Enhanced Ciliogenesis of Human Bronchial Epithelial Cells by Simulated Microgravity
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Bronchial Epithelial Cell Culture
2.1.1. Preparation of Humand Bronchial Epithelial Cell
2.1.2. Serum Starvation
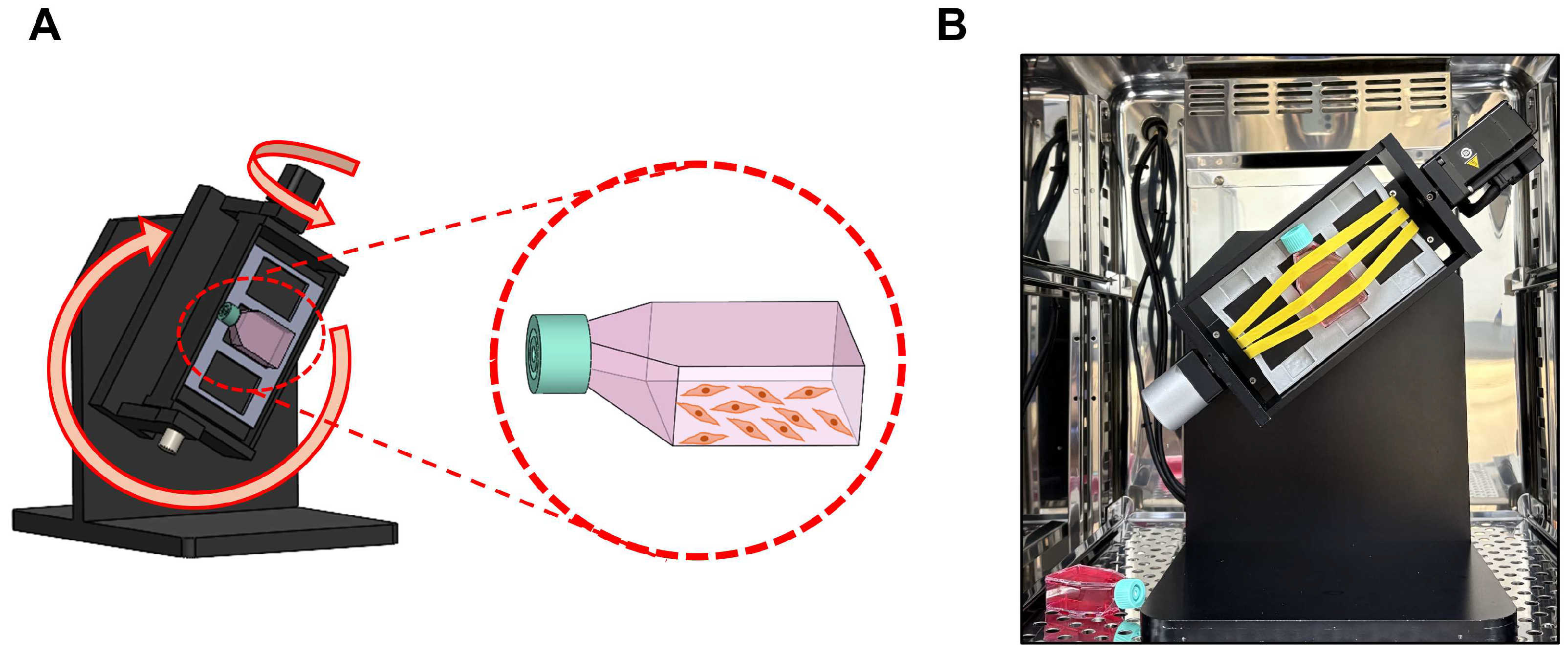
2.2. Three-Dimensional (3D) Clinostat
2.3. Cell Viability in SMG
2.4. Cell Proliferation in SMG
2.5. Gene Expression Analysis
2.6. Immunofluorescence
2.7. Cilia Length Measurement
2.8. Western Blot
2.9. Statistical Analysis
3. Results
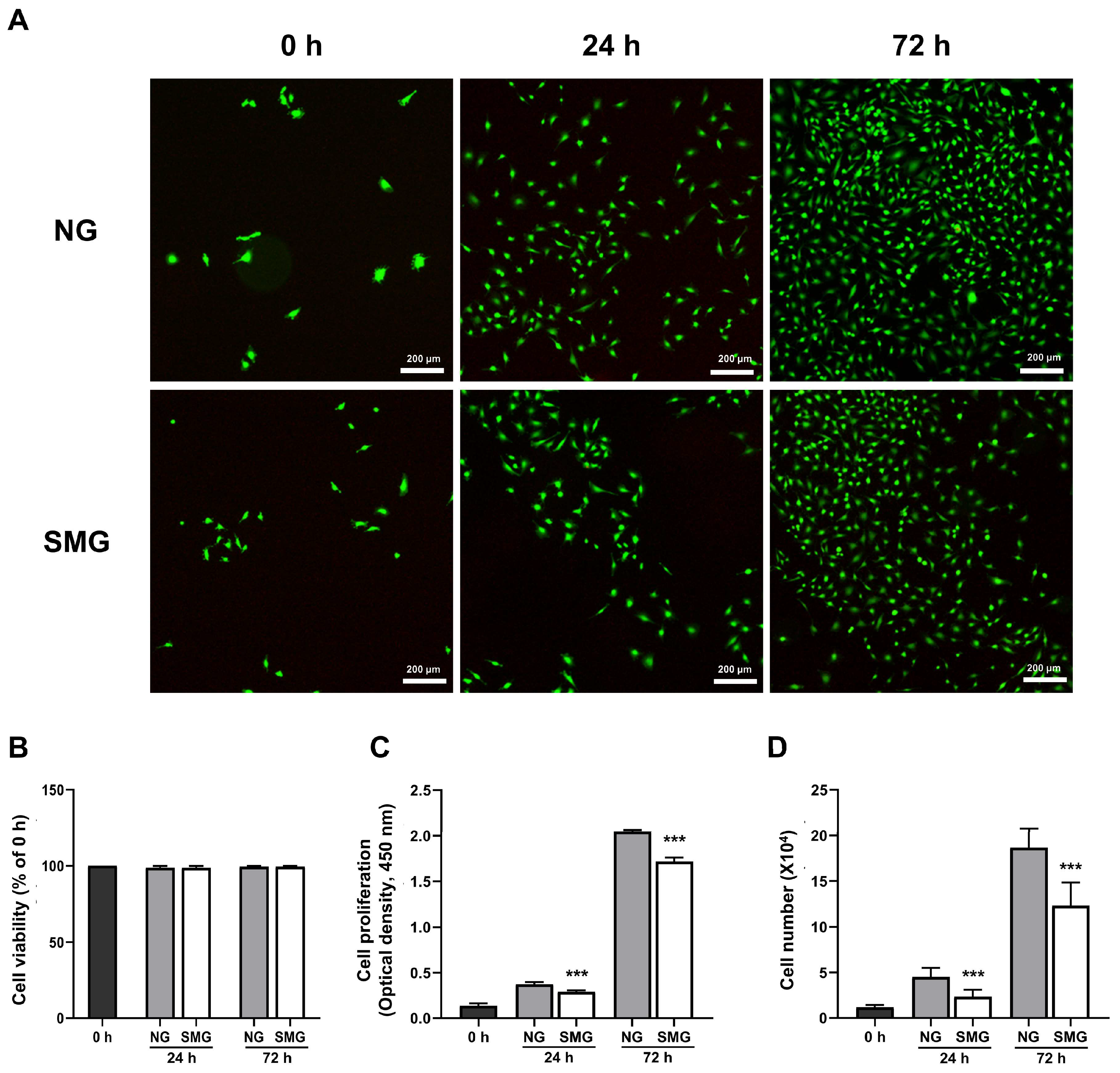
3.1. Changes in Cell Survival and Cell Proliferation Rate with SMG Exposure
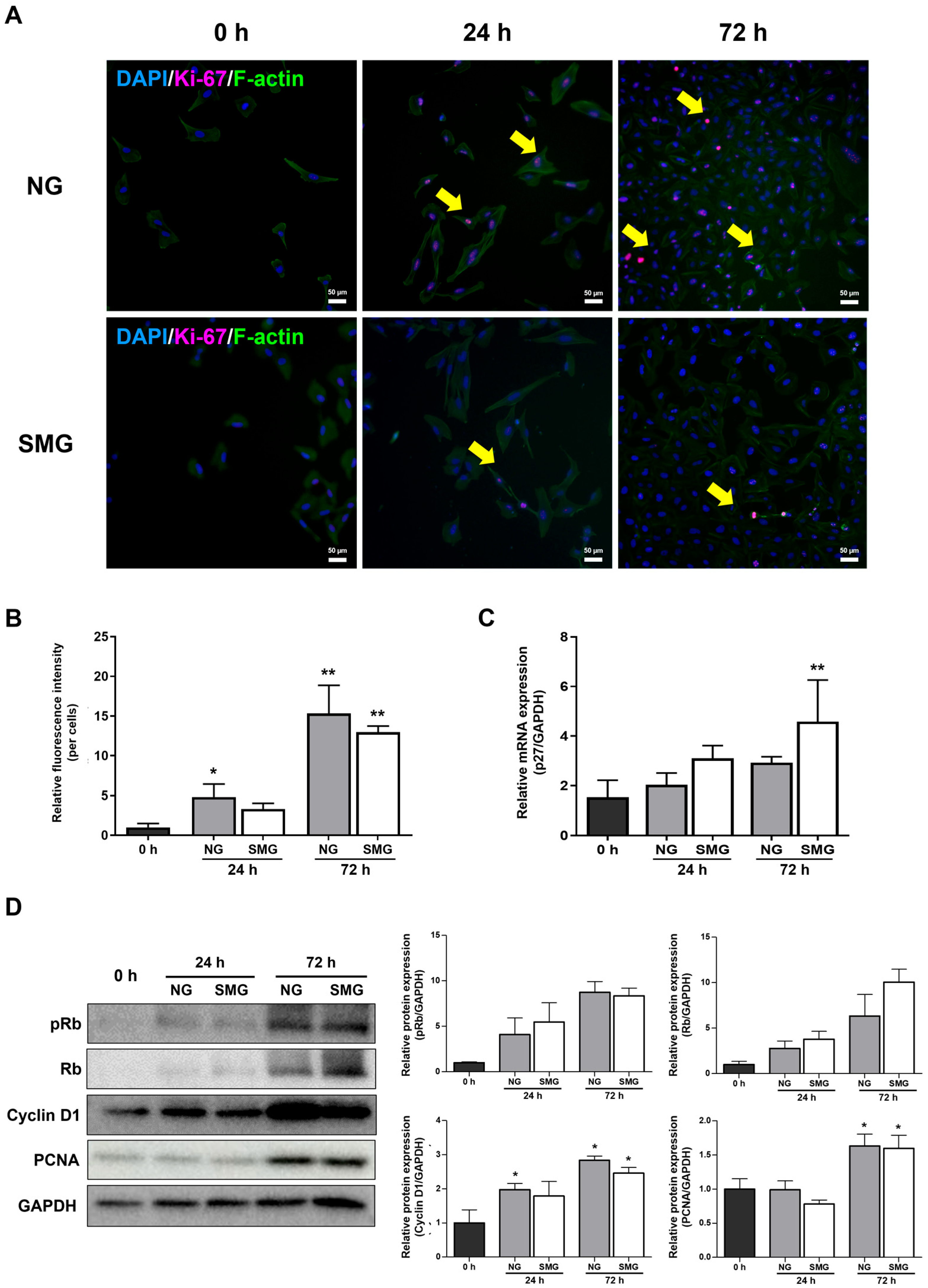
3.2. Change of Cell Cycle in Cells Exposed to SMG
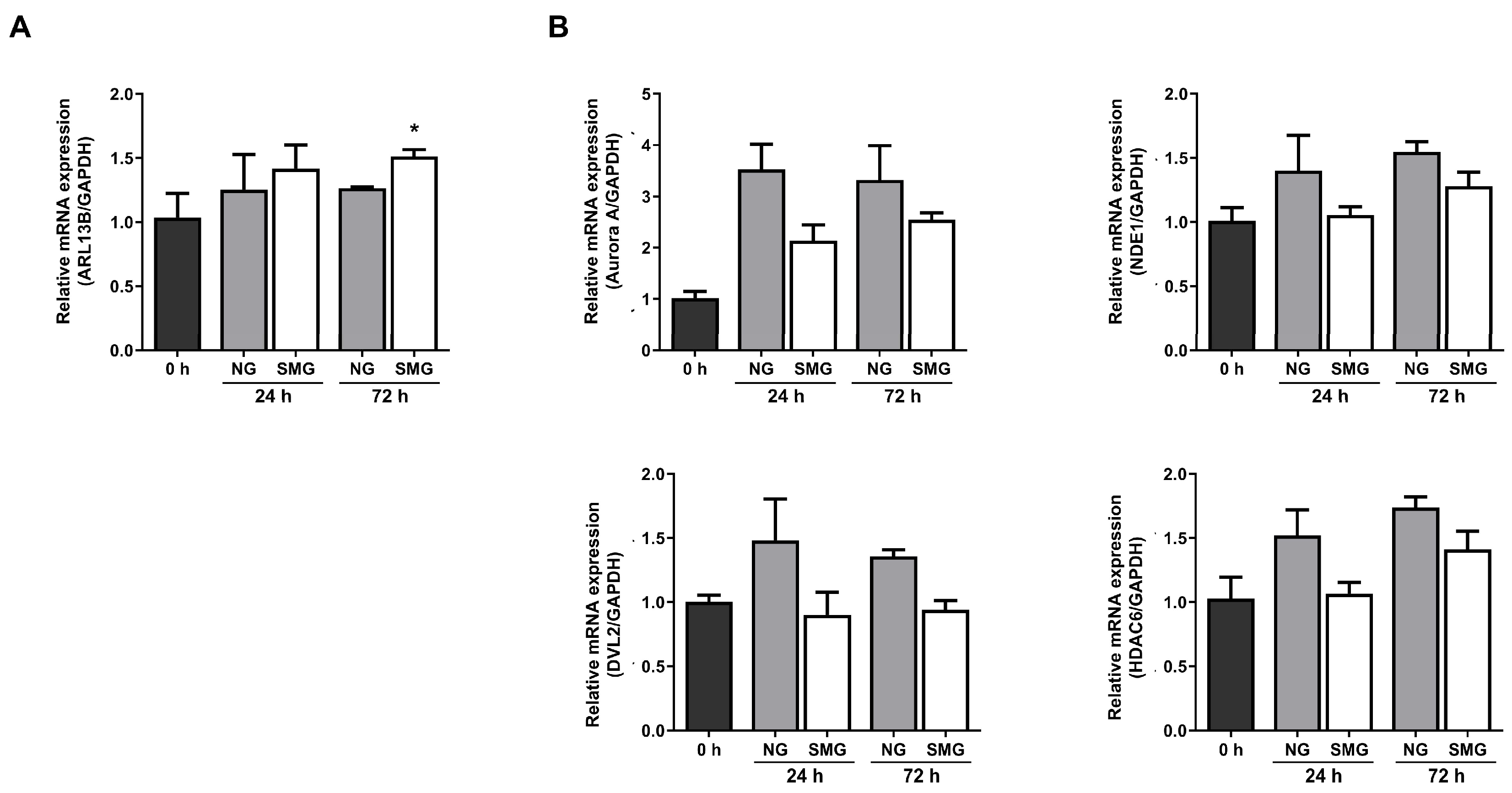
3.3. Change in Ciliogenesis Gene Expression Levels with SMG Exposure
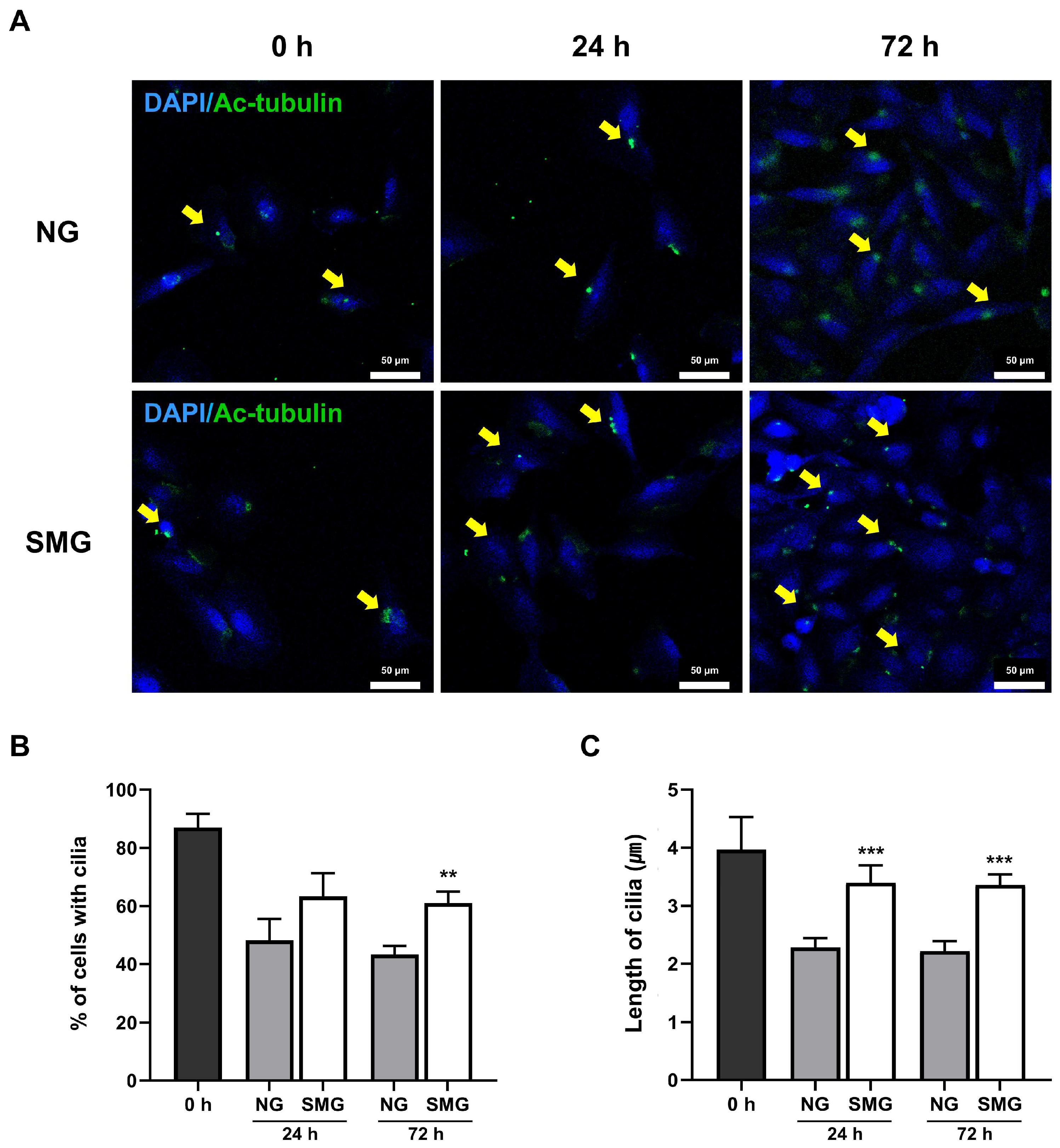
3.4. Change in Cilia Length with SMG Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NG | Normal gravity |
| SMG | Simulate microgravity |
| ARL13B | ADP-ribosylation factor-like protein 13B |
| NDE1 | Nuclear distribution protein nudE homolog 1 |
| HDAC6 | Histone deacetylase 6 |
| DVL2 | Dishevelled segment polarity protein 2 |
| Ac-tubulin | Acetylated α-tubulin |
| DAPI | 4′,6-diamidino-2-phenylindole |
References
- Han, S.J.; Jung, J.K.; Im, S.S.; Lee, S.R.; Jang, B.C.; Park, K.M.; Kim, J.I. Deficiency of primary cilia in kidney epithelial cells induces epithelial to mesenchymal transition. Biochem. Biophys. Res. Commun. 2018, 496, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, D.N.; Wang, A.M.; Strugnell, G.E. Expression of primary cilia in mammalian cells. Cell Biol. Int. 1996, 20, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Y.; Park, J.H. Mouse models of polycystic kidney disease induced by defects of ciliary proteins. BMB Rep. 2013, 46, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Basten, S.G.; Giles, R.H. Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis. Cilia 2013, 2, 6. [Google Scholar] [CrossRef]
