytochemical survey of the Ruspolia species sheds light on the diversity of products in this family to promote further investigations into the mechanism of action of ruspolinone and related natural products.
1. Introduction
Plants from the genus Ruspolia in the Acanthaceae family are distributed essentially in tropical regions of Africa, from Mali to Sudan in the north, and from Angola to the Republic of Mozambique in the south, as well as the island of Madagascar (Figure 1). The name Ruspolia was given by the German mycologist and botanist Gustav Lindau (1866–1923). It pays homage to the Italian botanist Francesco Maria Marescotti Ruspoli (1672–1731). Parenthetically, the name Ruspolia should not be confused with that of the three plant species Lopriorea ruspolii (family: Amaranthaceae), Moringa ruspoliana Engl. (family: Moringaceae), and Pterodiscus ruspolii Engl. (family: Pedaliaceae), named after another Italian naturalist, Prince Eugenio Ruspoli (1866–1893).

Figure 1.
Distribution of Ruspolia species in Africa (maps defined from World Flora Online (www.worldfloraonline.org, accessed on 3 January 2025) and selected views of the flowers of three Ruspolia species.
The Ruspolia genus includes five little-known species with an accepted name (Table 1). The major species is R. decurrens, a subshrub mainly found in central and south-tropical Africa. It grows primarily in the seasonally dry tropical biome. This vascular plant producing nice yellow flowers (Figure 1) can be found in riverine forests, shady places in woodland and rocky hills and on termite mounds, notably in Zambia and Zimbabwe [1,2]. The species R. hypocrateriformis (Vahl), also known as Justicia hypocrateriformis Vahl, is a medicinal plant used to prepare a herbal remedy for diarrhea in Cameroon folk medicine [1,2]. In the rural area of Bui Division of Cameroon, a decoction from the leaves and stems of R. hypocrateriformis (locally named “kifu ke menseh”) is used in combination with two other plants to treat anemia, hemiplegia, and neuralgia [3,4]. This fast-growing evergreen plant shows nice pink to red flowers producing a large amount of nectar largely consumed by butterflies. Ruspolia hypocrateriformis (Vahl) is not to be confused with the totally distinct species Rivea hypocrateriformis (Desr.) Choisy, an edible plant found in India [5]. The species R. australis is native to Tanzania and South Africa. It is a tall shrub known as red mock-plumbago, also producing scarlet red flowers. The species Ruspolia seticalyx is native to east and south tropical Africa, whereas Ruspolia paniculata is found essentially in Madagascar. There is also a species called Ruspolia humbertii Benoist (family: Acanthaceae), also originating in Madagascar, but it has an unchecked status in the World Flora Online repertoire. The main species in the genus is R. hypocrateriformis, which also presents a nutritional potential due to its content in mineral elements, macro-nutrients (carbohydrates and fibers), and vitamins, such as vitamin C (1.22 g/100 g) [6].

Table 1.
Ruspolia species.
It is worth noting that the name Ruspolia refers also to a genus of insects (family: Tettigoniidae; order: Orthoptera) including the two species R. differens and R. nitidula. The former is an edible grasshopper, very popular as a food supplement in central and eastern Africa [7]. The fortification of sorghum and wheat with longhorn R. differens powder (RDP) is considered for use as a supplementary food used to prevent protein-energy malnutrition in children. RDP-fortified biscuits are being commercialized [8]. The latter is also an edible grasshopper, used as a protein source in Uganda [9,10]. The grasshopper Ruspolia nitidula has expanded its area of distribution in western and central Europe in recent decades [11]. The Ruspolia species of insects can also be seen in India (Kashmir) [12]. Bioactive compounds can be found in edible insects, including for example antioxidant compounds isolated from R. differens [13]. There are a few others insect species (e.g., R. dubia, R. Yunnana) [14,15] but here the analysis will focus on the Ruspolia plant species only.
Among Ruspolia plant species, R. hypocrateriformis is the most important species from a medicinal viewpoint. In Cameroon, a decoction of the leaves of this plant is used for the treatment of anemia and fever associated with malaria, and for the management of diarrhea [16]. As mentioned above, R. hypocrateriformis is used also in combination with other plants to treat hemiplegia and neuralgia [3,4]. Ethnobotanical practices have been rarely reported with the other Ruspolia species. Nevertheless, several bioactive natural products have been discovered from these plants. The present analysis discusses the pharmacological properties of Ruspolia plant extracts to highlight their potential as a valuable source of natural remedies. The primary objective of the review is to provide an overview of the various phytochemicals isolated from Ruspolia plants, the associated chemistry, and their bioactive properties. The major objective of the research is to fill knowledge gaps in Ruspolia phytochemistry and pharmacology, to encourage further studies of these families of neglected medicinal plants.
2. Flavone Glycosides from Ruspolia
An ethanol leaf extract of R. hypocrateriformis has been shown to contain common phenolic compounds such as gallic acid and ferulic acid, and a few flavonoids such as quercetin. These chemicals contribute to the antioxidant properties of the plant extract [17]. This plant also contains flavonoid O-glycosides, such as the two glycosylated luteolin derivatives: luteolin 7-O-β-D-apiofuranosyl-(1→2)-β-D-xylopyranoside and luteolin 7-O-[-βD-glucopyranosyl-(1→2)-α-L-rhamnosyl-(1→6)]-βD-glucopyranoside. In addition, two specific glycosides have been isolated from the plant leaves and named justicialosides A and B. They bear a luteolin or a chrysoeriol flavonoid core linked to a disaccharide unit (7-O-α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside) (Figure 2). Their names derive from Justicia hypocrateriformis, the synonym for R. hypocrateriformis [18].
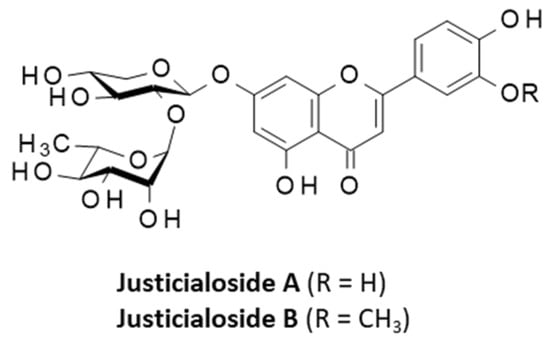
Figure 2.
Structures of two flavone glycosides isolated from Ruspolia hypocrateriformis.
