Abstract
Endometriosis is a multifaceted gynecological disorder defined by endometrium-like tissue outside the uterine cavity. It is mainly localized in the pelvis and creates a local inflammatory environment responsible for its manifestations and complications. In 30–50% of cases, endometriosis is associated with infertility. In 17–44% of cases, the ovaries are affected in the form of ovarian endometriomas (OEs). The symptoms of OEs are not very pronounced. The development is slow. Diagnosis is difficult because OEs resemble cystic ovarian pathology, which is so diverse. The actual diagnosis is possible through direct visualization or laparoscopy. Surgical treatment by cystectomy is common for OEs. Recently, other therapeutic modalities have emerged that have less impact on ovarian reserves and pregnancy rates. In this context, the review attempts to shed light on the best diagnostic and treatment methods for an insidious pathology with a major impact on fertility.
1. Introduction
Endometriosis is a systemic disease dependent on estrogen, characterized by pelvic pain and the impact on infertility, in which tissue resembling the inner lining of the uterus spreads outside the uterus [1]. This leads to an inflammatory condition due to endometrial-like glands and stroma in an abnormal location. The fallopian tubes, ovaries, and tissue lining the pelvis are often affected.
Endometriosis occurs most frequently in the pelvic region, but there are deep-infiltrating endometriosis, superficial implants, and solid nodules inside and outside the pelvis [2,3]. Implants can also occur throughout the abdomen, including the colon, in previous surgical wounds, and, in rare instances, in remote areas of the body, such as the cerebellum [4].
Typically, three different clinical manifestations are superficial endometrial implants in the peritoneum, OEs, and endometriotic nodules [5]. OEs consist of endometrium-like tissue in the form of ovarian cysts. They may be either invagination cysts or true cysts, with the cyst wall also containing endometrium-like tissue. These tumors are usually known as ‘chocolate cysts’ because of the viscous dark brown fluid they contain [6,7].
OEs signify a more advanced disease state in individuals with endometriosis and may result in complications, including diminished ovarian reserves [8,9].
Endometriosis is associated with an increased risk of infertility. Around 30–50% of people with endometriosis can become infertile. The probability of monthly conception in people without endometriosis is around 10–20%, while the probability in people with surgically confirmed endometriosis is around 1–10% [10]. In a population affected by subfertility, 17% have OEs [3,11].
This review aims to systematize the current knowledge on ovarian endometriomas (OEs), with a focus on etiology, diagnosis, and treatment for fertility preservation.
2. Epidemiology
Endometriosis mainly affects women of childbearing age. The estimated prevalence of endometriosis during a woman’s reproductive years is between 1 and 4%. OEs occur in 17–44% of women with endometriosis [12,13]. Bilateral OEs occur in 28% of these women [3].
The incidence of endometriosis is six times higher among first-degree relatives of women with severe endometriosis [14].
There are insufficient data to identify clear risk factors exclusively for OEs. Nonetheless, established general risk factors exist for the onset of endometriosis. Factors include nulliparity, early menarche, late menopause, short menstrual cycles, heavy menstrual bleeding, Müllerian anomalies, low body mass index, height exceeding 170 cm, high intake of trans-unsaturated fats, and in utero exposure to diethylstilbestrol [15,16,17]. A recent study found that the familial influence on the prevalence of endometriosis is negligible [18].
In addition to the risk factors associated with endometriosis, the disease carries other risks for patients, including infertility, persistent pelvic discomfort, dyspareunia, and dysmenorrhea.
3. Etiology
The many mechanisms of endometriosis development can explain the phenotypic heterogeneity observed in OEs.
Several theories have been described with supporting evidence.
- 1.
- Retrograde menstruation is the most widely accepted explanation [19]. Sampson hypothesizes that viable cells from the peritoneal fluid derived from retrograde menstruation can engraft, proliferate, and invade the peritoneal cavity [20]. Cells released from the endometrium during menstruation include epithelial cells, stromal fibroblasts/decidual cells, vascular cells, and various immune cells [21,22].
These cells can adhere to the surfaces of the pelvic organs. There, they can proliferate, thicken, and bleed with each menstrual cycle. Surprisingly, however, during menstruation, these cells do not adhere to the vaginal walls, which consist of stratified squamous, nonkeratinized epithelium and a high-pH vaginal environment. The prevalence of endometriosis in patients increases with the number of ovulatory menstrual cycles and with increasing life expectancy. Contradictions arise from the fact that partial retrograde menstruation is observed in most patients, although only 10% develop endometriosis [19].
- 2.
- Embryonic Müllerian remnants. This concept states that residual embryonic Müllerian migration cells retain the ability to grow into endometriotic lesions under the influence of estrogen, beginning around puberty or possibly in response to estrogen mimetics [12]. Epidemiologic studies indicate a twofold increased risk of endometriosis in women exposed to diethylstilbestrol in utero [23].
- 3.
- Genetic and epigenetic theory. Changes in the genetic regulatory networks have been found in the stromal cells of the endometrium [24]. Epigenetic alterations found in endometriosis include genomic DNA methylation [25]. In addition, aberrant DNA methylation in endometriosis induces the expression of many genes, including homeobox A10 (HOXA10), an estrogen beta receptor gene, a progesterone receptor, aromatase, HOXC6, and ALDH1A2 [26]. HOXA-10 is an important regulator of two essential processes during implantation: stromal cell proliferation and local immunosuppression [27].
- 4.
- Hematogenous and lymphatic spread. Another idea is that the menstrual tissue diffuses from the uterine cavity to distant areas via lymphatic vessels and veins, which explains the presence of implants outside the pelvis [28]. The blood contains endometrial cells derived from the endometrium. Microvascular studies revealed lymphatic movements from the uterine body to the ovary, indicating a possible involvement of the lymphatic system in the etiology of OEs [29].
- 5.
- Celomic metaplasia. Ferguson hypothesized that celomic metaplasia may play a role in the etiology of endometriosis. It is based on the hypothesis that the peritoneum harbors undifferentiated cells that can differentiate into endometriosis cells [5].
- 6.
- Neuroangiogenesis. Recent studies suggest that ectopic endometriotic implants attract a distinct neuronal and vascular supply via neuroangiogenesis. Endometriotic implants are complex multicellular structures characterized by vascularization that include the development of new blood vessels. It is postulated that the developing nerve fibers in the endometriotic implants influence dorsal root neurons in the central nervous system, thereby enhancing patients’ perception of pain [29,30].
Several chemokines have been shown to be elevated in the peritoneal fluid of women diagnosed with endometriosis. They may play a role in macrophage activation, inflammatory responses, adhesion of endometriotic tissue in the peritoneal cavity, and increased angiogenesis in the progression of endometriosis [31].
- 7.
- Stem cell theory. Progenitor cells within the endometrium and multipotent cells from the osseous sinus contribute to the composition of the eutopic endometrium [32].
- 8.
- Induction theory. Experts believe that hormones or immunologic factors may promote the transformation of peritoneal cells lining the internal abdominal cavity into cells resembling those of the endometrium [33].
In the typical endometrium, the expression of receptors for estrogens, androgens, progestins, and glucocorticoids is both cell-specific and dependent on the phase of the menstrual cycle. Estradiol promotes the synthesis of prostaglandin E2, which, in turn, increases aromatase activity. The expression of aromatase, a crucial enzyme in estrogen production, was detected in lesion-derived stromal cells more than 20 years ago [23].
These findings confirm the ability of endometriotic lesions to synthesize estradiol and validate treatments aimed at promoting a hypoestrogenic peritoneal microenvironment [19,34,35].
4. Infertility Related to OEs
The pathogenesis of OEs mirrors that of endometriosis. In endometriosis, the surrounding pelvic structures stick together, leading to the fusion of the fallopian tubes, ovaries, and intestines. Infertility due to OEs is characterized by deformation of the tubo-ovarian structure, impaired tubal motility, morphological distortion of the fallopian tubes, obstruction of oocyte retrieval, and peritubal adhesions.
Additional factors contributing to infertility in endometriosis include ovulatory dysfunction, abnormal folliculogenesis, anovulation, unruptured follicle syndrome, implantation failure due to endometrial dysfunction, peritoneal factors, local prostaglandin production, impaired coital function due to dyspareunia, and sperm inactivation due to macrophage phagocytosis [18]. The presence of OEs creates a pronounced prooxidant environment that negatively affects nearby ovarian follicles and, consequently, ovarian reserves [36].
The mechanism of this disease process involves the hormonal response of ectopic endometrial tissue. This tissue reacts to the cyclical hormonal fluctuations of a woman’s menstrual cycle similarly to the intrauterine endometrium. It will exhibit proliferative and secretory characteristics and undergo sloughing, like its behavior within the uterus. These oscillations result in differing quantities of cytokines and prostaglandin molecules.
Cytokines and prostaglandins serve as signaling chemicals that initiate an inflammatory response, hence producing inflammation at the site of the endometriotic implantation. This inflammatory reaction establishes the basis for new vascularization and the creation of fibrous tissue. This snowball effect subsequently generates adhesions and discomfort typically linked to this pathological process. These difficulties also result in the primary consequences of this condition, including infertility and chronic pelvic pain.
A significant relation has been demonstrated between OEs and pelvic deep-infiltrating endometriosis [2,37]. Furthermore, it is believed that dysregulation of the biomarkers involved in endometrial receptivity leads to endometrial progesterone resistance, increased cell proliferation, and decreased cell apoptosis, all of which ultimately contribute to subfertility [38].
Molecular, histological, and morphological findings suggest that OEs impair ovarian functions. The mechanism by which the OEs diminish the quantity of functional tissue—whether through a space-occupying action causing mechanical stretching damage or via a direct toxic effect—remains uncertain [32].
The presence of OEs leads to ovarian hypoperfusion and ischemia. The impairment of ovarian vascularity leads to a reduced gonadotropin response and, thus, to a decrease in follicular growth. One reason for the inadequate development of the follicles is seen in the fact that the mechanical pressure prevents the follicles from reaching a diameter of 18 mm [39].