- McGowan, C.H.; Russell, P. The DNA damage response: Sensing and signaling. Curr. Opin. Cell Biol. 2004, 16, 629–633. [Google Scholar] [CrossRef]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes Dev. 2011, 25, 409–433. [Google Scholar] [CrossRef]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Langerak, P.; Russell, P. Regulatory networks integrating cell cycle control with DNA damage checkpoints and double-strand break repair. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 3562–3571. [Google Scholar] [CrossRef]
- Medema, R.H.; Macurek, L. Checkpoint control and cancer. Oncogene 2012, 31, 2601–2613. [Google Scholar] [CrossRef]
- Carmena, M. Abscission checkpoint control: Stuck in the middle with Aurora B. Open Biol. 2012, 2, 120095. [Google Scholar] [CrossRef]
- Musacchio, A.; Salmon, E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007, 8, 379–393. [Google Scholar] [CrossRef]
- Deshpande, A.; Sicinski, P.; Hinds, P.W. Cyclins and cdks in development and cancer: A perspective. Oncogene 2005, 24, 2909–2915. [Google Scholar] [CrossRef]
- Sage, J. Cyclin C makes an entry into the cell cycle. Dev. Cell 2004, 6, 607–608. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Plas, D.R.; Rathmell, J.C.; Fox, C.J.; Harris, M.H.; Thompson, C.B. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell. Biol. 2001, 21, 5899–5912. [Google Scholar] [CrossRef] [PubMed]
- Deuel, T.F. Polypeptide growth factors: Roles in normal and abnormal cell growth. Annu. Rev. Cell Biol. 1987, 3, 443–492. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; Roan, E.; Navajas, D. Mechanobiology in lung epithelial cells: Measurements, perturbations, and responses. Compr. Physiol. 2012, 2, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L.; Barkauskas, C.E.; Chapman, H.A.; Epstein, J.A.; Jain, R.; Hsia, C.C.; Niklason, L.; Calle, E.; Le, A.; Randell, S.H.; et al. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 2014, 15, 123–138. [Google Scholar] [CrossRef]