These different flavonoids contribute to the antioxidant activity of the plant extract, and possibly to anti-anemia and antidiarrheal properties as well [2,6,19]. The glycoside moiety of the flavonoid contributes to its antioxidant action. Chrysoeriol is a multipotent flavone with anticancer, anti-inflammatory, antibacterial, antifungal, anti-osteoporosis, insecticide, and neuroprotective activities [20]. Chrysoeriol glycoside has been shown to inhibit the production superoxide anion by the xanthine/xanthine oxidase system more effectively than the aglycone. Similarly, the glycoside is a more efficient scavenger of 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) free radicals than the aglycone (chrysoeriol) itself [21]. A methanolic fraction of R. hypocrateriformis has revealed a modest antimalarial activity associated with inhibition of β-hematin formation (IC50 = 170 μg/mL) [22]. Justicialosides are phytochemicals specific to R. hypocrateriformis. Nevertheless, justicialoside A has been identified also from the leaves and stem bark of the African medicinal plant Pseudospondias microcarpa (A. Rich.) Engl. (Anacardiaceae) [23]. The pharmacological properties of the two justicialosides have not been specifically investigated. It would be useful to study their stress-reducing properties because a very similar flavone (acacetin) bearing exactly the same diglycoside moiety has been shown to reduce stress response and to promote longevity in a Caenorhabditis elegans model system. Notably, the product enhanced the levels of the antioxidant enzymes superoxide dismutase and catalase [24].
3. Pyrrolidine Alkaloids from Ruspolia
3.1. Isolation of the Natural Products
Ruspolinone, norruspolinone, and norruspoline are three pyrrolidine alkaloids isolated from R. hypocrateriformis [25]. There are also derivatives, such as N-methylruspolinone [26,27]. The pyrrolidinyl moiety of (nor)ruspolinone is reminiscent of that of the coca leaf alkaloids (nor)hygrine and the related alkaloids hygroline and pseudohygroline from the plants Carallia brachiata, Erythroxylum coca, Schizanthus hookeri, and Schizanthus tricolor [28,29,30,31]. They belong to a group of naturally occurring 2-(acylmethylene)pyrrolidine alkaloids which also includes dehydrodarlinine and dehydrodarlingianine derived from the plant Darlingia darlingiana found in Queensland (Australia) [26,32,33]. Phyllosterone (also named phyllostone) is a pyrrolidinyl-acetophenone derivative from Crytopcarya phyllostemon and is very similar to norruspoline [34]. This is also the case for the alkaloid ficuseptamine C isolated from the leaves of Ficus septica [35] (Figure 3). Norruspoline is structurally close to the pyrrolidine alkaloid anisotaline A (N-methyl norruspoline) isolated from the roots of the Chinese plant Anisodus tanguticus (Maxim.) Pascher. This compound has shown a very modest effect on the viability of human umbilical vein endothelial cells (HUVECs) in vitro [36]. These different pyrrolidine alkaloids have been described but they have been neglected from a pharmacological standpoint.
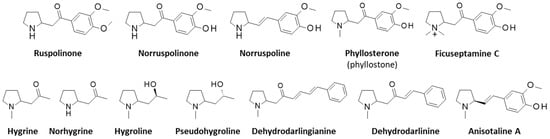
Figure 3.
Structures of pyrrolidine alkaloids related to ruspolinone.
Ruspolinone was initially isolated from the Ruspolia species but later the alkaloid was found in a few other plants, notably the fruits of the Vietnamese plant Boehmeria holosericea Blume (Urticaceae) [37], the leaves of Tephrosia pentaphylla (Roxb.) G.Don. (Fabaceae) [38], and the aerial parts of the invasive vine Vincetoxicum rossicum Kelopow [39]. This phenacylpyrrolidine has also been identified from endophytic fungi: (i) from a culture of Fusarium equiseti, initially isolated from the leaves of Ocimum gratissimum L. (clove basil, Lamiaceae) [40] and (ii) an endophytic fungus isolated from leaves of Newbouldia laevis [41]. Ruspolinone is generally considered as an intermediate in the biosynthesis of more complex alkaloids.
3.2. Total Synthesis of Ruspolinone and Analogues
Different methods have been proposed for the synthesis of ruspolinone and related pyrrolidine alkaloids. The chemistry of this group of pyrrolidine alkaloids has been well studied, in particular for (nor)hygrine-type natural products [42,43]. The synthesis of ruspolinone analogues has been less developed but, nevertheless, different access routes have been described. A total synthesis of both ruspolinone and norruspoline from 2-phenysulfonyl-piperidine derivatives has been proposed (Scheme 1). Norruspoline was obtained from the sulphonyl derivative (1) in the presence of β-bromo-3,4-dimethoxystyrene (2) and butyl lithium. The reaction afforded the ethenyl-pyrrolidine derivative (3) with a yield of 86% and the subsequent two-step deprotection of this intermediate with Na thiomethoxide in dry DMF followed with a treatment with aqueous sodium hydroxide (NaOH) afforded norruspoline as a major product with a yield of 53% and its isomer (4) (Scheme 1a). The synthesis of ruspolinone proceeded similarly from the intermediate (6), itself obtained from (1) in the presence of 1,2-dimethoxy-4-[1-[(1,2-dimethylethyl)silyloxy]ethenylbenzene (5) and magnesium bromide etherate at 0 °C. In this case, the final treatment of (6) with aqueous NaOH at reflux afforded ruspolinone with a yield of 80% (Scheme 1b) [44,45].
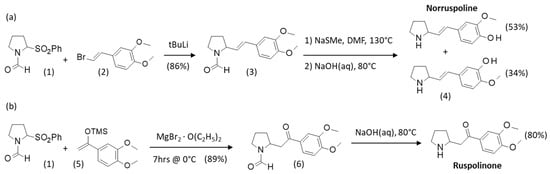
Scheme 1.
Synthetic routes to (a) norruspoline and (b) ruspolinone. Adapted from [43,44].
An alternative procedure was reported for the synthesis of ruspolinone starting from L-proline methyl ester (7), as depicted in Scheme 2. This seven-step procedure afforded the product with an overall yield of 26%, together with the related natural product phyllosterone (phyllostone) [46]. Compound (7) is converted into N-Boc prolinol (8) and then the O-tosylated derivative (9). Coupling of (9) with 2-(3′,4′-dimethoxyphenyl)-1,3-dithiane (10) in cold tetrahydrofuran (THF, −21 °C) in the presence of n-butyllithium in hexane afforded compound (11), purified by flash chromatography. The reaction of the dithiane (11) with N-chlorosuccinimoide (NCS) and silver nitrate in aqueous acetonitrile afforded the carbamate N-Boc-ruspolinone (12). Finally, the removal of the Boc protecting with trifluoroacetic acid (TFA) gave ruspolinone (Scheme 2a).