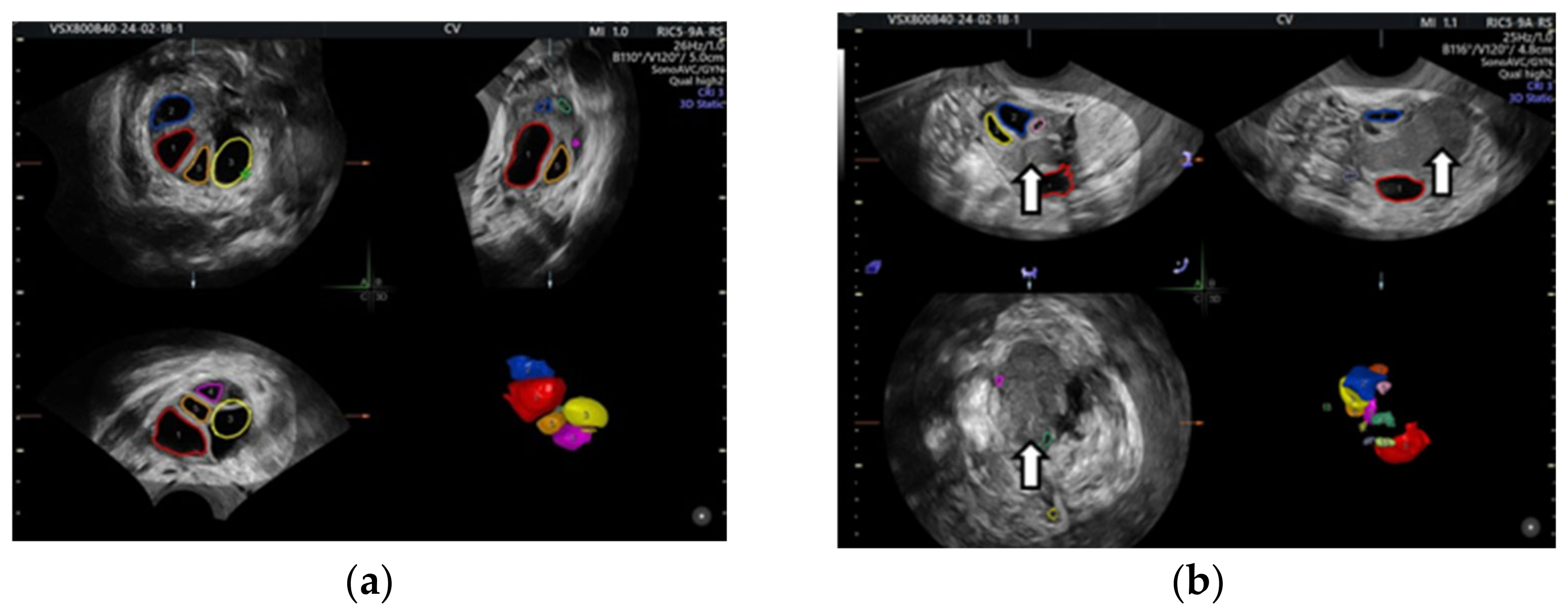
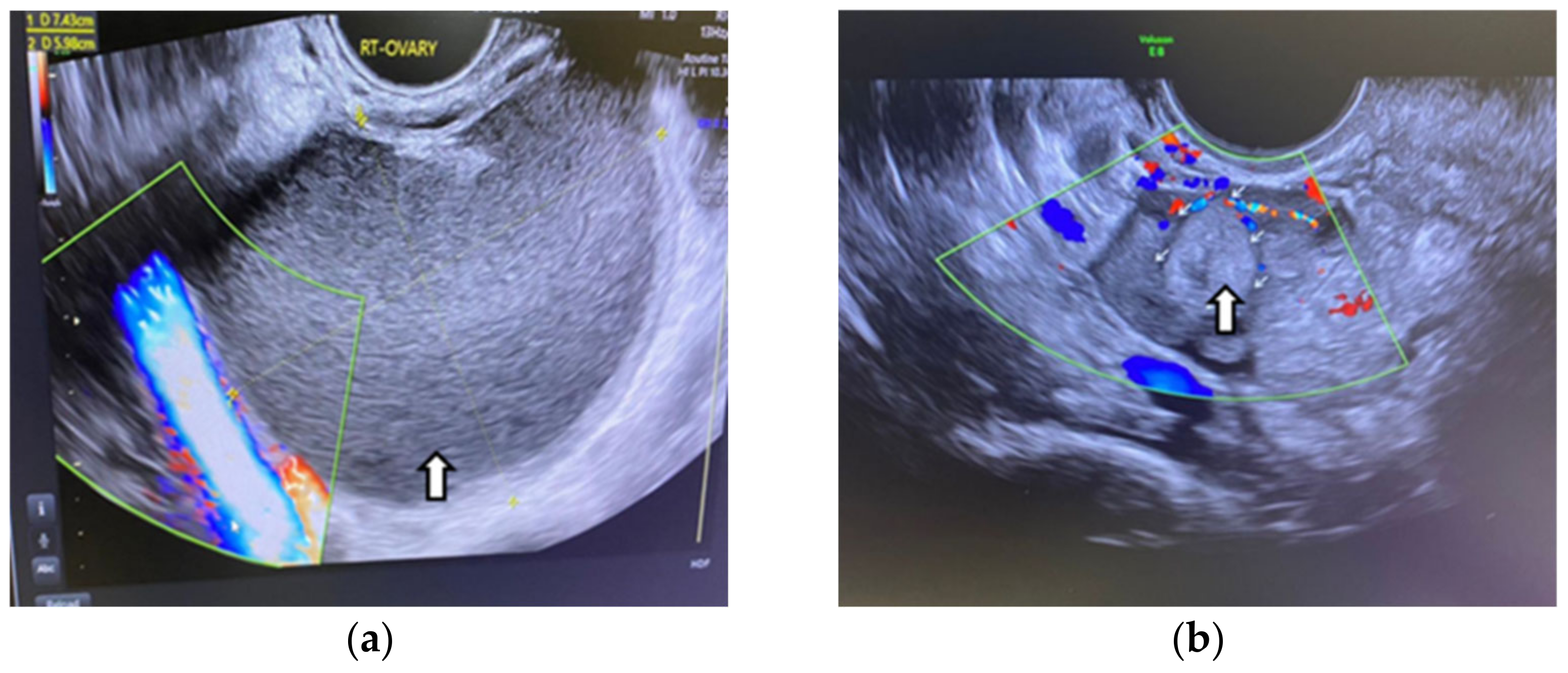
Even in the IVF cycle, the follicles stimulated with gonadotropin cannot grow sufficiently to be punctured. In the following figure, we show the case of a patient whom we stimulated for IVF but who was unable to get follicles to develop due to OE compression (Figure 1).
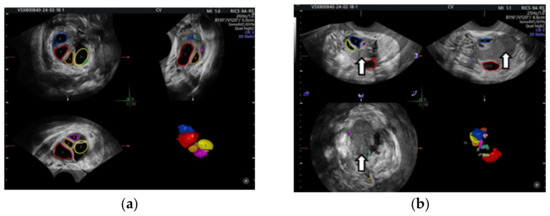
Figure 1.
Color-coded stimulated follicles in the IVF cycle: (a) right ovary with normally growing follicles, (b) in the left ovary the arrows show an ovarian endometrioma compressing the follicles—vaginal 3D ultrasound with SonoAVC software (SonoAVC, automatic volume calculation: GE Medical Systems).
In addition, the presence of OEs made egg retrieval difficult and, in certain cases, made the ovary inaccessible. The ectopic endometrial tissue interferes with the normal intraovarian processes of follicular and oocyte maturation, thereby compromising the quality of the retrieved eggs. This does not appear to affect their ability to fertilize and divide but results in functionally impaired embryos that have difficulty implanting [40].
It is hypothesized that the fluid in the OEs has a high concentration of iron, which triggers cytotoxic oxidative stress that impairs the number of ovarian follicles and leads to their reduction in size [41].
The discharge of toxic cyst contents into the surrounding ovarian parenchyma may result in oxidative stress, fibrosis, the depletion of cortical stroma, smooth muscle cell metaplasia, compromised vascularization, and, in later stages, diminished follicular maturation and atresia in early follicles [42].
Recent studies have shown that the number of primordial follicles in human ovaries decreases with OEs. Luteinized granulosa cells from women with endometriosis exhibit increased apoptosis; granulosa cells from women with endometriosis exhibit a decreased expression of P450 aromatase and show altered progesterone release, possibly impairing proper oocyte maturation [43].
In addition, cryopreserved human oocytes subjected to endometriotic fluid from patients with advanced disease stages exhibited excessive cellular fragmentation in embryos, potentially resulting in compromised embryo development through the induction of apoptosis in adjacent blastomeres or by modifying blastomere division [44,45].
Peritoneal-fluid-derived macrophages can release proteinases that exert a deleterious effect on ovarian tissue and lead to a reduction in ovarian reserve. The main pathophysiological processes are inflammation, fibrosis, adhesions, and surgical sequelae. Anatomical distortions and mechanical variables may hinder oocyte release from the ovary, obstruct tubal ovum pickup or transport, and/or impede sperm transfer into the fallopian tube [46].
5. Symptomatology
The symptoms of OEs must be seen in the context of endometriosis.
Endometriosis can be asymptomatic or symptomatic. Typical manifestations of endometriosis may include menorrhagia, dysmenorrhea, dyspareunia, adenomyosis, gastrointestinal symptoms during menstruation, urinary symptoms during menstruation, increased pelvic mass due to massive OEs, and infertility [2,47,48].
Pain correlates with the extent of tissue infiltration as it is thought to be related to the degree of peritoneal inflammation rather than the number of implants [49].
One of the hypotheses is that painful symptoms in a woman with an OE may be caused by associated deep-infiltrating lesions of endometriosis (DIE). Associations between OEs and DIE are commonly found and can involve two severe forms: intestinal endometriosis and ureteral endometriosis. A histological study of adhesions in women with endometriosis has shown that periovarian adhesions contain endometrial and inflammatory cells that can cause painful symptoms. The evidence for a relation between OEs and painful symptoms is poor [50].
OEs may be scarcely neurotrophic and consequently not significantly related to pain [51]. In fact, scholars did not find nerve fibers in OEs [52]. Pelvic adhesions are probably more important than the OEs’ diameter, which is not correlated with the pain degree [2]. Upon the rupture of an ovarian endometrioma, the viscous endometrial fluid may disseminate throughout the abdominal cavity, resulting in considerable pain and inflammation. These individuals frequently exhibit an acute surgical abdomen [53].
Special attention should be paid in cases with localized adnexal pain to exclude cases of ovarian cancer. Numerous recorded instances exist of OEs occurring within abdominal surgical incision scars. Endometrial implants have been recorded in the lung parenchyma and the brain. Therefore, endometriosis should be considered when a patient reports cyclic discomfort during menstruation, regardless of where the pain occurs [5,54].
Patients with OEs associated with endometriosis have a more severe disease state and generally suffer more from this condition than those with stage one or two of endometriosis [2,37,38].
6. Diagnosis
The diagnosis of endometriosis often begins with the appearance of symptoms. It is based on the patient’s symptoms, physical examination and ultrasound (US) findings, magnetic resonance imaging (MRI), and blood tests. A normal result in these procedures does not rule out the disease.
6.1. Examination of the Pelvis
6.1.1. Physical Examination
A thorough medical history and examination should form the basis of the examination. These are important in addition to the clinical symptoms of infertility described above.
A vaginal examination reveals cystic ovarian tumors, which may be associated with endometriosis, deposits at the vaginal level, and in the rectovaginal space, which is infiltrated. OEs may be palpable on bimanual examination if they are large enough. General pelvic discomfort or localized tenderness in the affected region is often observed. This may also be influenced by the time of the examination of the patient’s menstrual cycle. The patient typically has increased discomfort if the examination occurs shortly before the commencement of her menses. Additional potential findings during the bimanual examination may reveal a fixed or retroverted uterus, indicating scarring attributable to endometriosis. The uterosacral ligaments are hypertrophied, tender, and may be nodular [55,56,57].
