Use of Live Biopreservatives and Bacteriophages to Enhance the Safety of Meat Products
Abstract
1. Introduction
2. Antimicrobial Innovations
3. Use of Biopreservatives to Enhance Food Quality and Safety
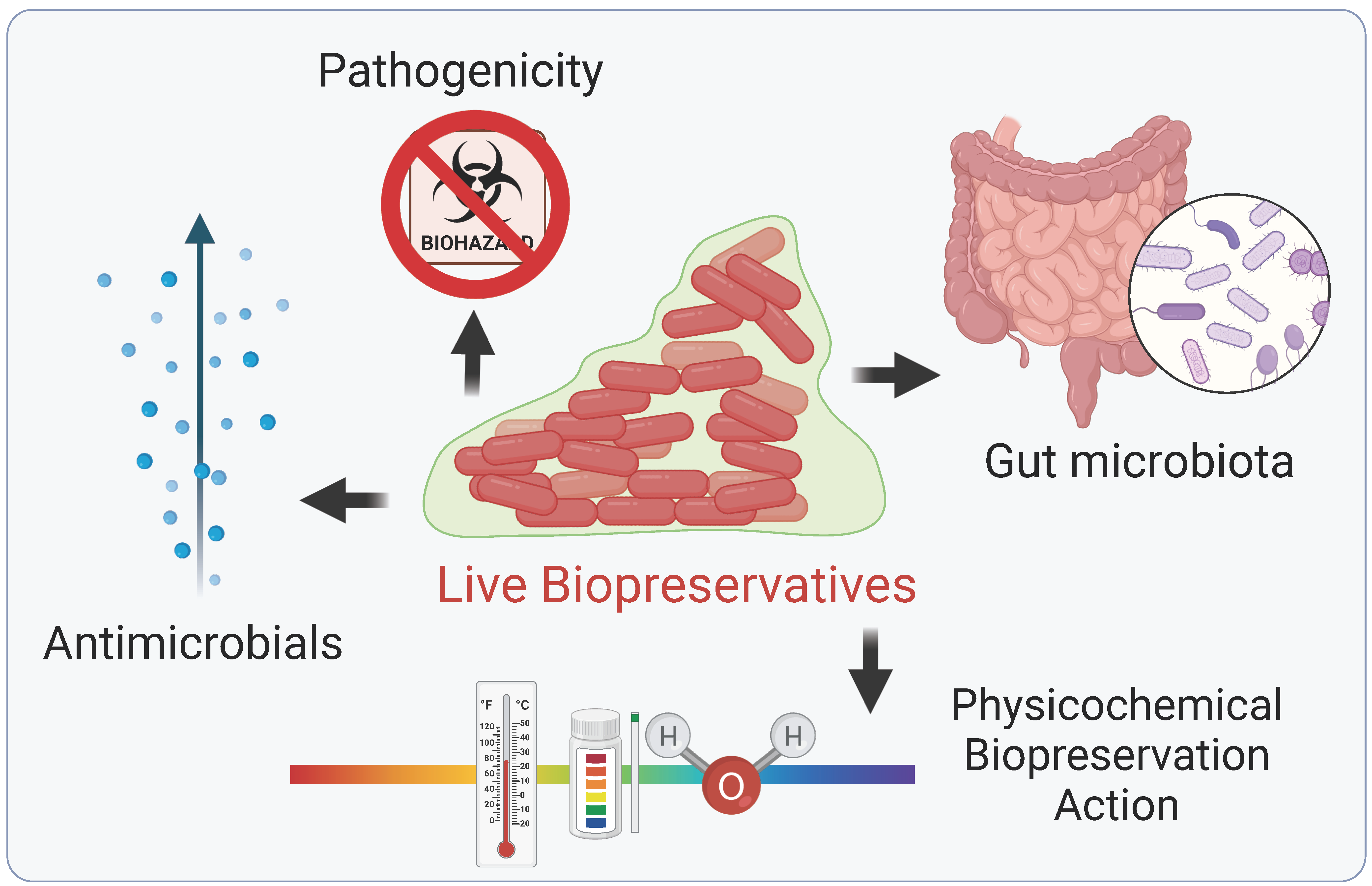
4. Live Biopreservatives (LBs)
5. Lactic Acid Bacteria to Enhance Meat Product Safety
6. Bacteriophages to Enhance Food Safety
7. Biodegradable Food Packaging for Meat Products
8. Future Perspectives
8.1. Scaling Up: Use of LAB and Bacteriocins in Real Meat Products