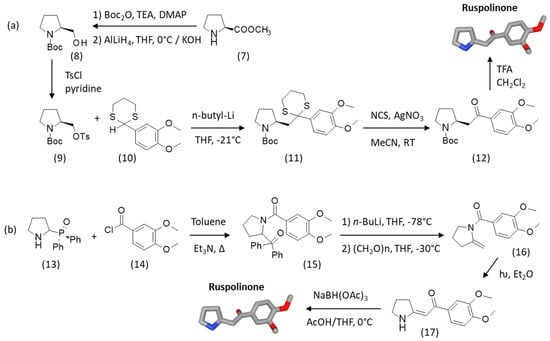
Scheme 2.
Alternative synthetic routes to ruspolinone. (a) Starting from L-proline methyl ester or (b) from 2-diphenylphosphinoyl pyrrolidine. Adapted from [45,47].
An alternative process to obtain ruspolinone consists in synthesizing an enaminoketone intermediate which is then selectively reduced in the presence of sodium triacetoxyborohydride (NaBH(OAc)3, also known as sodium triacetoxyhydroborate or STAB). The pyrrolidine enaminoketone moiety combines the nucleophilicity of the enamine with the eletrophilicity of the enone moiety [47]. 2-Diphenylphosphinoyl pyrrolidine (13) was treated with the substituted acyl chloride derivative (14) to afford the phosphorylated N-acylamine (15) with a yield of 75%. Treatment of phosphorylated amide (15) with n-butyllithium (n-BuLi) in THF and then with a pre-cooled THF solution of freshly depolymerized paraformaldehyde led to the desired pyrrolidine derivative (16) with a moderate yield of 55%. The irradiation at 254 nm of a deaerated solution of (16) in ether for about 30 min led to vinylogous amide (17). The final chemoselective reduction of the C=C unit of this enaminone (17) with sodium triacetoxyborohydride in a 3:1 mixture of acetic acid (AcOH) and THF at 0 °C led efficiently (91% yield) to the desired product ruspolinone. The same procedure can be adapted to the synthesis of both the corresponding piperidine and pyrrolidine derivatives [48].
More recently, Sirvent and coworkers have accomplished the synthesis of (-)-ruspolinone from N-tert-butanesulfinyl bromoaldimine (18) in the presence of a β-keto acid (19), affording the β-amino ketone derivative (20) in 82% yield. The imine (18) derived from 4-bromobutanal and (S)-tert-butanesulfinamide in toluene. After removal of the sulfinyl group of (20) under acidic conditions, the compound was transformed into (-)-ruspolinone in 92% yield, as represented in Scheme 3a [49]. A totally distinct synthetic route to (+)-ruspolinone from 4-chlorobutanal (21) and nonafluorobutane-1-sulfinamide (22) in the presence of titanium(IV) isopropoxide [Ti(OiPr)4] as a dehydrating agent, has been proposed, as depicted in Scheme 3b. The procedure involves the synthesis of a fluorous-tagged intermediate, separated via a process known as fluorous solid-phase extraction (F-SPE) which avoids column chromatography over multiple steps. The initial step leading to the sulfinamide derivative (23) is very efficient (98% yield). A Liebeskind−Srogl cross-coupling reaction of the thioester (23) with the boronic acid derivative (24) yielded the β-amino ketone (25) after F-SPE. Finally, the N-sulfinyl deprotection in the presence of cesium carbonate and intramolecular rearrangement afforded (+)-ruspolinone (Scheme 3b). A similar activated N-perfluorobutanesulfinamide intermediate was used to synthesize (+)-ruspolinone and the antibacterial alkaloid (+)-negamycin and the antidiabetic drug (-)-sitagliptin [50].
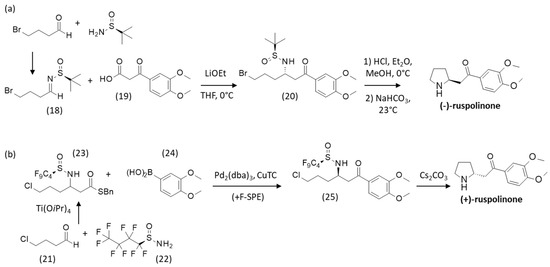
Scheme 3.
Synthetic routes to (a) (−)- and (b) (+)-ruspolinone. Adapted from [49].
Altogether, these different chemical processes underlined the attractiveness of the ruspolinone in terms of synthetic chemistry. There are multiple routes to access the product and derivatives. The process initially proposed by Brown and coworkers remains a straightforward and efficient route [44,45]. The (nor)ruspolinone chemistry has been well developed. Unfortunately, the same cannot be said for the pharmacology of these compounds, largely neglected until now.
3.3. Other Ruspolia Alkaloids
10H-quindoline (10H-indolo [3,2-b]quinoline), a tetracyclic alkaloid found in the Justicia species, particularly in J. betonica [51,52], has also been identified and isolated from the extract of the leaves of R. hypocrateriformis [17]. The pharmacological properties of 10H-quindoline have not been specifically investigated but the compound bears a close structural analogy with the well-known alkaloid cryptolepine (Figure 4) which exhibits many types of activities, including anti-bacterial, anti-fungal, anti-hyperglycemic, antidiabetic, anti-inflammatory, anti-hypotensive, antipyretic, and antimuscarinic properties [53]. Cryptolepine is a cytotoxic DNA-intercalating inhibitor of topoisomerase II [54,55,56]. It is therefore conceivable that 10H-quindoline can function also as a topo II poison, although other cytotoxic 10H-quindoline derivatives have revealed a mechanism of action independent from topoisomerase II poisoning [57]. Quindoline and derivatives have been shown to stabilize quadruplex DNA and to inhibit telomerase [58,59].
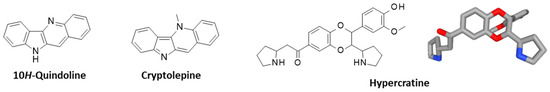
Figure 4.
Chemical structures of 10H-quindoline, cryptolepine, and hypercratine (with a molecular model).