Patients presenting after the rupture of OEs may show signs of an acute surgical abdomen on examination [52]. A combination of a physical examination and vaginal ultrasound is preferable [58].
6.1.2. Laboratory Tests
This may include a complete blood count, cancer antigen (CA-125), chemokine receptor (CCR1), urinalysis, and tests for sexually transmitted infections. CA-125 is often elevated in women with endometriosis; however, its specificity for the disease is limited [23]. In situ hybridization has demonstrated a doubling of CCR1 microRNA (miRNA) transcripts from peritoneal cells in endometriosis [59]. During the last decade, new diagnostic tools have been investigated to detect this debilitating disorder as early as possible [60,61,62]. Among these, miRNA analysis uses a saliva-based diagnostic miRNA signature for endometriosis in the diagnosis care pathways after an external validation to confirm these results [63]. An elevated white blood cell count would increase the suspicion of an infectious etiology for the patient’s pelvic pain. Hemoglobin levels can provide insight into the extent of blood loss, as these patients often suffer from menorrhagia and may, therefore, be anemic. Urinalysis is also essential to exclude urinary tract infections, as are tests for sexually transmitted diseases, including cervical cultures.
Histologic evidence is usually obtained by detecting extrauterine endometrial cells during laparoscopy.
6.1.3. Imaging
Originally, the US alone was not able to differentiate OEs from conditions such as tubo-ovarian abscesses, ruptured ectopic pregnancies, or other ovarian cysts and tumors [64,65]. The transvaginal US can accurately detect OEs with a sensitivity of 89% and a specificity of 91% and is considered the preferred imaging modality; however, smaller endometrial implants are not always detected [66].
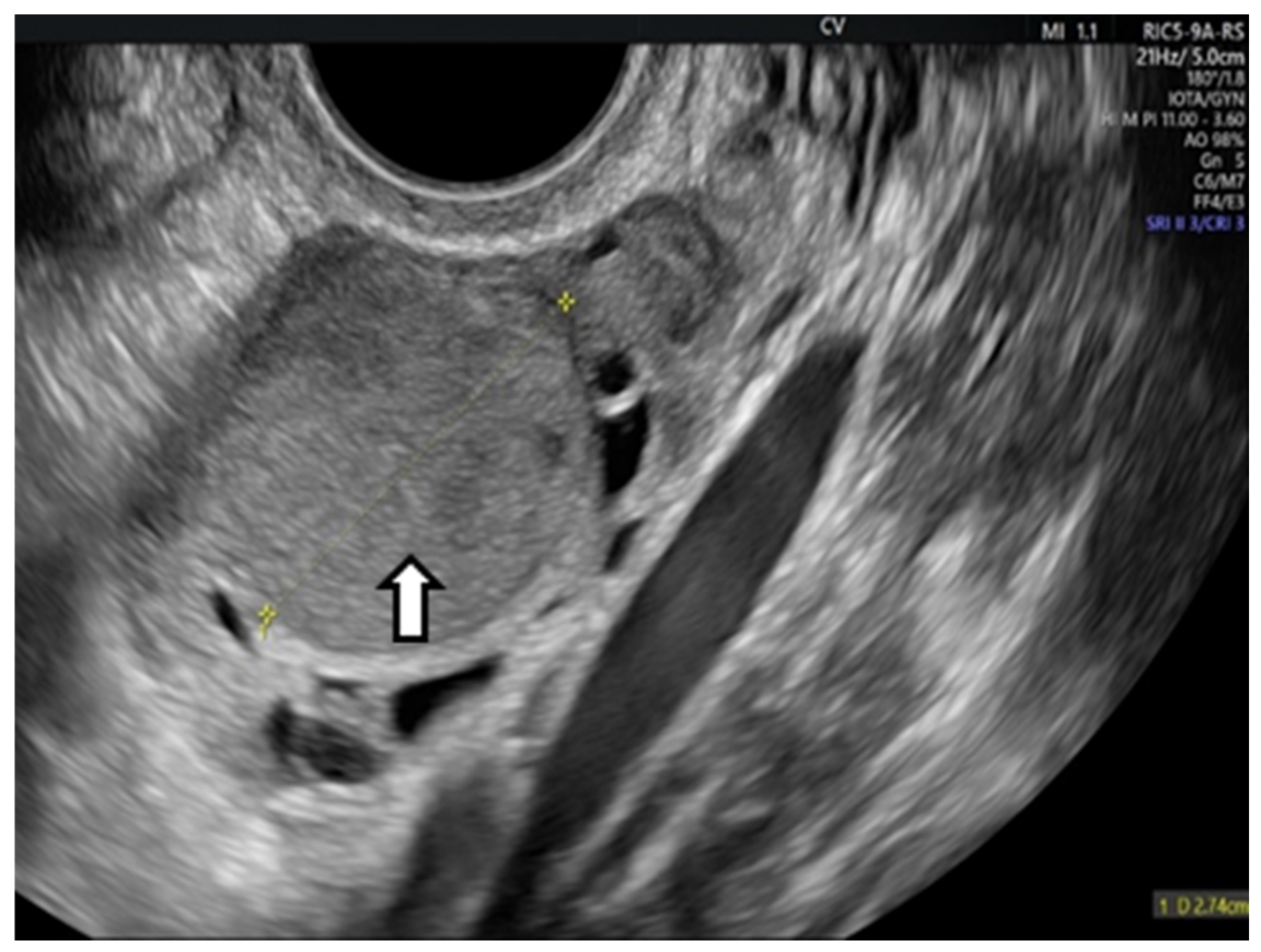
OEs generally manifest as uncomplicated cysts. Nonetheless, they can also be classified as multilocular cysts or cystic solid lesions. The standard US presentation of these lesions has low-level homogeneous echoes, sometimes referred to as a ground-glass appearance. This aligns with previous hemorrhagic debris. These lesions generally lack vascularity when assessed by Doppler flow imaging. In the next figure, we present one of our cases [66,67] (Figure 2).
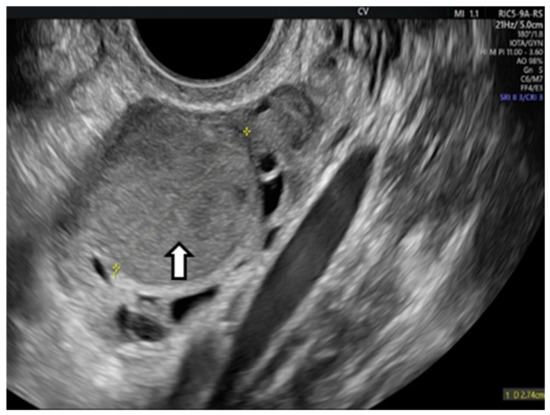
Figure 2.
Vaginal ultrasound image of the ovary. The arrows show the ovarian endometrioma with ground-glass appearance.
Also, the IOTA classification of simple descriptors, simple rules, and expert opinions performs well for classifying adnexal masses suspected to be OEs. The most common potentially malignant masses in these women were borderline ovarian tumors [68].
After the International Deep Endometriosis Analysis group, systemic endometriosis was diagnosed based on the presence of deeply infiltrative endometriotic nodules (for instance, uterosacral ligament, bowel, and bladder nodules), signs of pelvic adhesions (kissing ovaries or absent sliding of viscera, intestinal adhesions), or ovarian endometrioma or overt tubal disease [69,70,71,72,73].
Alternative imaging modalities include magnetic resonance imaging (MRI) and computed tomography (CT). MRI exhibits superior sensitivity in identifying pelvic masses compared to ultrasonography. Nevertheless, the expense of an MRI renders its advantages insufficient to justify the financial strain; hence, the US is utilized more frequently. Like US, an MRI is constrained in identifying widespread pelvic endometriosis and may primarily be advantageous for locating OEs. An MRI helps in the planning of surgery in certain individuals.
A CT scan, while exposing the patient to radiation, can reveal a pelvic mass, and the mass’s characteristics on the scan offer valuable insights into its kind. Consequently, a CT scan is not the optimal imaging technique for these patients.
A real-time dynamic assessment of pelvic organ motion is essential in all cases [64].
6.1.4. Laparoscoy
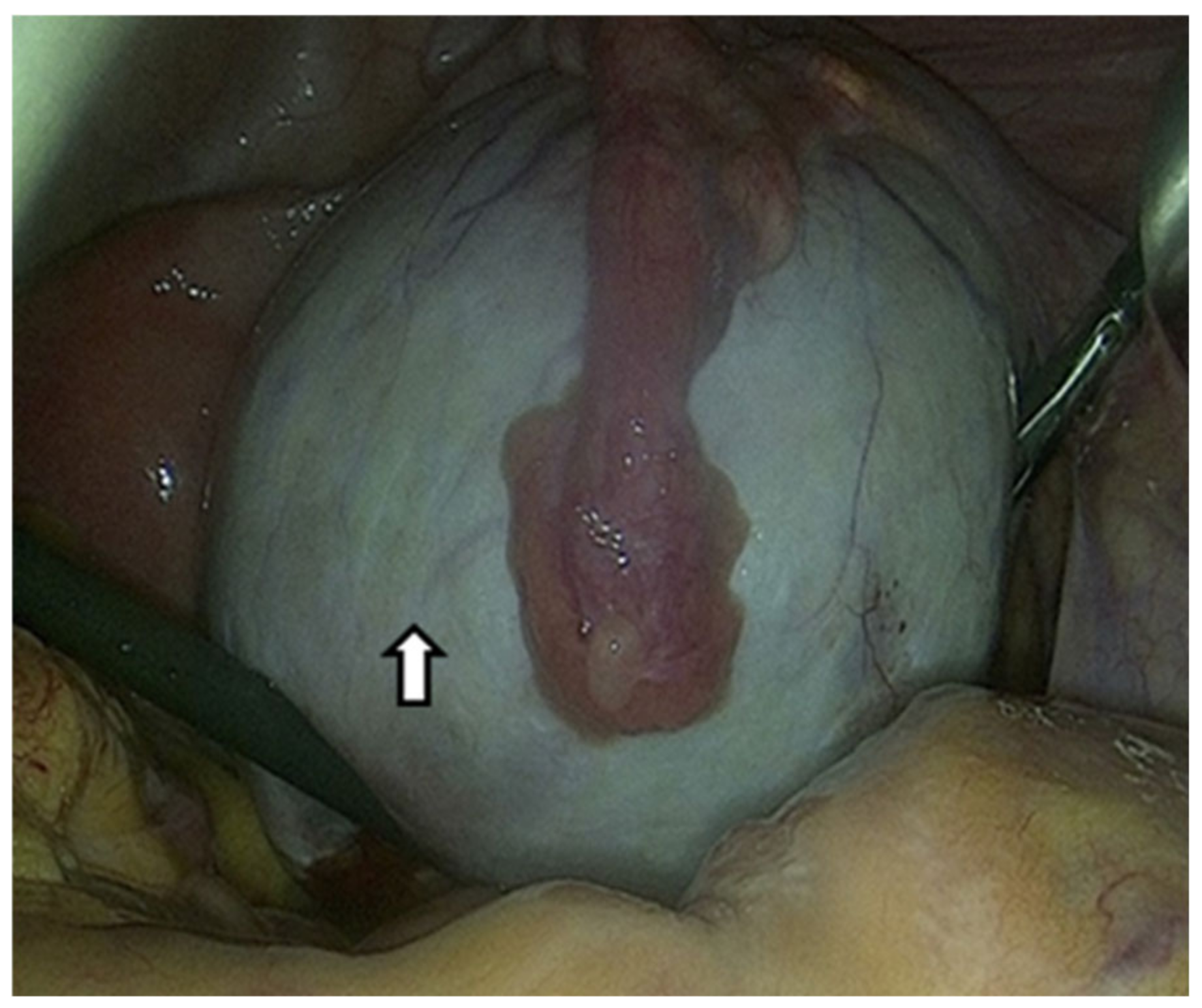
Due to the limited effectiveness of imaging techniques and laboratory tests in identifying endometriosis, the only method to confirm OEs is a surgical procedure that allows direct visualization and tissue sampling [74]. Laparoscopy can provide information on the location, extent, and size of OEs. The following figure shows an OE that we approached laparoscopically (Figure 3).