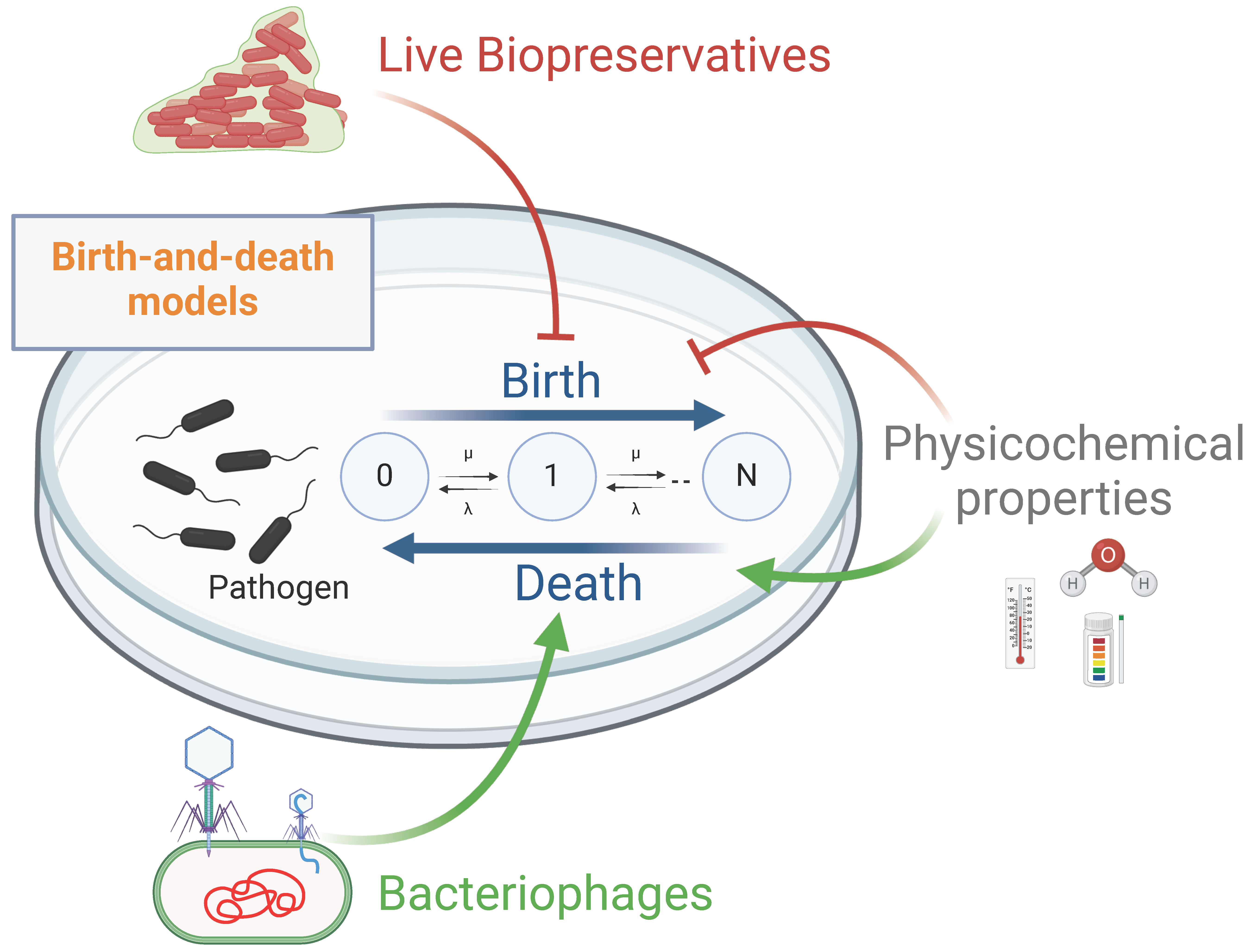
8.2. Computational Models for Assessing the Use of LBs and Bacteriophages
8.3. Assessment of the Potential Impact of LBs on Antimicrobial Resistance
8.4. Uses of LBs and Bacteriophages with Biodegradable Packaging for Meat
8.5. Socioeconomic Implications of LBs and Bacteriophages
9. Conclusions
- Is the combination of LBs (bacteriostatic) and bacteriophages (bacteriolytic) the most suitable strategy to improve quality and meat safety?