A rare bispyrrolidine alkaloid named hypercratine (Figure 4) was isolated from the roots of R. hypocrateriformis [60]. It is a benzodioxin derivative equipped with two pyrrolidinyl moieties, also identified in the leaves of the medicinal plant Justicia flava Vahl (Acanthaceae), possibly contributing to the tocolytic activity of the plant extract [61]. Justicia flava leaf extracts have been shown to potently inhibit uterine contractility in both a pregnant and a non-pregnant mouse uterus [62]. Hypercratine may contribute to the regulation of myometrial contractility. Its precise mechanism of action remains unknown at present but a binding to β2-adrenergic receptors (β2AR) is conceivable, by analogy with structurally related tocolytic agents such as salbutamol, isoproterenol, and terbutaline acting as β2AR agonists [63,64]. The targeting of the G protein-coupled receptor (GPCR) β2AR with the partial agonist salbutamol contributes to the successful use of this drug in treating asthma and chronic obstructive pulmonary disease (COPD). Hypercratine appears to be well adapted to bind tightly to β2AR, as illustrated in the molecular model shown in Figure 5. A docking analysis performed with β2AR bound to salbutamol (PDB: 7DHR) [65] revealed that the natural product can fit very well into the large salbutamol-binding cavity, engaging H-bonds or van der Waals contacts with much the same amino acid residues. A large set of molecular contacts stabilizes the hypercratine-β2AR complex and the two pyrrolidine units contribute significantly to its stability. The docking analysis suggests that the hypercratine-β2AR complex is more stable than that formed with the known β2AR ligands. The calculated empirical energy of interaction (ΔE) is largely more negative with hypercratine compared with terbutaline, ritodrine, isoproterenol, or salbutamol (Table 2). Hypercratine seems to be a bona fide β2AR ligand but its pharmacological effects, as an agonist or antagonist, remain to be characterized. Its benzodioxin structure is also reminiscent to that of the α2-adrenoceptor antagonists idazoxan and the antipsychotic derivative RX 821002 (2-methoxyidazoxan), which possesses high and selective affinities for D2-like and 5-HT(1A) receptors [66,67]. Hypercratine is a rare alkaloid totally neglected at present. This atypical natural product warrants further investigation.
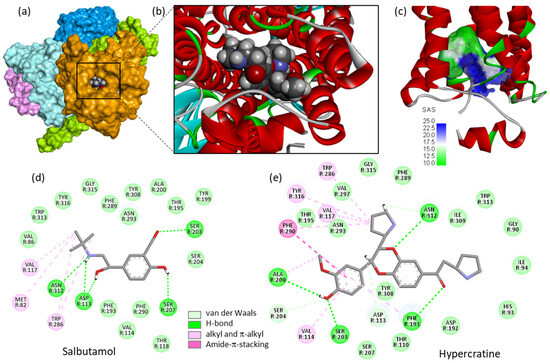
Figure 5.
Molecular model of hypercratine (compound CID: 177183) bound to the β2-adrenergic receptor (β2AR). (a) A surface of hypercratine bound to β2AR (PDB structure: 7DHR). (b) Close-up view of the ligand−protein interface. (c) The solvent-accessible surface (SAS) surrounding the drug binding zone (color code indicated). (d,e) Binding map contacts for salbutamol and hypercratine bound to β2AR (color code indicated). The docking analysis was performed as previously described with other protein−drug complexes [68,69].

Table 2.
Calculated potential energy of interaction (ΔE) and free energy of hydration (ΔG) for the interaction of hypercratine with the β2-adrenergic receptor (β2AR) *.
4. Discussion
Acanthaceae is a large plant family with over 2500 species, found primarily in subtropical and tropical regions. Several species of this family have been used traditionally to treat a variety of diseases and inflammatory conditions, including gastrointestinal and cardiovascular ailments. The family includes important and well-known genera, such as the genus Justicia with more than 600 species [1], and the genera Barleria [70], Blepharis [71], and Acanthus [72]. The family also comprises little-known genera such as Ruspolia Lindau., which belongs to Justicieae Dumort., the most taxonomically complex tribe in Acanthaceae [73]. Within the tribe Justicieae, the subtribe Graptophyllinae T. Anderson comprises 27 genera, including Ruspolia Lindau [74].
Plants from the genus Ruspolia have been little investigated thus far. The two main species R. decurrens and R. hypocrateriformis are emblematic of this family of plants essentially found in Africa. Different alkaloids and flavones have been isolated from these species, notably the leading product ruspolinone (Table 3). This small pyrrolidine alkaloid and its diverse analogues have attracted interest from medicinal chemists essentially, but the associated pharmacology remains largely unknown at present. There are efficient routes to access ruspoline and (nor)ruspolinone, but little mechanistic and pharmacological information is available. Ruspolinone is readily accessible and represents an intermediate in the synthesis of more complex indolizidine alkaloids, notably in the antofine series [75]. Its chemistry has been largely investigated, but the biological properties of this alkaloid remain to be discovered. It is high time to investigate the pharmacological effects of this series of pyrrolidine alkaloids.

Table 3.
Natural products isolated from Ruspolia hypocrateriformis and their bioactivities.
Ruspolinone seems to be an intermediate in the biosynthesis of hypercratine, a unique alkaloid isolated from Ruspolia. This rare benzodioxin alkaloid has been described in a single study, together with a few acetylated and methoxylated analogues [60]. It bears a relative similarity with the drug eliglustat, a benzodioxin derivative bearing a pyrrolidinyl moiety used to treat Gaucher disease type 1. Eliglustat (Cerdelga™) is a ceramide mimic inhibiting the enzyme UDP-glucose ceramide glucosyltransferase (UCCG) that synthesizes glucosylceramides. It therefore reduces accumulation of these lipids in the lysosome [76]. The drug has revealed interest for the treatment of cancers, notably in combinations with immune checkpoint inhibitors [77]. It would be interesting to investigate further the bioactivity and mechanism of the action of hypercratine in this context as a potential UCCG inhibitor, in parallel with its possible action as a modulator of β2-adrenergic receptors. It is to be hoped that the phytochemical survey reported here will help and encourage phytochemists and pharmacologists to investigate further Ruspolia species and associated natural products.
Overall, there is a need for a more in-depth exploration of the potential for pharmacological evaluation of the compounds isolated from Ruspolia plants, notably from the neglected medicinal species R. hypocrateriformis. The plant is abundant and accessible. The main alkaloids, notably ruspolinone and hypercratine, can be obtained via efficient synthetic procedures. These pyrrolidine alkaloids deserve further studies as anti-inflammatory, anticancer, and/or antimicrobial agents, and they can serve as a template for the design of novel pyrrolidine derivatives. Pyrrolidine molecules represent an important class of medicinal products [78,79,80]. There is a need for new scaffolds and lead molecules in this family.