- Croasdell Lucchini, A.; Gachanja, N.N.; Rossi, A.G.; Dorward, D.A.; Lucas, C.D. Epithelial Cells and Inflammation in Pulmonary Wound Repair. Cells 2021, 10, 339. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Ji, G.; Wang, S.; Mo, C.; Ding, B.S. Lung regeneration: Diverse cell types and the therapeutic potential. MedComm 2024, 5, e494. [Google Scholar] [CrossRef]
- Kim, C.F.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005, 121, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Bhogaraju, S.; Cajanek, L.; Fort, C.; Blisnick, T.; Weber, K.; Taschner, M.; Mizuno, N.; Lamla, S.; Bastin, P.; Nigg, E.A.; et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 2013, 341, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, T.C.; Ansari, T.W.; Barnes, N.C.; Jeffery, P.K. Inflammation in bronchial biopsies of subjects with chronic bronchitis: Inverse relationship of CD8+ T lymphocytes with FEV1. Am. J. Respir. Crit. Care Med. 1997, 155, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Jones-Freeman, B.; Starkey, M.R. Bronchioalveolar stem cells in lung repair, regeneration and disease. J. Pathol. 2020, 252, 219–226. [Google Scholar] [CrossRef]
- Zhang, P.; Kiseleva, A.A.; Korobeynikov, V.; Liu, H.; Einarson, M.B.; Golemis, E.A. Microscopy-Based Automated Live Cell Screening for Small Molecules That Affect Ciliation. Front. Genet. 2019, 10, 75. [Google Scholar] [CrossRef]
- Chung, J.H.; Ahn, C.B.; Son, K.H.; Yi, E.; Son, H.S.; Kim, H.S.; Lee, S.H. Simulated Microgravity Effects on Nonsmall Cell Lung Cancer Cell Proliferation and Migration. Aerosp. Med. Hum. Perform. 2017, 88, 82–89. [Google Scholar] [CrossRef]
- Ahn, C.B.B.; Lee, J.H.; Han, D.G.; Kang, H.W.; Lee, S.H.; Lee, J.I.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef]