- What is the impact of combining LBs and bacteriophages on the physicochemical, technological, and sensory properties of both fresh and fermented meat?
- How do LBs and bacteriophages perform in real meat products, considering food complexities?
- How does the combination of LBs with biodegradable food packaging improve meat safety and sustainability?
- What potential interactions can occur between LBs (and bacteriophages) with environmental plastic-degrading microbes on biodegradable plastic packaging materials?
- Does the use of LBs (and bacteriophages) pose a potential risk for the spread of antimicrobial resistance?
- Can mixed strategies involving LBs and bacteriophages effectively address the issue of reducing nitrites and nitrates in meat preservation?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- McMullen, L.M.; Stiles, M.E. Potential for Use of Bacteriocin-Producing Lactic Acid Bacteria in the Preservation of Meats. J. Food Prot. 1996, 59, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Wang, N.; Xiao, B.; Sun, X.; Li, J.; Zhong, K.; Yang, L.; Pang, X.; Huang, F.; et al. Critical review and recent advances of emerging real-time and non-destructive strategies for meat spoilage monitoring. Food Chem. 2024, 445, 138755. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Hogstrand, C.; Ron Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S.; et al. Risk assessment of N-nitrosamines in food. EFSA J. 2023, 21, e07884. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, G.; Barbieri, F.; Soglia, F.; Magnani, R.; Gardini, G.; Petracci, M.; Gardini, F.; Montanari, C. Safety and technological issues of dry fermented sausages produced without nitrate and nitrite. Food Res. Int. 2022, 160, 111685. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Romero, J.L.; Grande Burgos, M.J.; Pérez-Pulido, R.; Gálvez, A.; Lucas, R. Resistance to Antibiotics, Biocides, Preservatives and Metals in Bacteria Isolated from Seafoods: Co-Selection of Strains Resistant or Tolerant to Different Classes of Compounds. Front. Microbiol. 2017, 8, 1650. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Life|Special Issue: Antimicrobial Innovations. Available online: https://www.mdpi.com/journal/life/special_issues/IBAM7WRCGO (accessed on 14 December 2024).
- Muthuvelu, K.S.; Ethiraj, B.; Pramnik, S.; Raj, N.K.; Venkataraman, S.; Rajendran, D.S.; Bharathi, P.; Palanisamy, E.; Narayanan, A.S.; Vaidyanathan, V.K.; et al. Biopreservative technologies of food: An alternative to chemical preservation and recent developments. Food Sci. Biotechnol. 2023, 32, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P.; Omatola, C.A.; Olaniran, A.O. Applications of Lactic Acid Bacteria and Their Bacteriocins against Food Spoilage Microorganisms and Foodborne Pathogens. Molecules 2021, 26, 7055. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P. Recent approaches in food bio-preservation—A review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Bukvicki, D.; D’Alessandro, M.; Rossi, S.; Siroli, L.; Gottardi, D.; Braschi, G.; Patrignani, F.; Lanciotti, R. Essential Oils and Their Combination with Lactic Acid Bacteria and Bacteriocins to Improve the Safety and Shelf Life of Foods: A Review. Foods 2023, 12, 3288. [Google Scholar] [CrossRef] [PubMed]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Lee, N.-K.; Paik, H.-D. Status, antimicrobial mechanism, and regulation of natural preservatives in livestock food systems. Korean J. Food Sci. Anim. Resour. 2016, 36, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kasimin, M.E.; Shamsuddin, S.; Molujin, A.M.; Sabullah, M.K.; Gansau, J.A.; Jawan, R. Enterocin: Promising biopreservative produced by Enterococcus sp. Microorganisms 2022, 10, 684. [Google Scholar] [CrossRef]
- Ma, J.; Yu, W.; Hou, J.; Han, X.; Shao, H.; Liu, Y. Characterization and production optimization of a broad-spectrum bacteriocin produced by Lactobacillus casei KLDS 1.0338 and its application in soybean milk biopreservation. Int. J. Food Prop. 2020, 23, 677–692. [Google Scholar] [CrossRef]
- Webb, L.; Ma, L.; Lu, X. Impact of lactic acid bacteria on the control of Listeria monocytogenes in ready-to-eat foods. Food Qual. Saf. 2022, 6, fyac045. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Wu, T.; Song, Y.; Li, Y.; Huang, X.; Lu, H.; Xu, Z.Z. Systematic evaluation of antimicrobial food preservatives on glucose metabolism and gut microbiota in healthy mice. npj Sci. Food 2022, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Growth of spoilage bacteria during storage and transport of meat. EFSA J. 2016, 14, e04523. [Google Scholar] [CrossRef]
- Sirini, N.; Loyeau, P.; Ruiz, M.; Stegmayer, M.; Soto, L.; Werning, M.; Frizzo, L.; Ordoñez, V.; Fernández-López, J.; Rosmini, M. Development of Probiotic Fermented Sausages and Viability Monitoring of Supplemented Lactiplantibacillus plantarum BFL Strain. Fermentation 2022, 8, 526. [Google Scholar] [CrossRef]
- Tisserand, P. Use of Probiotics in Meat. WO2015048899A1, 9 April 2015. Available online: https://patents.google.com/patent/WO2015048899A1/en (accessed on 13 June 2023).