5. Conclusions
The phytochemical survey of the Ruspolia plant species has revealed the presence of bioactive products, mainly from the medicinal species R. hypocrateriformis with several pyrrolidine alkaloids and a few flavone glycosides. The main products correspond to ruspolinone and derivatives, for which there are efficient synthetic procedures to obtain the compounds and analogues. The analysis also sheds light on the alkaloid hypercratine, likely biosynthesized from ruspolinone, acting as a potential ligand to β2-adrenergic receptors. This compound and related Ruspolia alkaloids deserve further study to better define their mechanism of action and molecular targets. The analysis will encourage pharmacological investigations into the mode of action of Ruspolia-derived natural products.
Author Contributions
Conceptualization, C.B.; software, G.V.; investigation, C.B.; writing—original draft preparation, C.B.; writing—review and editing, C.B.; visualization, G.V. and C.B.; supervision, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Carneiro, M.R.B.; Sallum, L.O.; Martins, J.L.R.; Peixoto, J.C.; Napolitano, H.B.; Rosseto, L.P. Overview of the Justicia Genus: Insights into Its Chemical Diversity and Biological Potential. Molecules 2023, 28, 1190. [Google Scholar] [CrossRef] [PubMed]
- Agbor, G.A.; Longo, F.; Makong, E.A.; Tarkang, P.A. Evaluation of the antidiarrheal and antioxidant properties of Justicia hypocrateriformis. Pharm. Biol. 2014, 52, 1128–1133. [Google Scholar] [CrossRef]
- Noumi, E. Ethno-medico-botany, theory and philosophy: Case of traditional medicinal plant uses in hemiplegia and neuralgia, in Bui division, North West of Cameroon. World J. Pharm. Res. 2015, 4, 377–392. [Google Scholar]
- Makemteu, J.; Nana Piapleu, W.G.; Nkenmegne, S.; Yossa Nzeuwa, I.B.; Ngouana, V.; Tajeukem, V.C.; Noumi, E. Traditional use of medicinal plants in the town of Mbanga (Littoral- Cameroon). Int. J. Sci. Res. Updates 2022, 4, 173–190. [Google Scholar] [CrossRef]
- Mukim, M.; Kabra, A.; Hano, C.; Drouet, S.; Tungmunnithum, D.; Chaturvedi, M.; Patel, R.; Ayaz, M.; Shadrack, D.M. Rivea hypocrateriformis (Desr.) Choisy: An Overview of Its Ethnomedicinal Uses, Phytochemistry, and Biological Activities and Prospective Research Directions. J. Chem. 2022, 1, 9099672. [Google Scholar] [CrossRef]
- Orji, O.U.; Ibiam, U.A.; Uraku, A.J.; Obasi, O.D.; Aloke, C.E.; Awoke, J.N. Investigations of Phytochemical and Nutritional Composition of Ruspolia hypocrateriformis Leaf. IDOSR J. Appl. Sci. 2017, 2, 70–81. [Google Scholar]
- van Huis, A. Cultural significance of locusts, grasshoppers, and crickets in sub-Saharan Africa. J. Ethnobiol. Ethnomed. 2022, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Ronoh, A.K.; Serrem, C.A.; Tumwebaze, S.B.; Were, G.M. Effect of fortifying sorghum and wheat with Longhorn grasshopper (Ruspolia differens) powder on nutritional composition and consumer acceptability of biscuits. Food Sci. Nutr. 2024, 12, 3492–3507. [Google Scholar] [CrossRef] [PubMed]
- Bbosa, T.; Tamale Ndagire, C.; Muzira Mukisa, I.; Fiaboe, K.K.M.; Nakimbugwe, D. Nutritional Characteristics of Selected Insects in Uganda for Use as Alternative Protein Sources in Food and Feed. J. Insect Sci. 2019, 19, 23. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Mukisa, I.M.; Nakimbugwe, D. Nutritional composition, quality, and shelf stability of processed Ruspolia nitidula (edible grasshoppers). Food Sci. Nutr. 2016, 5, 103–112. [Google Scholar] [CrossRef]
- Kaláb, O.; Pyszko, P.; Kočárek, P. Estimation of the Recent Expansion Rate of Ruspolia nitidula (Orthoptera) on a Regional and Landscape Scale. Insects 2021, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Usmani, M.K.; Ali, M.; Dar, A.A. Description of a new species of the genus Ruspolia (Schulthess, 1898) (Conocephalinae: Copiphorini) from Kashmir, India. Zootaxa 2021, 4966, 483486. [Google Scholar] [CrossRef] [PubMed]
- Aiello, D.; Barbera, M.; Bongiorno, D.; Cammarata, M.; Censi, V.; Indelicato, S.; Mazzotti, F.; Napoli, A.; Piazzese, D.; Saiano, F. Edible Insects an Alternative Nutritional Source of Bioactive Compounds: A Review. Molecules 2023, 28, 699. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, M.; Aris-Brosou, S.; Xia, X. Testing alternative hypotheses on the origin and speciation of Hawaiian katydids. BMC Ecol. Evol. 2022, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, X.; Bian, X. The complete mitochondrial genome of Ruspolia yunnana (Orthoptera: Tettigoniidae: Conocephalinae). Mitochondrial DNA B Resour. 2022, 7, 1682–1684. [Google Scholar] [CrossRef] [PubMed]
- Adjanohoun, J.; Aboubakar, N.; Dramane, K.; Ebot, M.; Ekpere, J.; Enow-Orock, E.; Focho, D.; Gbile, Z.; Kamanyi, A.; Kamsu-Kom, J. Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanical and Floristic Studies in Cameroon. In Proceedings of the OUA/STRC Symposium on African Traditional Medicine and Medicinal Plants, Lagos, Nigeria, 1996; Volume 301. [Google Scholar]
- Awoke, J.N.; Orji, O.U.; Aja, P.M.; Ezeani, N.N.; Aloke, C.; Obasi, O.D. Ethanol leaf extract of Ruspolia hypocrateriformis abrogated hepatic redox imbalance and oxidative damage induced by heavy metal toxicity in rats. Arab J. Chem. 2020, 13, 8133–8145. [Google Scholar] [CrossRef]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Ismail, F.M.D.; Dempster, N.M.; Sarker, S.D. Ruspolia hypocrateriformis (Vahl) Milne-Redh. (Acanthaceae). Phytochem. Lett. 2019, 31, 101–103. [Google Scholar] [CrossRef]