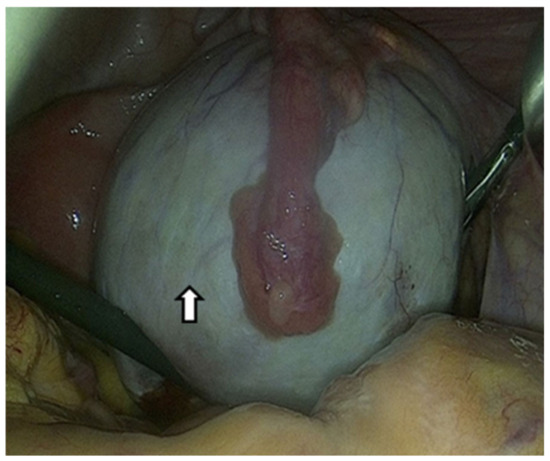
Figure 3.
The arrow shows an ovarian endometrioma that has developed in the ovary and is centrally covered by the fallopian tube laparoscopic image. Suspected diagnosis based on the chocolate content and pathological confirmation by a tissue sample.
6.1.5. Pathology
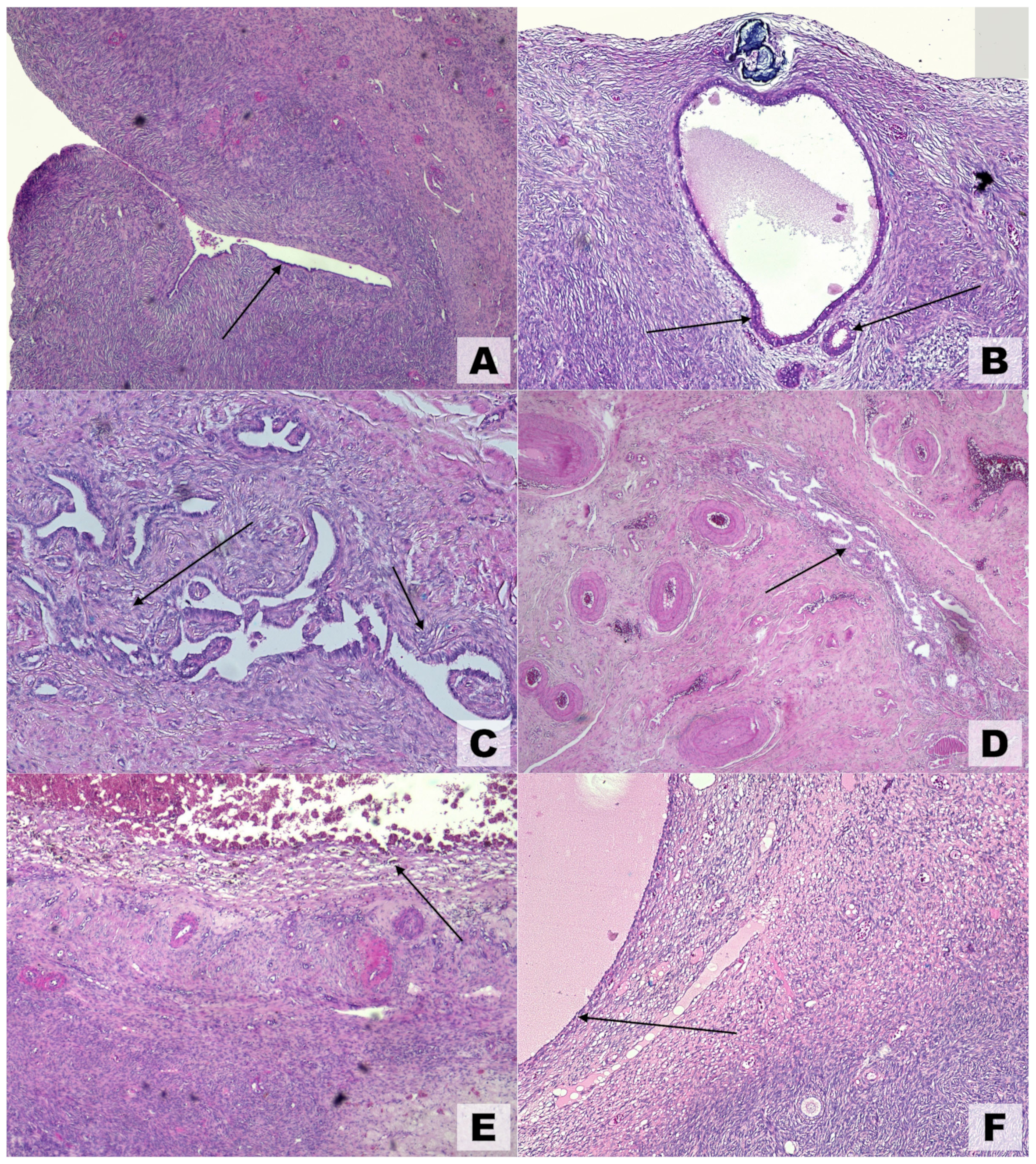
Because they are generated by extraovarian parenchyma, OEs have been referred to as pseudocysts and exhibit unique anatomical features that are not shared by other benign ovarian cysts. These results clarify why surgical enucleation entails ovarian reserve reduction, follicle loss, and partial gonadal cortex removal [75]. Macroscopic and ultrasonographic findings are typically corroborated by microscopic pathological observations (Figure 4). Various theories have been proposed to explain the development of ovarian endometriosis. One such theory, illustrated in Figure 4A, suggests the colonization of an ovarian cortical invagination by endometrial-like cells [20]. Another theory, depicted in Figure 4B, proposes an internal (embryonal) origin for endometriosis, where the condition arises without evidence of external colonization.
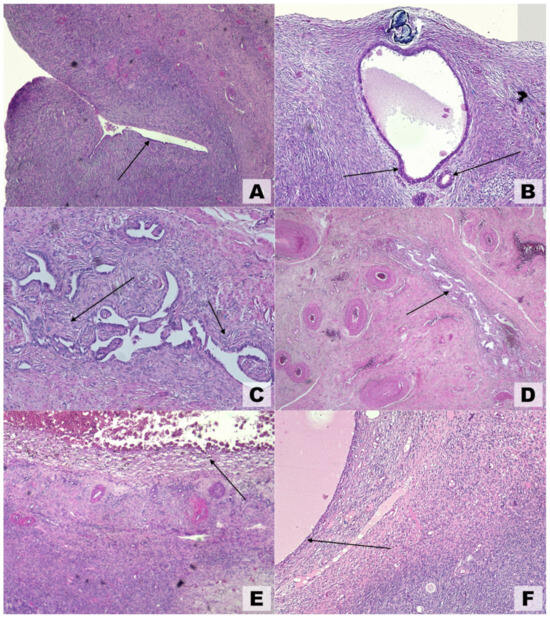
Figure 4.
Microscopic findings in ovarian endometriosis using hematoxylin and eosin (H&E) staining. (A) Invagination of the ovarian cortex with colonization by endometrial-like epithelial cells (arrow), magnification 4×. (B) Early-stage endometriosis localized to the ovarian cortex; arrows highlight endometrial-like glands, magnification 10×. (C) Advanced cortical endometriosis characterized by stromal alterations that followed glandular changes (arrows), magnification 5×. (D) Medullary endometriosis (arrow) involving deeper ovarian layers, magnification 4×. (E) Hemorrhagic ovarian endometrioma displaying hemosiderin-laden macrophages (arrow) and degenerating blood components, magnification 4×. (F) Inclusion cyst shown for comparison, lacking endometrial-like epithelial lining (arrow), magnification 4×.
However, they may also be accompanied by blue, red, white, or unpigmented endometriotic implantation lesions on the peritoneum. The presence of OEs, significant adhesions, and peritoneal deformities indicate more serious endometriosis. This aspect is obvious when we look at the following table that presents the revised American Society of Reproductive Medicine (ASRM) staging system (Table 1) [38].

Table 1.
Stages of endometriosis.
The visualized lesions might undergo biopsy and subsequent pathological evaluation for endometrial glands and stroma [76]. Should the patient encounter infertility concerns, chromotubation may be performed concurrently to evaluate tubal patency.
6.2. Differential Diagnosis
When assessing individuals with suspected OEs, it is crucial to investigate all potentially associated diseases [15]. Patients often present with non-specific pelvic symptoms. Therefore, other causes of pelvic pain should also be considered in the differential diagnosis. These include ectopic pregnancy, pelvic inflammatory disease, appendicitis, diverticulitis, ovarian torsion, urinary tract infection, ovarian cysts, and sexually transmitted infections [50,77].
As mentioned in the assessment section, OEs show a distinct ground-glass appearance in the US image. These observations are similarly evident in hemorrhagic cysts, and frequently, the differentiation between the two is not established until surgical intervention [78].
When a patient presents with symptoms of an acute surgical abdomen and a ruptured OE is suspected, it is essential to consider ruptured ectopic pregnancy and ovarian torsion as primary differential diagnoses [50,79]. These are all surgical emergencies that require immediate transfer to the operating room.
7. Complications
Complications can be divided into clinical conditions due to the OEs’ size, the endometriotic tissue penetration, o adhesion, perforation, or a malignant change.
7.1. Risk to Fertility
According to previous studies, endometriosis impairs the function of the ovaries by damaging the ovarian tissue. To prevent spontaneous ovulation, endometriosis can impair physiological reproductive processes [80]. Reduced ovarian reserves may be caused by endometriosis and associated structural tissue changes in the ovarian cortex [81].
According to reports, women with endometriosis have a monthly fertility rate of 2 to 10%, which is lower than the rate of 15 to 20% in healthy women. Furthermore, the more severe the disease, the lower the fertility rate [40].
7.2. Risk of Infection or Contamination During IVF Cycles
Several substances found in OEs are thought to be harmful to eggs. Smaller studies suggest that such contamination may decrease the fertilization or pregnancy rate, so it is necessary to avoid the accidental puncture of the OEs and to wash the oocyte immediately if contamination is detected [82].