- Rouhi, M.; Sohrabvandi, S.; Mortazavian, A.M. Probiotic fermented sausage: Viability of probiotic microorganisms and sensory characteristics. Crit. Rev. Food Sci. Nutr. 2013, 53, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Bachtarzi, N.; Gomri, M.A.; Meradji, M.; Gil-Cardoso, K.; Ortega, N.; Chomiciute, G.; Del Bas, J.M.; López, Q.; Martínez, V.; Kharroub, K. In vitro assessment of biofunctional properties of Lactiplantibacillus plantarum strain Jb21-11 and the characterization of its exopolysaccharide. Int. Microbiol. 2023, 27, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, J.C.; Martínez-Tapia, A.; Lazcano-Hernández, G.; García-Pérez, B.E.; Castrejón-Jiménez, N.S. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals 2021, 11, 979. [Google Scholar] [CrossRef]
- Vera Peña, M.Y.; Cortés Rodríguez, M.; Valencia-García, F.E. Secado por atomización de bacterias ácido lácticas: Una revisión. Ing. Cienc. 2019, 15, 179–213. [Google Scholar] [CrossRef]
- Fischer, S.W.; Titgemeyer, F. Protective cultures in food products: From science to market. Foods 2023, 12, 1541. [Google Scholar] [CrossRef]
- Austrich-Comas, A.; Serra-Castelló, C.; Jofré, A.; Gou, P.; Bover-Cid, S. Control of Listeria monocytogenes in chicken dry-fermented sausages with bioprotective starter culture and high-pressure processing. Front. Microbiol. 2022, 13, 983265. [Google Scholar] [CrossRef]
- Serra-Castelló, C.; Costa, J.C.C.P.; Jofré, A.; Bolívar, A.; Pérez-Rodríguez, F.; Bover-Cid, S. A mathematical model to predict the antilisteria bioprotective effect of Latilactobacillus sakei CTC494 in vacuum packaged cooked ham. Int. J. Food Microbiol. 2022, 363, 109491. [Google Scholar] [CrossRef]
- Kaveh, S.; Hashemi, S.M.B.; Abedi, E.; Amiri, M.J.; Conte, F.L. Bio-Preservation of Meat and Fermented Meat Products by Lactic Acid Bacteria Strains and Their Antibacterial Metabolites. Sustainability 2023, 15, 10154. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, T.-T.; Guo, R.-R.; Ye, Q.; Zhao, H.-L.; Huang, X.-H. The regulation of key flavor of traditional fermented food by microbial metabolism: A review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- García, P.; Martínez, B.; Obeso, J.M.; Rodríguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Ebner, P.D.; Syed, Q.A.; Ubaid ur Rahman, H. Employing list-shield bacteriophage as a bio-control intervention for Listeria monocytogenes from raw beef surface and maintain meat quality during refrigeration storage. LWT 2020, 132, 109784. [Google Scholar] [CrossRef]
- Amjad, N.; Naseer, M.S.; Imran, A.; Menon, S.V.; Sharma, A.; Islam, F.; Tahir, S.; Shah, M.A. A mini-review on the role of bacteriophages in food safety. CyTA-J. Food 2024, 22, 2357192. [Google Scholar] [CrossRef]
- Garvey, M. Bacteriophages and Food Production: Biocontrol and Bio-Preservation Options for Food Safety. Antibiotics 2022, 11, 1324. [Google Scholar] [CrossRef]
- Ranveer, S.A.; Dasriya, V.; Ahmad, M.F.; Dhillon, H.S.; Samtiya, M.; Shama, E.; Anand, T.; Dhewa, T.; Chaudhary, V.; Chaudhary, P.; et al. Positive and negative aspects of bacteriophages and their immense role in the food chain. npj Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef]
- Moon, S.H.; Waite-Cusic, J.; Huang, E. Control of Salmonella in chicken meat using a combination of a commercial bacteriophage and plant-based essential oils. Food Control 2020, 110, 106984. [Google Scholar] [CrossRef]
- Yeh, Y.; Purushothaman, P.; Gupta, N.; Ragnone, M.; Verma, S.C.; de Mello, A.S. Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products. Meat Sci. 2017, 127, 30–34. [Google Scholar] [CrossRef]