- Orji, O.U.; Ibiam, U.A.; Aja, P.M.; Obasi, O.D.; Ezeani, N.; Aloke, C.; Anayo, S.; Inya-Agha, O.R. Effect of Ethanol Extract of Ruspolia hypocrateriformis Leaf on Haematological Parameters in Lead Poisoned Albino Rats. World J. Med. Sci. 2016, 13, 225–235. [Google Scholar]
- Aboulaghras, S.; Sahib, N.; Bakrim, S.; Benali, T.; Charfi, S.; Guaouguaou, F.E.; Omari, N.E.; Gallo, M.; Montesano, D.; Zengin, G.; et al. Health Benefits and Pharmacological Aspects of Chrysoeriol. Pharmaceuticals 2022, 15, 973. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.I.; Kumar, M.S.; Unnikrishnan, M.K.; Mohan, H. Effect of O-glycosilation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorg. Med. Chem. 2003, 11, 2677–2685. [Google Scholar] [CrossRef]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Ismail, F.M.D.; Evans, A.R.; Tchinda, A.T.; Tarkang, A.P.; Nnanga, E.N.; Sarker, S.D. Haem Polymerization Inhibitory Activity and Cytotoxicity of Six Medicinal Plants Used in Cameroon for the Management of Malaria. Acta Pharm. Sci. 2022, 60, 235–245. [Google Scholar] [CrossRef]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Daud Ismail, F.M.; Dempster, N.M.; Nnanga, E.N.; Sarker, S.D. Phenolic compounds from the leaves and stem bark of Pseudospondias microcarpa (A. Rich.) Engl. (Anacardiaceae). Biochem. System Ecol. 2020, 91, 104078. [Google Scholar] [CrossRef]
- Asthana, J.; Yadav, D.; Pant, A.; Yadav, A.K.; Gupta, M.M.; Pandey, R. Acacetin 7-O-α-l-rhamnopyranosyl (1-2) β-D-xylopyranoside Elicits Life-Span Extension and Stress Resistance in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1160–1168. [Google Scholar] [CrossRef]
- Roessler, F.; Ganzinger, D.; Johne, S.; Schöpp, E.; Hesse, M. Ruspolia hypercrateriformis M.R.: Isolierung und Strukturaufklärung von neuen Pyrrolidin-Alkaloiden. Helvet Chim. Acta 1978, 61, 1200–1206. [Google Scholar] [CrossRef]
- Ghirlando, R.; Howard, A.S.; Katz, R.B.; Michael, J.P. The application of the sulfide contraction to the synthesis of some simple pyrrolidine alkaloids. Tetrahedron 1984, 40, 2879–2884. [Google Scholar] [CrossRef]
- Langenskiold, T.; Lounasmaa, H. Novel applications of the modified Polonovski reaction. IV Preparation of (+)-hygrine and (+)-N-methylruspolinone. Heterocycles 1983, 20, 671. [Google Scholar]
- Bhat, C.; Bhat, S.I.; Budanur, B.M. Hygroline and pseudohygroline: Isolation, biological perspectives and synthesis. Synthetic Commun Rev. 2022, 53, 85–102. [Google Scholar] [CrossRef]
- Cretton, S.; Genta-Jouve, G.; Kaiser, M.; Mäser, P.; Muñoz, O.; Bürgi, T.; Cuendet, M.; Christen, P. Hygroline derivatives from Schizanthus tricolor and their anti-trypanosomatid and antiplasmodial activities. Phytochemistry. 2021, 192, 112957. [Google Scholar] [CrossRef] [PubMed]
- Bhoite, S.P.; Kamble, R.B.; Suryavanshi, G.M. An enantioselective synthesis of (+)-hygroline and (+)-pseudohygroline via Keck allylation and CBS reduction. Tetrahedron. Lett. 2015, 56, 4704–4705. [Google Scholar] [CrossRef]
- Liniger, M.; Estermann, K.; Altmann, K.H. Total synthesis of hygrolines and pseudohygrolines. J. Org. Chem. 2013, 78, 11066–11070. [Google Scholar] [CrossRef]
- Shih, Y.C.; Tsai, P.H.; Hsu, C.C.; Chang, C.W.; Jhong, Y.; Chen, Y.C.; Chien, T.C. Biomimetic Approach Toward the Total Synthesis of rac-2-(Acylmethylene)pyrrolidine Alkaloids. J. Org. Chem. 2015, 80, 6669–6678. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, J.J.; Puglis, J.M. The stereochemistry of nitrone-diene cycloadditions. Synthesis of the alkaloids of Darlingia darlingiana. Tetrahedron. Lett. 1986, 27, 1265–1268. [Google Scholar] [CrossRef]
- Cave, A.; Leboeuf, M.; Moskowitz, H.; Ranaivo, A.; Bick, I.R.C.; Sinchai, W.; Nieto, M.; Sevenet, T.; Cabalion, P. Alkaloids of Cryptocarya phyllostemon. Austral. J. Chem. 1989, 42, 2243–2263. [Google Scholar] [CrossRef]
- Ueda, J.Y.; Takagi, M.; Shin-ya, K. Aminocaprophenone- and pyrrolidine-type alkaloids from the leaves of Ficus septica. J. Nat. Prod. 2009, 72, 2181–2183. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.Y.; Peng, C.; Shu, H.Z.; Liu, Z.H.; Zhou, Q.M.; Xiong, L. New indolizidine- and pyrrolidine-type alkaloids with anti-angiogenic activities from Anisodus tanguticus. Biomed. Pharmacother. 2023, 167, 115481. [Google Scholar] [CrossRef]
- Ai, D.T.T.; Van, T.T.T.; Huong, D.T.M.; Litaudon, M.; Tram, L.H.; Cuong, P.V. Chemical constituents of Boehmeria holosericea Blume (Urticaceae). Vietnam J. Chem. 2018, 56, 172–175. [Google Scholar] [CrossRef]
- Paikroa, K.; Dhabe, A.S. Phytochemical analysis of Tephrosia pentaphylla (Roxb.) G.Don. BioInfoLet 2022, 19, 435–439. [Google Scholar]
- Kempthorne, C.J.; St Pierre, M.; Le, A.; Livingstone, S.; McNulty, J.; Cadotte, M.W.; Liscombe, D.K. Mass spectrometry-based metabolomics for the elucidation of alkaloid biosynthesis and function in invasive Vincetoxicum rossicum populations. Phytochemistry 2024, 221, 114051. [Google Scholar] [CrossRef] [PubMed]
- Adione, N.M.; Onyeka, I.P.; Abba, C.C.; Okoye, N.N.; Okolo, C.C.; Eze, P.M.; Umeokoli, B.O.; Anyanwu, O.O.; Okoye, F.B.C. Detection, isolation and identification of more bioactive compounds from Fusarium equiseti, an endophytic isolated from Ocimum gratissimum. GSC Biol. Pharm. Sci. 2022, 20, 130–140. [Google Scholar] [CrossRef]