Infection in OEs can result from an accidental puncture during oocyte retrieval or iatrogenic damage after oocyte retrieval. Although prophylactic antibiotics cannot completely prevent these infections, they are important to reduce the risk of infection when an endometrial puncture is suspected [40].
7.3. Risk of Egg Retrieval
Ovaries are sometimes displaced to regions that are difficult to access (e.g., behind the uterus) and do not glide due to adhesions; the presence of OEs is thought to exacerbate difficulties with egg retrieval. This can lead to certain problems. First and foremost, there is a risk of rupture of the OEs. This event can lead to chemical peritonitis and unexpected severe pain [83]. Damage to other organs, particularly the bowel, is a possible second concern.
7.4. Risk for Pregnancy
Delayed implantation can lead to placenta previa or placental insufficiency, which can cause intrauterine growth restriction, preeclampsia, and obstetric hemorrhage. Recurrent pregnancy loss and spontaneous abortions can also be the result of endometriosis-related implantation that occurs outside the typical implantation window. According to Leone Roberti et al., premature decidual senescence can lead to premature birth [84]. Despite conflicting results from epidemiologic research, there is currently no evidence that preventive surgery halts the deleterious effects of endometriosis on pregnancy outcomes [81].
7.5. Risk of Disease Development During IVF Cycles
As endometriosis is an estrogen-dependent disease, the question arises as to how regulated ovarian stimulation for ART may affect the progression or recurrence of the disease. The natural history of endometriotic lesions did not appear to be affected by gonadotropin treatment, and the patients who received ART had a cumulative recurrence rate comparable to the control group [85].
Pregnancy in mothers who already have endometriosis has become an important issue due to the effectiveness of ART. According to Ueda’s results, in these situations, 52% of patients experienced a decrease in OEs’ size, 28% had no change, and 20% had an increase [86]. The OEs showed signs of rupture, abscesses, and decidualization. According to other studies, endometriosis during pregnancy can be studied with caution as most lesions do not shrink or change in size [87].
7.6. Other Complications
Risk of adhesion—OEs can lead to local problems because endometrial tissue adheres and penetrates other organs, e.g., injury to the rectum, bladder, or fallopian tubes.
Risk of perforation—in more severe cases, these OES can perforate and cause symptoms of an acute surgical abdomen.
Risk of torsion—if the OEs measure 6 cm or larger, the patient has an elevated risk of ovarian torsion, a surgical emergency that may result in the loss of the ovary [88].
Risk of malignant change—OEs present a minor risk of malignancy, particularly epithelial ovarian malignancies [89]. Nonetheless, other research suggests that women with endometriosis have a fourfold increased risk of certain forms of ovarian cancer: clear cell and endometrioid ovarian cancer [90]. In a specific study from Finland, an increased risk was only found in women with OEs [91]. Gene activation of KRAS and PI3K/AKT may be linked to ovarian cancer and a history of ovarian mucinous adenomas. Moreover, genes like PTEN and ARID1A have been associated with the etiology of endometrial cancer [92]. Nevertheless, these lesions by themselves are not considered premalignant lesions, and no staging evaluation or screening is necessary.
8. Treatment
8.1. Surgical Treatment
If a patient’s endometriosis is so advanced that OEs have developed, surgery is often preferred, especially laparoscopic or robotic procedures.
If a patient’s discomfort is intense and they do not wish to preserve future fertility, some patients opt for a total hysterectomy with bilateral salpingo-ophorectomy as a more conclusive treatment.
For conservative treatment, there are other surgical procedures: cystectomy, enucleation, partial oophorectomy, ablation by laser or by plasma energy or electrocoagulation, and laparoscopic fulguration. The disadvantages include a high recurrence rate and a decrease in ovarian reserves [3].
The European Society of Human Reproduction and Embryology (ESHRE) guideline recommends considering surgery prior to assisted reproductive technology only in women with ovarian masses larger than 3 cm and only to relieve endometriosis-related symptoms or improve follicular accessibility [93].
Ovarian cystectomy is the favored method for recurrence and the rate of spontaneous pregnancy post-surgery [3]. The primary concern of surgical OE excision, particularly in women facing infertility and contemplating in vitro fertilization (IVF), is its impact on ovarian reserves. Cystectomy often leads to ovarian impairment and reduced ovarian reserves. Three months after ovarian cystectomy, serum levels of the anti-Müllerian hormone (AMH) decreased by 36% and 49% in patients with unilateral and bilateral ovarian masses, respectively, due to the surgical removal of endometrial and normal ovarian tissue [8,9,94]. In addition, the remaining normal ovarian tissue is usually coagulated to control bleeding, which further reduces the ovarian reserve [95]. In older women and women with larger ovarian masses, bilateral lesions, and advanced stages of disease, the ovarian reserve may be more severely reduced. Therefore, cystectomy must be chosen with extreme caution in women who wish to have children or are infertile. To preserve the ovarian reserve during a cystectomy, hemostasis was introduced by suturing the ovaries or by using a hemostatic agent [96].
For extensive OEs, a three-stage protocol may be recommended, starting with a laparoscopic procedure to drain the cyst, followed by three months of treatment with gonadotropin-releasing hormone (GnRH) agonists and a second laparoscopy to ablate the reduced ovarian endometrioma [97].
Ablation is a technique for incising OEs to extract the internal fluid and ablate the endometrial lining, and it is seen as a superior alternative to cystectomy concerning ovarian reserves. Ablation may be executed with bipolar coagulation, laser vaporization, or plasma energy [98].
When surgically resecting OEs, it is crucial to remove the cyst wall and not just aspirate the cyst contents. This has been shown to reduce the recurrence rate. However, resection of OEs has been shown to increase the spontaneous conception rate in patients with reproductive problems [99].
The reduced AMH levels indicate that both ablation and cystectomy impair ovarian reserves. Recent studies have shown that the size of OEs correlates with reduced AMH levels after ablation or a cystectomy [100]. Consequently, for OEs of ≥5 cm, ablation may be preferable to a cystectomy to preserve serum AMH levels [101].
In addition, there is a documented incidence of 2 to 3% of patients experiencing ovarian failure after the removal of bilateral ovarian masses [102]. Given the limited data available, OEs are often treated expectantly in patients under the care of a fertility specialist. Exceptions are made for severe symptoms or complications during egg retrieval due to OEs [96].
Endometriosis may result in many adhesions and endometriotic implants. Occasionally, these lesions may affect the colon or the bladder. In advanced illnesses, intestinal blockages or ureteral involvement may occur. In such instances, general surgery or urology may need to participate in executing the requisite surgical interventions [103,104].
8.2. Medical Treatment
Although medical treatment can reduce the size of OEs, its main aim is to control the symptoms and slow the progression of the underlying disease.
8.2.1. Suppression of Ovarian Function
Since steroidogenesis is disturbed in endometriosis, hormone suppression therapy is considered. Hormone treatment options include oral contraceptives, progestins, aromatase inhibitors, gonadotropin-releasing hormone (GnRH) agonists and antagonists, and androgens [105]. These medical treatments are primarily based on methods that inhibit ovarian function. They reduce the effect of estrogen on the endometriotic tissue and reduce the pain associated with endometriosis. However, the drugs administered for this purpose usually lead to contraception or subfertility, so these treatments are not helpful for the treatment of endometriosis-associated infertility [93]. Eight international guidelines recommend progestogens as the first-line medical treatment for endometriosis [106].
Hormone therapy as a neoadjuvant or adjuvant to surgical therapy is not effective in the treatment of infertility [107]. However, a recent Cochrane review found that drug suppression before surgery can prevent recurrence and increase pregnancy rates [107]. An effective adjuvant postoperative strategy to prevent or reduce the frequency and severity of recurrent dysmenorrhea and morphologic recurrence of endometriosis is long-term contraceptive use [108].
8.2.2. Symptomatic Therapy
Non-steroidal anti-inflammatory drugs and analgesics are very effective against endometriosis-related pain. They are recommended as first-line medical therapies due to their easy accessibility as over-the-counter medications and low side-effect profile [50].
8.2.3. Other Medical Therapies
According to an international survey, 49% of people with endometriosis have used cannabis to treat their symptoms [109]. Other medications have also been used, such as selective estrogen or progesterone receptor modulators, which act on estrogen or progesterone receptors [106]. In addition, palmitoylethanolamide has anti-inflammatory and analgesic properties [110].
8.3. Other Alternative Treatments
8.3.1. Sclerotherapy Is a Non-Surgical Approach to the Treatment of OEs
The epithelial cells lining the walls of ovarian OEs play a crucial role in the cellular mechanisms of sclerotherapy. Sufficient interaction between the sclerosing agent and the cyst wall activates the coagulation cascade, producing inflammatory mediators, which then trigger fibrosis by the cells lining the endometrioma wall, eventually causing adhesion of the walls. The result is the destruction of pseudo-capsule cytoarchitecture [111].
Agents used in sclerotherapy include ethanol, methotrexate, interleukin-2, recombinant interleukins, metronidazole, tetracycline, leuprolide, and cefoperazone-sulbactam. The predominant method is transvaginal sclerotherapy with ethanol. The optimal ethanol concentration and quantity for the sclerotherapy of OEs have not yet been determined [112].
In sclerotherapy, an OE is punctured directly transvaginally or percutaneously to extract the internal fluid, and then a sclerosing agent, like ethanol, is introduced into the cyst cavity and removed again after a certain period (“flushing”). A direct puncture can be performed with a long aspiration needle (16–17 gage) or a flexible catheter (catheter-guided sclerotherapy). US-guided aspiration and ethanol sclerotherapy is indicated for OEs with an average diameter of 4–8 cm, and the reduction in size at 6 months can be major (7–8 times), as we see in the next figure from our previous article [9] (Figure 5).
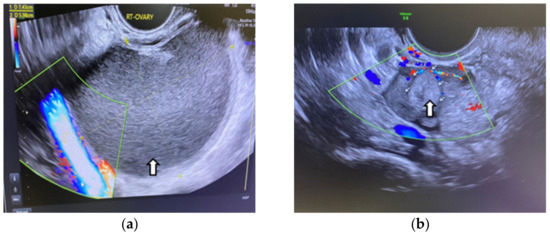
Figure 5.
Vaginal ultrasound image of an ovarian endometrioma treated by sclerotherapy: (a) Arrow shows the original aspect of the endometrioma (b) Arrows show the endometrioma 6 months after ethanolic sclerotherapy. A considerable reduction in size can be seen.
Although there is no evidence of malignancy or pelvic abscess, many gynecologists avoid puncturing OEs [12].
Sclerotherapy with ethanol is associated with a minimal recurrence rate of 7.4% at 6 months [9]. The recurrence rate correlates with the duration of instillation, as prolonged instillation is associated with an increase in cystic fibrosis. Cohen et al. found that ethanol and tetracycline can cause abdominal pain and increase the recurrence rate, while methotrexate can cause postoperative fever [111].