- Mangieri, N.; Picozzi, C.; Cocuzzi, R.; Foschino, R. Evaluation of a Potential Bacteriophage Cocktail for the Control of Shiga-Toxin Producing Escherichia coli in Food. Front. Microbiol. 2020, 11, 1801. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Gliźniewicz, M.; Olszewska, P.; Miłek, D.; Czajkowski, A.; Serwin, N.; Cecerska-Heryć, E.; Rakoczy, R. Response Surface Methodology Application for Bacteriophage-Antibiotic Antibiofilm Activity Optimization. Microorganisms 2023, 11, 2352. [Google Scholar] [CrossRef] [PubMed]
- Kandeepan, G. Biodegradable nanocomposite packaging films for meat and meat products: A review. J. Packag. Technol. Res. 2021, 5, 143–166. [Google Scholar] [CrossRef]
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- Dörnyei, K.R.; Uysal-Unalan, I.; Krauter, V.; Weinrich, R.; Incarnato, L.; Karlovits, I.; Colelli, G.; Chrysochou, P.; Fenech, M.C.; Pettersen, M.K.; et al. Sustainable food packaging: An updated definition following a holistic approach. Front. Sustain. Food Syst. 2023, 7, 1119052. [Google Scholar] [CrossRef]
- Herbes, C.; Beuthner, C.; Ramme, I. How green is your packaging—A comparative international study of cues consumers use to recognize environmentally friendly packaging. Int. J. Consum. Stud. 2020, 44, 258–271. [Google Scholar] [CrossRef]
- A Critical Review on Biodegradable Food Packaging for Meat: Materials, Sustainability, Regulations, and Perspectives in the EU|Request PDF. Available online: https://www.researchgate.net/publication/371534548_A_critical_review_on_biodegradable_food_packaging_for_meat_Materials_sustainability_regulations_and_perspectives_in_the_EU (accessed on 15 June 2023).
- Back, A.; Borges, F.; Mangavel, C.; Paris, C.; Rondags, E.; Kapel, R.; Aymes, A.; Rogniaux, H.; Pavlović, M.; van Heel, A.J.; et al. Recombinant pediocin in Lactococcus lactis: Increased production by propeptide fusion and improved potency by co-production with PedC. Microb. Biotechnol. 2016, 9, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, T.M.; O’Connor, P.M.; Lawton, E.; Thapa, D.; Mesa-Pereira, B.; Abulu, S.; Hill, C.; Ross, R.P.; Oliveira, R.P.S.; Cotter, P.D. An oxidation resistant pediocin PA-1 derivative and penocin A display effective anti-Listeria activity in a model human gut environment. Gut Microbes 2022, 14, 2004071. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.; Todorov, S.D.; Chihib, N.E.; Drider, D.; Nero, L.A. Lactic Acid Bacteria (LAB) and Their Bacteriocins as Alternative Biotechnological Tools to Control Listeria monocytogenes Biofilms in Food Processing Facilities. Mol. Biotechnol. 2018, 60, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.; McMeekin, T.A. Predictive microbiology. Int. J. Food Microbiol. 1994, 23, 241–264. [Google Scholar] [CrossRef]
- Aspridou, Z.; Koutsoumanis, K. Variability in microbial inactivation: From deterministic Bigelow model to probability distribution of single cell inactivation times. Food Res. Int. 2020, 137, 109579. [Google Scholar] [CrossRef]
- González, S.C.; Possas, A.; Carrasco, E.; Valero, A.; Bolívar, A.; Posada-Izquierdo, G.D.; García-Gimeno, R.M.; Zurera, G.; Pérez-Rodríguez, F. “MicroHibro”: A software tool for predictive microbiology and microbial risk assessment in foods. Int. J. Food Microbiol. 2019, 290, 226–236. [Google Scholar] [CrossRef]
- Crawford, F.W.; Ho, L.S.T.; Suchard, M.A. Computational methods for birth-death processes. Wiley Interdiscip. Rev. Comput. Stat. 2018, 10, e1423. [Google Scholar] [CrossRef] [PubMed]
- Puigbò, P.; Lobkovsky, A.E.; Kristensen, D.M.; Wolf, Y.I.; Koonin, E.V. Genomes in turmoil: Quantification of genome dynamics in prokaryote supergenomes. BMC Biol. 2014, 12, 66. [Google Scholar] [CrossRef]
- Shim, H.; Fishwick, P.A. Visualization and interaction design for ecosystem modeling. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3685–3693. ISBN 9780080454054. [Google Scholar]