- Poon, C. Factors implicating the validity and interpretation of mechanobiology studies in simulated microgravity environments. Eng. Rep. 2020, 2, e12242. [Google Scholar] [CrossRef]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Sanon, S.; Hart, D.A.; Tredget, E.E. Molecular and cellular biology of wound healing and skin regeneration. In Skin Tissue Engineering and Regenerative Medicine; Academic Press: New York, NY, USA, 2016; Volume 10. [Google Scholar]
- Gontcharov, I.B.; Kovachevich, I.V.; Pool, S.L.; Navinkov, O.L.; Barratt, M.R.; Bogomolov, V.V.; House, N. In-flight medical incidents in the NASA-Mir program. Aviat. Space Environ. Med. 2005, 76, 692–696. [Google Scholar]
- Agren, M.S.; Steenfos, H.H.; Dabelsteen, S.; Hansen, J.B.; Dabelsteen, E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J. Investig. Dermatol. 1999, 112, 463–469. [Google Scholar] [CrossRef]
- Brem, H.; Golinko, M.S.; Stojadinovic, O.; Kodra, A.; Diegelmann, R.F.; Vukelic, S.; Entero, H.; Coppock, D.L.; Tomic-Canic, M. Primary cultured fibroblasts derived from patients with chronic wounds: A methodology to produce human cell lines and test putative growth factor therapy such as GMCSF. J. Transl. Med. 2008, 6, 75. [Google Scholar] [CrossRef]
- Liang, L.; Stone, R.C.; Stojadinovic, O.; Ramirez, H.; Pastar, I.; Maione, A.G.; Smith, A.; Yanez, V.; Veves, A.; Kirsner, R.S.; et al. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair. Regen. 2016, 24, 943–953. [Google Scholar] [CrossRef]
- Maione, A.G.; Smith, A.; Kashpur, O.; Yanez, V.; Knight, E.; Mooney, D.J.; Veves, A.; Tomic-Canic, M.; Garlick, J.A. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair. Regen. 2016, 24, 630–643. [Google Scholar] [CrossRef]
- Cialdai, F.; Colciago, A.; Pantalone, D.; Rizzo, A.M.; Zava, S.; Morbidelli, L.; Celotti, F.; Bani, D.; Monici, M. Effect of Unloading Condition on the Healing Process and Effectiveness of Platelet Rich Plasma as a Countermeasure: Study on In Vivo and In Vitro Wound Healing Models. Int. J. Mol. Sci. 2020, 21, 407. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, V.; Cucina, A.; Dinicola, S.; Fabrizi, G.; Catizone, A.; Gesualdi, L.; Ceccarelli, S.; Harrath, A.H.; Alwasel, S.H.; Ricci, G.; et al. Microgravity Modifies the Phenotype of Fibroblast and Promotes Remodeling of the Fibroblast-Keratinocyte Interaction in a 3D Co-Culture Model. Int. J. Mol. Sci. 2022, 23, 2163. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T.; Ho, C.N.Q.; Hoang, S.N.; Doan, C.C.; Nguyen, M.T.; Van, H.D.; Ly, C.N.; Le, C.P.M.; Hoang, H.N.Q.; Nguyen, H.T.M.; et al. Morphological Changes of 3T3 Cells under Simulated Microgravity. Cells 2024, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Glazier, J.B.; Hughes, J.M.; Maloney, J.E.; West, J.B. Vertical gradient of alveolar size in lungs of dogs frozen intact. J. Appl. Physiol. 1967, 23, 694–705. [Google Scholar] [CrossRef]
- Bryan, A.C.; Milic-Emili, J.; Pengelly, D. Effect of gravity on the distribution of pulmonary ventilation. J. Appl. Physiol. 1966, 21, 778–784. [Google Scholar] [CrossRef]
- Prisk, G.K. Pulmonary challenges of prolonged journeys to space: Taking your lungs to the moon. Med. J. Aust. 2019, 211, 271–276. [Google Scholar] [CrossRef]
- Yang, J.Q.; Jiang, N.; Li, Z.P.; Guo, S.; Chen, Z.Y.; Li, B.B.; Chai, S.B.; Lu, S.Y.; Yan, H.F.; Sun, P.M.; et al. The effects of microgravity on the digestive system and the new insights it brings to the life sciences. Life Sci. Space Res. 2020, 27, 74–82. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Wolverton, C.; Wyatt, S.E.; Hasenstein, K.H.; van Loon, J. Comparison of Microgravity Analogs to Spaceflight in Studies of Plant Growth and Development. Front. Plant Sci. 2019, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y. Technology using simulated microgravity. Regen. Ther. 2023, 24, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Reddel, R.R.; Ke, Y.; Gerwin, B.I.; McMenamin, M.G.; Lechner, J.F.; Su, R.T.; Brash, D.E.; Park, J.B.; Rhim, J.S.; Harris, C.C. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988, 48, 1904–1909. [Google Scholar] [PubMed]