A retrospective review of recurrent OE cases found that the 1-to-10 min wash method had a non-significantly increased recurrence rate at one year compared to the retention method (32.1% vs. 13.3%) [112]. Consequently, it is uncertain whether the irrigation technique has a higher recurrence rate compared to the retention technique in the sclerotherapy of OEs [113].
Sclerotherapy for the treatment of ovarian masses up to 8 cm in size is a simple procedure that significantly reduces the likelihood of recurrence while preserving the ovarian reserve [114]. Laparoscopic cystectomy comes with the resources, costs, and risks associated with any surgical procedure. In addition, the ovarian reserve is significantly impaired, resulting in reduced fertility in patients who have undergone surgery. Sclerotherapy is suitable for individuals seeking IVF treatment [9,115].
8.3.2. Drainage of Cysts
The simplest and least invasive technique for removing cysts is technical cyst drainage, in which a needle is inserted into the cyst wall to aspirate the contents. This procedure can be performed either transvaginal or laparoscopic under ultrasound guidance.
However, it has been observed that the recurrence rate of OE can be between 80% and 100% [116].
Assisted reproductive techniques (ART) are therapeutic modalities that correct the effects of OEs on fertility.
Controlled ovarian stimulation and intrauterine insemination (IUI) can be a useful alternative treatment for patients with mild-to-moderate endometriosis and infertility [117]. In women with severe endometriosis, such as OEs, IUI is generally not used due to impaired tubal function and concerns about pelvic adhesions. In these cases, IVF should be considered [118].
As mentioned above, OEs appear to reduce ovarian reserves, which may lead to a reduced ovarian response to IVF gonadotropin stimulation. However, other findings contradict this conclusion. According to a retrospective study of IVF treatment cycles, the size or amount of OEs did not correlate with the number of oocytes retrieved [119].
Although moderate-to-severe endometriosis appears to affect IVF outcomes, a systematic review comparing the ART outcomes of women with OEs without surgical treatment with those of women who underwent OE removal prior to IVF found similar clinical pregnancy rates, live birth rates, and the average number of eggs retrieved [120].
According to the ESHRE guidelines, surgical removal is not routinely recommended before ART is considered. When deciding whether to surgically remove endometriotic lesions, clinicians are advised to assess ovarian reserves. To determine the best treatment option, it is recommended to monitor AMH levels for at least three months after surgery [93].
If conception does not occur within six to twelve months after OE surgery, IVF is recommended. The various options for preserving fertility include the cryopreservation of embryos or eggs and the cryopreservation of ovarian tissue [121].
In summary, the treatment of OEs can be presented schematically, depending on the patient’s symptoms and her desire for future fertility (Table 2).

Table 2.
Surgical treatment of ovarian endometrioma.
9. Prognosis
The general prognosis for people with endometriosis is positive. It is a non-threatening disease. Nevertheless, it is a persistent disease that can progress. Patients with OEs indicate a more severe disease state and, thus, may experience greater long-term repercussions from the condition. Although treatment can be temporarily effective for patients, the disease is unfortunately characterized by a high relapse rate. Fortunately, symptoms improve in most women during menopause, which is due to the absence of cyclical hormonal signals [3].
10. Conclusions
OEs are a frequently encountered pathology in gynecological practice. The presence of OEs reduces the number of ovarian follicles and creates a toxic environment with negative effects on the oocytes and the development of the embryos resulting from their fertilization. The diagnosis is difficult because the symptoms appear gradually, and the differential diagnosis with extensive cystic ovarian pathologies is controversial. Treatment depends on the clinical context and the patient’s desire to preserve her fertility. Treatment is mainly surgical, and the most important question for gynecologists is whether OEs should be removed or not. Of the available surgical methods, cystectomy appears to be advantageous in terms of the lower recurrence rate and the likelihood of spontaneous conception. Current therapeutic options have evolved, and recently, sclerotherapy using US-guided punctures has gained acceptance. Drug therapy can be used as an adjuvant therapy. ART is a viable alternative for the treatment of infertility associated with OEs when other procedures fail to deliver results.
Author Contributions
Conceptualization, C.-C.V. and A.C.-V.; methodology, C.-C.V.; software, L.D.; validation, C.-C.V., A.C.-V. and L.D.; formal analysis, L.B.; investigation, L.D. and M.-S.Ș.; resources, C.-C.V.; data curation, L.D.; writing—original draft preparation, C.-C.V. and L.B.; writing—review and editing, M.-S.Ș. and A.C.-V.; visualization, L.D. and L.B.; supervision, A.C.-V. and M.-S.Ș.; project administration, C.-C.V.; funding acquisition, C.-C.V. All authors have read and agreed to the published version of the manuscript.
Funding
The article processing charge (APC) was funded by the University of Medicine and Pharmacy in Craiova, Romania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Audebert, A.; Petousis, S.; Margioula-Siarkou, C.; Ravanos, K.; Prapas, N.; Prapas, Y. Anatomic distribution of endometriosis: A reappraisal based on series of 1101 patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 36–40. [Google Scholar] [CrossRef]
- Vercellini, P.; Fedele, L.; Aimi, G.; Pietropaolo, G.; Consonni, D.; Crosignani, P.G. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: A multivariate analysis of over 1000 patients. Hum. Reprod. 2007, 22, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Working Group of ESGE, ESHRE and WES; Saridogan, E.; Becker, C.M.; Feki, A.; Grimbizis, G.F.; Hummelshoj, L.; Keckstein, J.; Nisolle, M.; Tanos, V.; Ulrich, U.A.; et al. Recommendations for the Surgical Treatment of Endometriosis. Part 1: Ovarian Endometrioma. Hum. Reprod. Open 2017, 2017, hox016. [Google Scholar] [CrossRef] [PubMed]
- Meggyesy, M.; Friese, M.; Gottschalk, J.; Kehler, U. Case Report of Cerebellar Endometriosis. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti, C.; Johnson, N.P.; Petrozza, J.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Lee, T.T.M.; Missmer, S.; Vermeulen, N.; et al. An International Terminology for Endometriosis, 2021. Facts Views Vis. Obgyn 2021, 13, 295–304. [Google Scholar] [CrossRef]
- Exacoustos, C.; De Felice, G.; Pizzo, A.; Morosetti, G.; Lazzeri, L.; Centini, G.; Piccione, E.; Zupi, E. Isolated Ovarian Endometrioma: A History Between Myth and Reality. J. Minim. Invasive Gynecol. 2018, 25, 884–891. [Google Scholar] [CrossRef]
- Hwu, Y.M.; Wu, F.S.; Li, S.H.; Sun, F.J.; Lin, M.H.; Lee, R.K. The impact of endometrioma and laparoscopic cystectomy on serum anti-Müllerian hormone levels. Reprod. Biol. Endocrinol. 2011, 9, 80. [Google Scholar] [CrossRef]
- Vaduva, C.C.; Dira, L.; Carp-Veliscu, A.; Goganau, A.M.; Ofiteru, A.M.; Siminel, M.A. Ovarian reserve after treatment of ovarian endometrimas by ethanolic sclerotherapy compared to surgical treatment. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5575–5582. [Google Scholar]
- Daniilidis, A.; Angioni, S.; Di Michele, S.; Dinas, K.; Gkrozou, F.; D’Alterio, M.N. Deep Endometriosis and Infertility: What Is the Impact of Surgery? J. Clin. Med. 2022, 11, 6727. [Google Scholar] [CrossRef]
- Barnhart, K.; Dunsmoor-Su, R.; Coutifaris, C. Effect of endometriosis on in vitro fertilization. Fertil. Steril. 2002, 77, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, S.; Zahiri Sorouri, Z.; Askari, E.; Poordast, T.; Chamanara, K. The success of various endometrioma treatments in infertility: A systematic review and meta-analysis of prospective studies. Reprod. Med. Biol. 2019, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.D.; Seaman, H.E.; de Vries, C.S.; Wright, J.T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008, 115, 1382–1391. [Google Scholar] [CrossRef]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Malspeis, S.; Willett, W.C.; Hunter, D.J. Reproductive history and endometriosis among premenopausal women. Obstet. Gynecol. 2004, 104 Pt 1, 965–974. [Google Scholar] [CrossRef]
- Ozkan, S.; Murk, W.; Arici, A. Endometriosis and infertility: Epidemiology and evidence-based treatments. Ann. N. Y. Acad. Sci. 2008, 1127, 92–100. [Google Scholar] [CrossRef]
- Nouri, K.; Ott, J.; Krupitz, B.; Huber, J.C.; Wenzl, R. Family incidence of endometriosis in first-, second-, and third-degree relatives: Case-control study. Reprod. Biol. Endocrinol. 2010, 8, 85. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Yovich, J.L.; Rowlands, P.K.; Lingham, S.; Sillender, M.; Srinivasan, S. Pathogenesis of endometriosis: Look no further than John Sampson. Reprod. Biomed. Online 2020, 40, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Dorien, F.O.; Roskams, T.; Van den Eynde, K.; Vanhie, A.; Peterse, D.P.; Meuleman, C.; Tomassetti, C.; Peeraer, K.; D’Hooghe, T.M.; Fassbender, A. The Presence of Endometrial Cells in Peritoneal Fluid of Women with and Without Endometriosis. Reprod. Sci. 2017, 24, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.M.; Maybin, J.A.; Murray, A.A.; Nicol, M.; Walker, C.; Saunders, P.T.K.; Rossi, A.G.; Critchley, H.O.D. Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model. Sci. Rep. 2017, 7, 17416. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Michels, K.B.; Hunter, D.J. In utero exposures and the incidence of endometriosis. Fertil. Steril. 2004, 82, 1501–1508. [Google Scholar] [CrossRef]
- Dyson, M.T.; Roqueiro, D.; Monsivais, D.; Ercan, C.M.; Pavone, M.E.; Brooks, D.C.; Kakinuma, T.; Ono, M.; Jafari, N.; Dai, Y.; et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014, 10, e1004158. [Google Scholar] [CrossRef] [PubMed]
- Izawa, M.; Taniguchi, F.; Harada, T. GATA6 expression promoted by an active enhancer may become a molecular marker in endometriosis lesions. Am. J. Reprod. Immunol. 2019, 81, e13078. [Google Scholar] [CrossRef]