- Wachenheim, D.E.; Patterson, J.A.; Ladisch, M.R. Analysis of the logistic function model: Derivation and applications specific to batch cultured microorganisms. Bioresour. Technol. 2003, 86, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, P. Analytical solution for a hybrid Logistic-Monod cell growth model in batch and continuous stirred tank reactor culture. Biotechnol. Bioeng. 2020, 117, 873–878. [Google Scholar] [CrossRef]
- Puigbò, P.; Leino, L.I.; Rainio, M.J.; Saikkonen, K.; Saloniemi, I.; Helander, M. Does glyphosate affect the human microbiota? Life 2022, 12, 707. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The spread of antibiotic resistance genes in vivo model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef]
- Cen, T.; Zhang, X.; Xie, S.; Li, D. Preservatives accelerate the horizontal transfer of plasmid-mediated antimicrobial resistance genes via differential mechanisms. Environ. Int. 2020, 138, 105544. [Google Scholar] [CrossRef]
- Liu, J.; Gefen, O.; Ronin, I.; Bar-Meir, M.; Balaban, N.Q. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 2020, 367, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Song, H.; Lu, J.; Yuan, Z.; Guo, J. Copper nanoparticles and copper ions promote horizontal transfer of plasmid-mediated multi-antibiotic resistance genes across bacterial genera. Environ. Int. 2019, 129, 478–487. [Google Scholar] [CrossRef]
- Sharma, S.; Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Natural antimicrobials from fruits and plant extract for food packaging and preservation. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2024; pp. 133–152. ISBN 9780323900447. [Google Scholar]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Yang, X.-G.; Wen, P.-P.; Yang, Y.-F.; Jia, P.-P.; Li, W.-G.; Pei, D.-S. Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects. Front. Microbiol. 2022, 13, 1001750. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Wei, J.; Jiang, L.; Jiang, L.; Sun, Y.; Zeng, Z.; Wang, Z. Phage-inspired strategies to combat antibacterial resistance. Crit. Rev. Microbiol. 2024, 50, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of phage therapy across the world. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef] [PubMed]
- McCammon, S.; Makarovs, K.; Banducci, S.; Gold, V. Phage therapy and the public: Increasing awareness essential to widespread use. PLoS ONE 2023, 18, e0285824. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.V.; Martins, E.; Moreira, I.M.F.B.; de Carvalho, A.F. Strategies for the development of bioprotective cultures in food preservation. Int. J. Microbiol. 2022, 2022, 6264170. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Marca, C.; Domenech-Coca, C.; Nakamura, M.; Ortega-Olivé, N.; Puigbò, P. Use of Live Biopreservatives and Bacteriophages to Enhance the Safety of Meat Products. Life 2025, 15, 197. https://doi.org/10.3390/life15020197
Rodríguez-Marca C, Domenech-Coca C, Nakamura M, Ortega-Olivé N, Puigbò P. Use of Live Biopreservatives and Bacteriophages to Enhance the Safety of Meat Products. Life. 2025; 15(2):197. https://doi.org/10.3390/life15020197
Chicago/Turabian StyleRodríguez-Marca, Cristina, Cristina Domenech-Coca, Miho Nakamura, Nàdia Ortega-Olivé, and Pere Puigbò. 2025. "Use of Live Biopreservatives and Bacteriophages to Enhance the Safety of Meat Products" Life 15, no. 2: 197. https://doi.org/10.3390/life15020197
APA StyleRodríguez-Marca, C., Domenech-Coca, C., Nakamura, M., Ortega-Olivé, N., & Puigbò, P. (2025). Use of Live Biopreservatives and Bacteriophages to Enhance the Safety of Meat Products. Life, 15(2), 197. https://doi.org/10.3390/life15020197