- Han, X.; Na, T.; Wu, T.; Yuan, B.Z. Human lung epithelial BEAS-2B cells exhibit characteristics of mesenchymal stem cells. PLoS ONE 2020, 15, e0227174. [Google Scholar] [CrossRef]
- Tan, S.; Pei, W.; Huang, H.; Zhou, G.; Hu, W. Additive effects of simulated microgravity and ionizing radiation in cell death, induction of ROS and expression of RAC2 in human bronchial epithelial cells. NPJ Microgravity 2020, 6, 34. [Google Scholar] [CrossRef]
- Wang, P.; Tian, H.; Zhang, J.; Qian, J.; Li, L.; Shi, L.; Zhao, Y. Spaceflight/microgravity inhibits the proliferation of hematopoietic stem cells by decreasing Kit-Ras/cAMP-CREB pathway networks as evidenced by RNA-Seq assays. FASEB J. 2019, 33, 5903–5913. [Google Scholar] [CrossRef]
- Hoang, H.N.Q.; Ho, C.N.Q.; Dang, L.T.T.; Phan, N.L.C.; Doan, C.C.; Nguyen, H.T.M.; Le, C.P.M.; Hoang, S.N.; Le, L.T. The Proliferation of Chang Liver Cells After Simulated Microgravity Induction. Curr. Issues Mol. Biol. 2025, 47, 164. [Google Scholar] [CrossRef]
- Boers, J.E.; Ambergen, A.W.; Thunnissen, F.B. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1998, 157, 2000–2006. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Chen, J.L.; Hong, F.F.; Chen, P.; Wang, J.F. Effects of simulated microgravity on the expression profiles of RNA during osteogenic differentiation of human bone marrow mesenchymal stem cells. Cell Prolif. 2019, 52, e12539. [Google Scholar] [CrossRef]
- Deng, B.; Liu, R.; Tian, X.; Han, Z.; Chen, J. Simulated microgravity inhibits the viability and migration of glioma via FAK/RhoA/Rock and FAK/Nek2 signaling. Vitro Cell. Dev. Biol. Anim. 2019, 55, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Inagaki, M. Primary ciliary signaling: Links with the cell cycle. Trends Cell Biol. 2021, 31, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, T.; Pagano, M. The SCF ubiquitin ligase: Insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 2004, 5, 739–751. [Google Scholar] [CrossRef]