- Naqvi, H.; Ilagan, Y.; Krikun, G.; Taylor, H.S. Altered genome-wide methylation in endometriosis. Reprod. Sci. 2014, 21, 1237–1243. [Google Scholar] [CrossRef]
- Celik, O.; Unlu, C.; Otlu, B.; Celik, N.; Caliskan, E. Laparoscopic endometrioma resection increases peri-implantation endometrial HOXA-10 and HOXA-11 mRNA expression. Fertil. Steril. 2015, 104, 356–365. [Google Scholar] [CrossRef]
- Macer, M.L.; Taylor, H.S. Endometriosis and infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Yu, J.; Francisco, A.M.C.; Patel, B.G.; Cline, J.M.; Zou, E.; Berga, S.L.; Taylor, R.N. IL-1β Stimulates Brain-Derived Neurotrophic Factor Production in Eutopic Endometriosis Stromal Cell Cultures: A Model for Cytokine Regulation of Neuroangiogenesis. Am. J. Pathol. 2018, 188, 2281–2292. [Google Scholar] [CrossRef]
- Nishida, M.; Nasu, K.; Narahara, H. Role of chemokines in the pathogenesis of endometriosis. Front. Biosci. (Schol. Ed.) 2011, 3, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Bonavina, G.; Taylor, H.S. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Istrate-Ofiţeru, A.-M.; Mogoantă, C.A.; Zorilă, G.-L.; Roşu, G.-C.; Drăguşin, R.C.; Berbecaru, E.-I.; Zorilă, M.V.; Comănescu, C.M.; Mogoantă, S.; Vaduva, C.-C.; et al. Clinical Characteristics and Local Histopathological Modulators of Endometriosis and Its Progression. Int. J. Mol. Sci. 2024, 25, 1789. [Google Scholar] [CrossRef]
- Bourdel, N.; Alves, J.; Pickering, G.; Ramilo, I.; Roman, H.; Canis, M. Systematic review of endometriosis pain assessment: How to choose a scale? Hum. Reprod. Update 2015, 21, 136–152. [Google Scholar] [CrossRef]
- Garzon, S.; Laganà, A.S.; Barra, F.; Casarin, J.; Cromi, A.; Raffaelli, R.; Uccella, S.; Franchi, M.; Ghezzi, F.; Ferrero, S. Novel drug delivery methods for improving efficacy of endometriosis treatments. Expert Opin. Drug Deliv. 2021, 18, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Decherney, A.H. Medical Management of Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 485–496. [Google Scholar] [CrossRef]
- Schliep, K.; Chen, Z.; Stanford, J.; Xie, Y.; Mumford, S.; Hammoud, A.; Johnstone, E.B.; Dorais, J.; Varner, M.; Louis, G.B.; et al. Endometriosis diagnosis and staging by operating surgeon and expert review using multiple diagnostic tools: An inter-rater agreement study. BJOG 2017, 124, 220–229. [Google Scholar] [CrossRef]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Trinchant, R.; García-Velasco, J.A. Oocyte Quality in Women with Endometriosis. Gynecol. Obstet. Investig. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Benaglia, L.; Paffoni, A.; Busnelli, A.; Vigano, P.; Vercellini, P. Risks of conservative management in women with ovarian endometriomas undergoing IVF. Hum. Reprod. Update 2015, 21, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Schubert, B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil. Steril. 2010, 93, 2431–2432. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Viganò, P.; Somigliana, E.; Panina-Bordignon, P.; Vercellini, P.; Candiani, M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Update 2014, 20, 217–230. [Google Scholar] [CrossRef]
- Takeuchi, A.; Koga, K.; Satake, E.; Makabe, T.; Taguchi, A.; Miyashita, M.; Takamura, M.; Harada, M.; Hirata, T.; Hirota, Y.; et al. Endometriosis Triggers Excessive Activation of Primordial Follicles via PI3K-PTEN-Akt-Foxo3 Pathway. J. Clin. Endocrinol. Metab. 2019, 104, 5547–5554. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Somigliana, E.; Vercellini, P.; Pagliardini, L.; Candiani, M.; Vigano, P. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact. J. Steroid Biochem. Mol. Biol. 2016, 155 Pt A, 35–46. [Google Scholar] [CrossRef]
- Paffoni, A.; Bolis, V.; Ferrari, S.; Benaglia, L.; Vercellini, P.; Somigliana, E. The Gametotoxic Effects of the Endometrioma Content: Insights From a Parthenogenetic Human Model. Reprod. Sci. 2019, 26, 573–579. [Google Scholar] [CrossRef]
- Kitajima, M.; Defrère, S.; Dolmans, M.-M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef]
- Foti, P.V.; Farina, R.; Palmucci, S.; Vizzini, I.A.A.; Libertini, N.; Coronella, M.; Spadola, S.; Caltabiano, R.; Iraci, M.; Basile, A.; et al. Endometriosis: Clinical features, MR imaging findings and pathologic correlation. Insights Into Imaging 2018, 9, 149–172. [Google Scholar] [CrossRef]
- Hansen, K.E.; Kesmodel, U.S.; Baldursson, E.B.; Kold, M.; Forman, A. Visceral syndrome in endometriosis patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 198–203. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Oosterlynck, D.; D’Hooghe, T.; Meuleman, C. Deeply infiltrating endometriosis is a disease whereas mild endometriosis could be considered a non-disease. Ann. N. Y. Acad. Sci. 1994, 734, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier, A.; Chapron, C. Endometriosis and pelvic pain: Epidemiological evidence of the relationship and implications. Hum. Reprod. Update 2005, 11, 595–606. [Google Scholar] [CrossRef]
- Anaf, V.; Simon, P.; El Nakadi, I.; Fayt, I.; Simonart, T.; Buxant, F.; Noel, J.C. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum. Reprod. 2002, 17, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Al-Fozan, H.; Bakare, S.; Chen, M.F.; Tulandi, T. Nerve fibers in ovarian dermoid cysts and endometriomas. Fertil. Steril. 2004, 82, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Y.J. Rupture of ovarian endometriotic cyst complicated with endometriosis: A case report. World J. Clin. Cases 2021, 9, 8524–8530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanada, T.; Park, J.; Hagiwara, M.; Ikeda, N.; Nagai, T.; Matsubayashi, J.; Saito, K. CT and MRI findings of bronchopulmonary endometriosis: A case presentation. Acta Radiol. Open 2018, 7, 2058460118801164. [Google Scholar] [CrossRef] [PubMed]
- Kingsberg, S.A.; Janata, J.W. Female sexual disorders: Assessment, diagnosis, and treatment. Urol. Clin. N. Am. 2007, 34, 497–506. [Google Scholar] [CrossRef]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E.; on behalf of the ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- Mounsey, A.L.; Wilgus, A.; Slawson, D.C. Diagnosis and management of endometriosis. Am. Fam. Physician 2006, 74, 594–600. [Google Scholar]
- Cranney, R.; Condous, G.; Reid, S. An update on the diagnosis, surgical management, and fertility outcomes for women with endometrioma. Acta Obstet. Gynecol. Scand. 2017, 96, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Wieser, F.; Dogan, S.; Klingel, K.; Diedrich, K.; Taylor, R.N.; Hornung, D. Expression and regulation of CCR1 in peritoneal macrophages from women with and without endometriosis. Fertil. Steril. 2005, 83, 1878–1881. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood Biomarkers for the Non-Invasive Diagnosis of Endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef]
- Surrey, E.; Carter, C.M.; Soliman, A.M.; Khan, S.; DiBenedetti, D.B.; Snabes, M.C. Patient-Completed or Symptom-Based Screening Tools for Endometriosis: A Scoping Review. Arch. Gynecol. Obstet. 2017, 296, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Xu, D.; Nagel, S.C.; Bromfield, J.J.; Pelch, K.E.; Wilshire, G.B.; Joshi, T. GenomeForest: An Ensemble Machine Learning Classifier for Endometriosis. AMIA Summits Transl. Sci. Proc. 2020, 2020, 33–42. [Google Scholar] [PubMed]
- Bendifallah, S.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J. Clin. Med. 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melinte-Popescu, A.S.; Popa, R.F.; Harabor, V.; Nechita, A.; Harabor, A.; Adam, A.M.; Vasilache, I.A.; Melinte-Popescu, M.; Vaduva, C.; Socolov, D. Managing Fetal Ovarian Cysts: Clinical Experience with a Rare Disorder. Medicina 2023, 59, 715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albu, D.F.; Albu, C.C.; Gogănău, A.M.; Albu, S.D.; Mogoantă, L.; Edu, A.; DiŢescu, D.; Văduva, C.C. Borderline Brenner tumors associated with ovarian cyst—Case presentation. Rom. J. Morphol. Embryol. 2016, 57, 893–898. [Google Scholar]
- Noventa, M.; Scioscia, M.; Schincariol, M.; Cavallin, F.; Pontrelli, G.; Virgilio, B.; Vitale, S.G.; Laganà, A.S.; Dessole, F.; Cosmi, E.; et al. Imaging Modalities for Diagnosis of Deep Pelvic Endometriosis: Comparison between Trans-Vaginal Sonography, Rectal Endoscopy Sonography and Magnetic Resonance Imaging. A Head-to-Head Meta-Analysis. Diagnostics 2019, 9, 225. [Google Scholar] [CrossRef]
- Young, S.W.; Groszmann, Y.; Dahiya, N.; Caserta, M.; Yi, J.; Wasson, M.; Patel, M.D. Sonographer-acquired ultrasound protocol for deep endometriosis. Abdom. Radiol. 2020, 45, 1659–1669. [Google Scholar] [CrossRef]
- Cohen Ben-Meir, L.; Mashiach, R.; Eisenberg, V.H. External Validation of the IOTA Classification in Women with Ovarian Masses Suspected to Be Endometrioma. J. Clin. Med. 2021, 10, 2971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abrao, M.S.; Goncalves, M.O.D.C.; Dias, J.A., Jr.; Podgaec, S.; Chamie, L.P.B.R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum. Reprod. 2007, 22, 3092–3097. [Google Scholar] [CrossRef]
- Guerriero, S.; Ajossa, S.; Gerada, M.; Virgilio, B.; Angioni, S.; Melis, G.B. Diagnostic value of transvaginal ’tenderness-guided’ ultrasonography for the prediction of location of deep endometriosis. Hum. Reprod. 2008, 23, 2452–2457. [Google Scholar] [CrossRef]
- Martin, D.C.; Batt, R.E. Retrocervical, retrovaginal pouch, and rectovaginal septum endometriosis. J. Am. Assoc. Gynecol. Laparosc. 2001, 8, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.; Alcazar, J.L. High sliding sign: A new soft marker of uterine fundus compromise in deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2015, 45, 624. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Alcazar, J.L.; Ajossa, S.; Pilloni, M.; Melis, G.B. Three-dimensional sonographic characteristics of deep endometriosis. J. Ultrasound Med. 2009, 28, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Rolla, E. Endometriosis: Advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Research 2019, 8, F1000 Faculty Rev-529. [Google Scholar] [CrossRef]
- Vercellini, P.; Frontino, G.; Pietropaolo, G.; Gattei, U.; Daguati, R.; Crosignani, P.G. Deep endometriosis: Definition, pathogenesis, and clinical management. J. Am. Assoc. Gynecol. Laparosc. 2004, 11, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.M.; Arambage, K.; Correa, F.J.; Olive, D.; Farquhar, C.; Garry, R.; Barlow, D.H.; Jacobson, T.Z. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 2014, 4, CD011031. [Google Scholar]