- Eze, P.M.; Nnanna, J.C.; Okezie, U.; Buzugbe, H.S.; Abba, C.C.; Chukwunwejim, C.R.; Okoye, F.B.C.; Esimone, C.O. Screening of metabolites from endophytic fungi of some Nigerian medicinal plants for antimicrobial activities. EuroBiotech J. 2019, 3, 10–18. [Google Scholar] [CrossRef]
- Smolobochkin, A.V.; Gazizov, A.S.; Burilov, A.R.; Pudovik, M.A. Norhygrine Alkaloid and Its Derivatives: Synthetic Approaches and Applications to the Natural Products Synthesis. Helv Chim. Acta 2022, 105, e202100158. [Google Scholar] [CrossRef]
- Bhat, C.; Tilve, S.G. Recent advances in the synthesis of naturally occurring pyrrolidines, pyrrolizidines and indolizidine alkaloids using proline as a unique chiral synthon. RSC Adv. 2014, 4, 5405. [Google Scholar] [CrossRef]
- Brown, D.S.; Hansson, T.; Ley, S.V. Direct Substitution of 2-Benzenesulphonylpiperidines and -pyrrolidines by Carbon Nucleophiles: Synthesis of the Pyrrolidine Alkaloid Ruspolinone. Synlett 1990, 1990, 48–49. [Google Scholar] [CrossRef]
- Brown, D.S.; Charreau, P.; Hansson, T.; Ley, S.V. Substitution reactions of 2-phenylsylfonyl-piperidines and -pyrrolodines with carbon nucleophiles: Synthesis of the pyrrolidine alklaoids noruspoline and ruspolinone. Tetrahedron 1991, 47, 1311–1328. [Google Scholar] [CrossRef]
- Jones, K.; Woo, K.C. A total synthesis of (-)-ruspolinone. Tetrahedron 1991, 47, 7179–7184. [Google Scholar] [CrossRef]
- Negri, G.; Kascheres, C.; Kascheres, A.J. Recent Development in Preparation Reactivity and Biological Activity of Enaminoketones and Enaminothiones and Their Utilization to Prepare Heterocyclic Compounds. J. Heterocyclic Chem. 2004, 41, 461. [Google Scholar] [CrossRef]
- Couture, A.; Deniau, E.; Grandclaudon, P.; Lebrun, S. Dramatically Different Photochemical Behaviour of 1-Aroyl-2-methylene Piperidine and Pyrrolidine Derivatives. An Expeditious Synthesis of Ruspolinone. Tetrahedron Lett. 1996, 37, 7749–7752. [Google Scholar] [CrossRef]
- Sirvent, A.; Hernandez-Ibanez, S.; Yus, M.; Foubelo, F. Pyrrolidine and Indolizidine Alkaloids from Chiral N-tert-Butanesulfinyl Imines Derived from 4-Halobutanal. Synthesis 2021, 53, 1749–1759. [Google Scholar]
- Namitharan, K.; Cellnik, T.; Mukanova, A.; Kim, S.; Healy, A.R. A Dual Role for the N-Perfluorobutanesulfinamide Auxiliary in an Asymmetric Decarboxylative Mannich Reaction. Org Lett. 2024, 26, 8810–8815, preprint: ChemRxiv. 2024. [Google Scholar] [CrossRef]
- Manokari, M.; Latha, R.; Priyadharshini, S.; Cokul, R.M.; Beniwal, P.; Manjunatha, R.Y.; Rajput, B.S.; Shekhawat, M.S. A comprehensive review on a less explored medicinally important plant Justicia betonica L. World Sci. News 2019, 131, 110–122. [Google Scholar]
- Subbaraju, G.V.; Kavitha, J.; Rajasekhar, D.; Jimenez, J.I. Jusbetonin, the first indolo [3,2-b]quinoline alkaloid glycoside, from Justicia betonica. J. Nat. Prod. 2004, 67, 461–462. [Google Scholar] [CrossRef]
- Tudu, C.K.; Bandyopadhyay, A.; Kumar, M.; Radha; Das, T.; Nandy, S.; Ghorai, M.; Gopalakrishnan, A.V.; Proćków, J.; Dey, A. Unravelling the pharmacological properties of cryptolepine and its derivatives: A mini-review insight. Naunyn Schmiedebergs Arch Pharmacol. 2023, 396, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Bonjean, K.; De Pauw-Gillet, M.C.; Defresne, M.P.; Colson, P.; Houssier, C.; Dassonneville, L.; Bailly, C.; Greimers, R.; Wright, C.; Quetin-Leclercq, J.; et al. The DNA intercalating alkaloid cryptolepine interferes with topoisomerase II and inhibits primarily DNA synthesis in B16 melanoma cells. Biochemistry. 1998, 37, 5136–5146. [Google Scholar] [CrossRef] [PubMed]
- Dassonneville, L.; Bonjean, K.; De Pauw-Gillet, M.C.; Colson, P.; Houssier, C.; Quetin-Leclercq, J.; Angenot, L.; Bailly, C. Stimulation of topoisomerase II-mediated DNA cleavage by three DNA-intercalating plant alkaloids: Cryptolepine, matadine, and serpentine. Biochemistry 1999, 38, 7719–7726. [Google Scholar] [CrossRef] [PubMed]
- Dassonneville, L.; Lansiaux, A.; Wattelet, A.; Wattez, N.; Mahieu, C.; Van Miert, S.; Pieters, L.; Bailly, C. Cytotoxicity and cell cycle effects of the plant alkaloids cryptolepine and neocryptolepine: Relation to drug-induced apoptosis. Eur J. Pharmacol. 2000, 409, 9–18. [Google Scholar] [CrossRef]
- Chen, J.; Deady, L.W.; Desneves, J.; Kaye, A.J.; Finlay, G.J.; Baguley, B.C.; Denny, W.A. Synthesis of substituted indeno[1,2-b]quinoline-6-carboxamides, [1]benzothieno[3,2-b]quinoline-4-carboxamides and 10H-quindoline-4-carboxamides: Evaluation of structure-activity relationships for cytotoxicity. Bioorg. Med. Chem. 2000, 8, 2461–2466. [Google Scholar] [CrossRef]
- Zhou, J.L.; Lu, Y.J.; Ou, T.M.; Zhou, J.M.; Huang, Z.S.; Zhu, X.F.; Du, C.J.; Bu, X.Z.; Ma, L.; Gu, L.Q.; et al. Synthesis and evaluation of quindoline derivatives as G-quadruplex inducing and stabilizing ligands and potential inhibitors of telomerase. J. Med. Chem. 2005, 48, 7315–7321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Zhu, X.F.; Lu, Y.J.; Deng, R.; Huang, Z.S.; Mei, Y.P.; Wang, Y.; Huang, W.L.; Liu, Z.C.; Gu, L.Q.; et al. Senescence and telomere shortening induced by novel potent G-quadruplex interactive agents, quindoline derivatives, in human cancer cell lines. Oncogene 2006, 25, 503–511. [Google Scholar] [CrossRef]