- Lee, K.H.; Johmura, Y.; Yu, L.R.; Park, J.E.; Gao, Y.; Bang, J.K.; Zhou, M.; Veenstra, T.D.; Yeon Kim, B.; Lee, K.S. Identification of a novel Wnt5a-CK1varepsilon-Dvl2-Plk1-mediated primary cilia disassembly pathway. EMBO J. 2012, 31, 3104–3117. [Google Scholar] [CrossRef]
- Yu, F.; Ran, J.; Zhou, J. Ciliopathies: Does HDAC6 Represent a New Therapeutic Target? Trends Pharmacol. Sci. 2016, 37, 114–119. [Google Scholar] [CrossRef]
- Goto, H.; Inoko, A.; Inagaki, M. Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell. Mol. Life Sci. 2013, 70, 3893–3905. [Google Scholar] [CrossRef]
- Janke, C.; Bulinski, J.C. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2011, 12, 773–786. [Google Scholar] [CrossRef]
- Song, Y.; Brady, S.T. Post-translational modifications of tubulin: Pathways to functional diversity of microtubules. Trends Cell Biol. 2015, 25, 125–136. [Google Scholar] [CrossRef]
- Goto, H.; Inaba, H.; Inagaki, M. Mechanisms of ciliogenesis suppression in dividing cells. Cell. Mol. Life Sci. 2017, 74, 881–890. [Google Scholar] [CrossRef]
- Kim, S.; Zaghloul, N.A.; Bubenshchikova, E.; Oh, E.C.; Rankin, S.; Katsanis, N.; Obara, T.; Tsiokas, L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat. Cell Biol. 2011, 13, 351–360. [Google Scholar] [CrossRef]
- Larkins, C.E.; Aviles, G.D.; East, M.P.; Kahn, R.A.; Caspary, T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol. Biol. Cell 2011, 22, 4694–4703. [Google Scholar] [CrossRef] [PubMed]
- Veland, I.R.; Awan, A.; Pedersen, L.B.; Yoder, B.K.; Christensen, S.T. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009, 111, p39–p53. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xie, Y.; He, J.; Zhou, J.; Gao, Y.; Wei, W.; Ding, N.; Ma, H.; Xian, C.J.; Chen, K.; et al. Microgravity induces inhibition of osteoblastic differentiation and mineralization through abrogating primary cilia. Sci. Rep. 2017, 7, 1866. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Y.; Chen, K.; He, J.; Feng, X.; Wei, W.; Hua, J.; Wang, J. Primary cilia act as microgravity sensors by depolymerizing microtubules to inhibit osteoblastic differentiation and mineralization. Bone 2020, 136, 115346. [Google Scholar] [CrossRef]