- Mobeen, S.; Apostol, R. Ovarian Cyst. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Patel, M.D.; Feldstein, V.A.; Filly, R.A. The likelihood ratio of sonographic findings for the diagnosis of hemorrhagic ovarian cysts. J. Ultrasound Med. 2005, 24, 607–614, quiz 15. [Google Scholar] [CrossRef]
- Costea, D.; Serbanescu, L.; Badiu, D.; Ardeleanu, V.; Branescu, C.; Zgura, A.; Costea, A. Pain Managemnent in the Right Iliac Fossa During the COVID-19 Pandemic. J. Mind Med. Sci. 2022, 9, 162–167. [Google Scholar] [CrossRef]
- Benaglia, L.; Somigliana, E.; Vercellini, P.; Abbiati, A.; Ragni, G.; Fedele, L. The rate of spontaneous ovulation is negatively affected by endometriotic ovarian cysts. Hum. Reprod. 2009, 24, 2183–2186. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.R.; Lee, D.; Jee, B.C. Treatment options and considerations for infertility due to endometriosis. Clin. Exp. Reprod. Med. 2020, 47, 1–11. [Google Scholar]
- Benaglia, L.; Cardellicchio, L.; Guarneri, C.; Paffoni, A.; Restelli, L.; Somigliana, E.; Fedele, L. IVF outcomes in women with accidental contamination of follicular fluid with endometrioma contents. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Liou, J.D.; Hsieh, C.L.; Shiau, C.S.; Lo, L.M.; Chang, M.Y. Long-term follow-up of patients surgically treated for ruptured ovarian endometriotic cysts. Taiwan. J. Obstet. Gynecol. 2011, 50, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Leone Roberti Maggiore, U.; Ferrero, S.; Mangili, G.; Bergamini, A.; Inversetti, A.; Giorgione, V.; Viganò, P.; Candiani, M. A systematic review on endometriosis during pregnancy: Diagnosis, misdiagnosis, complications and outcomes. Hum. Reprod. Update 2016, 22, 70–103. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.E.; Rizzello, F.; Gianfranco, S. Does controlled ovarian hyperstimulation in women with a history of endometriosis influence recurrence rate? J Womens Health 2010, 19, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Enomoto, T.; Miyatake, T.; Fujita, M.; Yamamoto, R.; Kanagawa, T.; Shimizu, H.; Kimura, T. A retrospective analysis of ovarian endometriosis during pregnancy. Fertil. Steril. 2010, 94, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Benaglia, L.; Somigliana, E.; Calzolari, L.; Busnelli, A.; Cardellicchio, L.; Ragni, G.; Fedele, L. The disappearing endometrioma: The intriguing effect of pregnancy on small endometriotic ovarian cysts. Gynecol. Endocrinol. 2013, 29, 863–866. [Google Scholar] [CrossRef]
- Sasaki, K.J.; Miller, C.E. Adnexal torsion: Review of the literature. J. Minim. Invasive Gynecol. 2014, 21, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Centini, G.; Schettini, G.; Pieri, E.; Giorgi, M.; Lazzeri, L.; Martire, F.G.; Mancini, V.; Raimondo, D.; Seracchioli, R.; Habib, N.; et al. Endometriosis-Related Ovarian Cancer: Where Are We Now? A Narrative Review towards a Pragmatic Approach. J. Clin. Med. 2024, 13, 1933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Saavalainen, L.; Lassus, H.; But, A.M.; Tiitinen, A.; Härkki, P.; Gissler, M.; Pukkala, E.; Heikinheimo, O. Risk of Gynecologic Cancer According to the Type of Endometriosis. Obstet. Gynecol. 2018, 131, 1095–1102. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Noske, A.; Dedes, K.J.; Fink, D.; Imesch, P. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinOEs. Int. J. Mol. Sci. 2013, 14, 18824–18849. [Google Scholar] [CrossRef]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, G.; Safinataj, M.; Shahesmaeili, A.; Allahqoli, L.; Salehiniya, H.; Alkatout, I. Effect of laparoscopic cystectomy on ovarian reserve in patients with ovarian cyst. Front. Endocrinol. 2022, 13, 964229. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Berlanda, N.; Benaglia, L.; Viganò, P.; Vercellini, P.; Fedele, L. Surgical excision of endometriomas and ovarian reserve: A systematic review on serum antimüllerian hormone level modifications. Fertil. Steril. 2012, 98, 1531–1538. [Google Scholar] [CrossRef]
- Pais, A.S.; Flagothier, C.; Tebache, L.; Almeida Santos, T.; Nisolle, M. Impact of Surgical Management of Endometrioma on AMH Levels and Pregnancy Rates: A Review of Recent Literature. J. Clin. Med. 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Tsolakidis, D.; Pados, G.; Vavilis, D.; Athanatos, D.; Tsalikis, T.; Giannakou, A.; Tarlatzis, B.C. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: A prospective randomized study. Fertil. Steril. 2010, 94, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Lousse, J.C.; Jadoul, P.; Donnez, O.; Squifflet, J. Laparoscopic management of endometrioms using a combined technique of excisional (cystectomy) and ablative surgery. Fertil. Steril. 2010, 94, 28–32. [Google Scholar] [CrossRef]
- Daniilidis, A.; Grigoriadis, G.; Kalaitzopoulos, D.R.; Angioni, S.; Kalkan, Ü.; Crestani, A.; Merlot, B.; Roman, H. Surgical Management of Ovarian Endometrioma: Impact on Ovarian Reserve Parameters and Reproductive Outcomes. J. Clin. Med. 2023, 12, 5324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Zhang, S.; Zhao, Z.; Wang, C.; Xu, S.; Wang, F. Impact of cystectomy versus ablation for endometrioma on ovarian reserve: A systematic review and meta-analysis. Fertil. Steril. 2022, 118, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Achilli, C.; Bergamini, V.; Candiani, M.; Garavaglia, E.; Lazzeri, L.; Lecce, F.; Maiorana, A.; Maneschi, F.; Marana, R.; et al. Comparison between the stripping technique and the combined excisional/ablative technique for the treatment of bilateral ovarian endometriomas: A multicentre RCT. Hum. Reprod. 2016, 31, 339–344. [Google Scholar] [CrossRef][Green Version]
- Alammari, R.; Lightfoot, M.; Hur, H.C. Impact of Cystectomy on Ovarian Reserve: Review of the Literature. J. Minim. Invasive Gynecol. 2017, 24, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Lang, E. Endometriosis as a rare cause of small bowel obstruction. ANZ J. Surg. 2020, 90, E137–E138. [Google Scholar] [CrossRef]
- Endo, Y.; Akatsuka, J.; Obayashi, K.; Takeda, H.; Hayashi, T.; Nakayama, S.; Suzuki, Y.; Hamasaki, T.; Kondo, Y. Efficacy of Laparoscopic Partial Cystectomy with a Transurethral Resectoscope in Patients with Bladder Endometriosis: See-Through Technique. Urol. Int. 2020, 104, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.L.; Schorge, J.O.; Halvorson, L.M.; Hamid, C.A.; Corton, M.M.; Schaffer, J.I. (Eds.) Williams Gynecology, 4th ed.; McGraw-Hill Education: Berkshire, UK, 2020. [Google Scholar]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of endometriosis: A review with comparison of 8 guidelines. BMC Women’s Health 2021, 21, 397. [Google Scholar] [CrossRef]
- Chen, I.; Veth, V.B.; Choudhry, A.J.; Murji, A.; Zakhari, A.; Black, A.Y.; Agarpao, C.; Maas, J.W. Pre- and postsurgical medical therapy for endometriosis surgery. Cochrane Database Syst. Rev. 2020, 11, CD003678. [Google Scholar] [CrossRef] [PubMed]
- Seracchioli, R.; Mabrouk, M.; Manuzzi, L.; Vicenzi, C.; Frasca, C.; Elmakky, A.; Venturoli, S. Post-operative use of oral contraceptive pills for prevention of anatomical relapse or symptom-recurrence after conservative surgery for endometriosis. Hum. Reprod. 2009, 24, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Armour, M.; Sinclair, J.; Cheng, J.; Davis, P.; Hameed, A.; Meegahapola, H.; Rajashekar, K.; Suresh, S.; Proudfoot, A.; Leonardi, M. Endometriosis and Cannabis Consumption During the COVID-19 Pandemic: An International Cross-Sectional Survey. Cannabis Cannabinoid Res. 2022, 7, 473–481. [Google Scholar] [CrossRef]
- Alonso, A.; Gunther, K.; Maheux-Lacroix, S.; Abbott, J. Medical management of endometriosis. Curr. Opin. Obstet. Gynecol. 2024, 36, 353–361. [Google Scholar] [CrossRef]
- Cohen, A.; Almog, B.; Tulandi, T. Sclerotherapy in the management of ovarian endometrioma: Systematic review and meta-analysis. Fertil. Steril. 2017, 108, 117–124.e5. [Google Scholar] [CrossRef]
- Noma, J.; Yoshida, N. Efficacy of ethanol sclerotherapy for ovarian endometriomas. Int. J. Gynaecol. Obstet. 2001, 72, 35–39. [Google Scholar] [CrossRef]
- Huang, L.; Chang, M.-Y.; Shiau, C.-S.; Hsieh, T.-T. Changes in anti-müllerian hormone after ultrasound guided aspiration and ethanol sclerotic therapy of ovarian cyst. Taiwan. J. Obstet. Gynecol. 2021, 60, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Lee, I.; Han, K.; Seo, S.K.; Kim, M.-D.; Lee, J.K.; Kwon, J.H.; Kim, G.M.; Lee, J.; Won, J.Y. Comparison of the therapeutic efficacy and ovarian reserve between catheter-directed sclerotherapy and surgical excision for ovarian endometrioma. Eur. Radiol. 2021, 31, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tejedor, A.; Martinez-Garcia, J.M.; Candas, B.; Suarez, E.; Mañalich, L.; Gomez, M.; Merino, E.; Castellarnau, M.; Regueiro, P.; Carreras, M.; et al. Ethanol Sclerotherapy versus Laparoscopic Surgery for Endometrioma Treatment: A Prospective, Multicenter, Cohort Pilot Study. J. Minim. Invasive Gynecol. 2020, 27, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Perrone, U.; Ferrero, S.; Gazzo, I.; Izzotti, A.; Leone Roberti Maggiore, U.; Gustavino, C.; Ceccaroni, M.; Bogliolo, S.; Barra, F. Endometrioma surgery: Hit with your best shot (But know when to stop). Best Pract. Res. Clin. Obstet. Gynaecol. 2024, 96, 102528. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–598. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.K.; Lee, J.R.; Jee, B.C. Management of endometriosis-related infertility: Considerations and treatment options. Clin. Exp. Reprod. Med. 2020, 47, 1–11, Erratum in: Clin. Exp. Reprod. Med. 2020, 47, 153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esinler, I.; Bozdag, G.; Arikan, I.; Demir, B.; Yarali, H. Endometrioma ≤3 cm in diameter per se does not affect ovarian reserve in intracytoplasmic sperm injection cycles. Gynecol. Obstet. Investig. 2012, 74, 261–264. [Google Scholar] [CrossRef]
- Hamdan, M.; Dunselman, G.; Li, T.C.; Cheong, Y. The impact of endometrioma on IVF/ICSI outcomes: A systematic review and metaanalysis. Hum. Reprod. Update 2015, 21, 809–825. [Google Scholar] [CrossRef]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).