- Neukomm, G.; Roessler, F.; Johne, S.; Hesse, M. Contributions to the Structure of Hypercratine, another Alkaloid of Ruspolia hypercrateriformis. Planta Med. 1983, 48, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Bafor, E.E.; Ukpebor, F.; Omoruyi, O.; Ochoyama, E.; Omogiade, G.; Ekufu, J.; Edrada-Ebel, R. Tocolytic activity assessment of the methanol leaf extract of Justicia flava Vahl (Acanthaceae) on mouse myometrial contractility and preliminary mass spectrometric determination of secondary metabolites. J. Ethnopharmacol. 2019, 243, 112087. [Google Scholar] [CrossRef] [PubMed]
- Bafor, E.E.; Prendergast, C.; Wray, S. Justicia flava leaf extract potently relaxes pregnant human myometrial contractility: A lead plant for drug discovery of new tocolytic drugs. Exp. Physiol. 2020, 105, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Yin, J.; Yao, Q.; Wang, T.; Chen, J.; Liang, Q.; Li, Q.; Zhao, X. Development of an Allostery Responsive Chromatographic Method for Screening Potential Allosteric Modulator of Beta2-adrenoceptor from a Natural Product-Derived DNA-Encoded Chemical Library. Anal. Chem. 2022, 94, 9048–9057. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Shi, B.; Yao, Q.; Wang, T.; Ji, X.; Zhang, Y.; Wang, J.; Zhao, X. Early potential evaluation of lead compounds from a DNA-encoded library by the determination of their thermodynamics through a chromatographic method based on immobilized β2-adrenoceptor. Bioorg. Med. Chem. 2022, 68, 116864. [Google Scholar] [CrossRef]
- Yang, F.; Ling, S.; Zhou, Y.; Zhang, Y.; Lv, P.; Liu, S.; Fang, W.; Sun, W.; Hu, L.A.; Zhang, L.; et al. Different conformational responses of the β2-adrenergic receptor-Gs complex upon binding of the partial agonist salbutamol or the full agonist isoprenaline. Natl. Sci. Rev. 2021, 8, nwaa284. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.W.; Harris, J. RX 821002 as a tool for physiological investigation of alpha(2)-adrenoceptors. CNS Drug Rev. 2002, 8, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Claustre, Y.; Peretti, D.D.; Brun, P.; Gueudet, C.; Allouard, N.; Alonso, R.; Lourdelet, J.; Oblin, A.; Damoiseau, G.; Françon, D.; et al. SSR181507, a dopamine D(2) receptor antagonist and 5-HT(1A) receptor agonist. I: Neurochemical and electrophysiological profile. Neuropsychopharmacology 2003, 28, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Vergoten, G.; Bailly, C. Insights into the Mechanism of Action of the Degraded Limonoid Prieurianin. Int. J. Mol. Sci. 2024, 25, 3597. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Binding of Vialinin A and p-Terphenyl Derivatives to Ubiquitin-Specific Protease 4 (USP4): A Molecular Docking Study. Molecules 2022, 27, 5909. [Google Scholar] [CrossRef]
- Gangaram, S.; Naidoo, Y.; Dewir, Y.H.; El-Hendawy, S. Phytochemicals and Biological Activities of Barleria (Acanthaceae). Plants 2021, 11, 82. [Google Scholar] [CrossRef]
- Dirar, A.I.; Adhikari-Devkota, A.; Kunwar, R.M.; Paudel, K.R.; Belwal, T.; Gupta, G.; Chellappan, D.K.; Hansbro, P.M.; Dua, K.; Devkota, H.P. Genus Blepharis (Acanthaceae): A review of ethnomedicinally used species, and their phytochemistry and pharmacological activities. J. Ethnopharmacol. 2021, 265, 113255. [Google Scholar] [CrossRef]
- Matos, P.; Batista, M.T.; Figueirinha, A. A review of the ethnomedicinal uses, chemistry, and pharmacological properties of the genus Acanthus (Acanthaceae). J. Ethnopharmacol. 2022, 293, 115271. [Google Scholar] [CrossRef]
- Yaradua, S.S.; Yessoufou, K. Chloroplast genome of Ecbolium viride (Forssk.) Alston: Plastome evolution and phylogenomics of Justiceae (Acanthaceae, Acanthoideae). Genome 2024, 67, 267–280. [Google Scholar] [CrossRef]
- Manzitto-Tripp, E.A.; Darbyshire, I.; Daniel, T.F.; Kiel, C.A.; McDade, L.A. Revised classification of Acanthaceae and worldwide dichotomous keys. Taxon 2022, 71, 103–153. [Google Scholar] [CrossRef]
- Ambrosini, L.M.; Cernak, T.A.; Lambert, T.H. Total synthesis of the tylophora alkaloids rusplinone, 13aα-secoantofine, and antofine using a multicatalytic oxidative aminochlorocarbonylation/Friedel–Crafts reaction. Tetrahedron 2010, 66, 4882–4887. [Google Scholar] [CrossRef]
- Scott, L.J. Eliglustat: A Review in Gaucher Disease Type 1. Drugs 2015, 75, 1669–1678. [Google Scholar] [CrossRef]
- Dong, L.; Cao, Z.; Chen, M.; Liu, Y.; Ma, X.; Lu, Y.; Zhang, Y.; Feng, K.; Zhang, Y.; Meng, Z.; et al. Inhibition of glycosphingolipid synthesis with eliglustat in combination with immune checkpoint inhibitors in advanced cancers: Preclinical evidence and phase I clinical trial. Nat Commun. 2024, 15, 6970. [Google Scholar] [CrossRef] [PubMed]
- Poyraz, S.; Döndaş, H.A.; Döndaş, N.Y.; Sansano, J.M. Recent insights about pyrrolidine core skeletons in pharmacology. Front. Pharmacol. 2023, 14, 1239658. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Singh, I.; Tandon, N.; Tandon, R. Structure activity relationship (SAR) and anticancer activity of pyrrolidine derivatives: Recent developments and future prospects (A review). Eur. J. Med. Chem. 2023, 246, 114954. [Google Scholar] [CrossRef]
- Li Petri, G.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Top Curr. Chem. 2021, 379, 34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).