| Gene | Sequence (5′→3′) | Tm (°C) |
|---|---|---|
| GAPDH | F: ACCAGGTGGTCTCCTCTGAC | 57 |
| R: TGCTTAGCCAAATTCGTTG | ||
| ARL13B | F: AGCCTGTCAGGTTGGCAAAT | 57 |
| R: ACGCTGCTCTGTTGTCTCTT | ||
| Aurora A | F: AAGACTTGGGTCCTTGGGTC | 57 |
| R: GTCCATGATGCCTCTAGCTGT | ||
| HDAC6 | F: GCCTCAATCACTGAGACATCC | 57 |
| R: GGTGCCTTCTTGGTGACAACT | ||
| DVL2 | F: AGAGACAGCAGTGAGCATGG | 57 |
| R: GGAATCTGTGACGCTGCTGA | ||
| NDE1 | F: GACACCATGCCACAAGGAGA | 57 |
| R: TCCATGCGAAGGCGGTTATT | ||
| p27 | F: ATGTCAAACGTGCGAGTGTC | 58 |
| R: TCTCTGCAGTGCTTCTCCAA | ||
| E-cadherin | F: CATCTTTGTGCCTCCTGAAA | 56 |
| R: TGGGCAGTGTAGGATGTGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, S.H.; Hwang, S.; Choi, S.Y.; Kim, H.J.; Kim, J.H.; Lee, S.H.; Lee, J.W.; Son, K.H. Enhanced Ciliogenesis of Human Bronchial Epithelial Cells by Simulated Microgravity. Life 2025, 15, 1864. https://doi.org/10.3390/life15121864
Bang SH, Hwang S, Choi SY, Kim HJ, Kim JH, Lee SH, Lee JW, Son KH. Enhanced Ciliogenesis of Human Bronchial Epithelial Cells by Simulated Microgravity. Life. 2025; 15(12):1864. https://doi.org/10.3390/life15121864
Chicago/Turabian StyleBang, Seung Hyun, Soyoung Hwang, Seon Young Choi, Hyun Joo Kim, Joo Hyung Kim, Sung Ho Lee, Jin Woo Lee, and Kuk Hui Son. 2025. "Enhanced Ciliogenesis of Human Bronchial Epithelial Cells by Simulated Microgravity" Life 15, no. 12: 1864. https://doi.org/10.3390/life15121864
APA StyleBang, S. H., Hwang, S., Choi, S. Y., Kim, H. J., Kim, J. H., Lee, S. H., Lee, J. W., & Son, K. H. (2025). Enhanced Ciliogenesis of Human Bronchial Epithelial Cells by Simulated Microgravity. Life, 15(12), 1864. https://doi.org/10.3390/life15121864






