Intracavernosal Botulinum Toxin Injection for Erectile Dysfunction: A Comprehensive Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Registration
2.2. Study Selection and Eligibility Criteria
2.3. Study Selection Process
2.4. Data Extraction
- -
- Study characteristics: First author, publication year, country, and study design.
- -
- Patient demographics: Sample size, mean age, and ED etiology and severity.
- -
- Intervention details: BoNT-A formulation (e.g., onabotulinumtoxinA), total dose, number and frequency of injections, injection technique, and any concomitant ED therapies.
- -
- Outcome data: As outlined below.
2.5. Outcomes
- -
- The safety and adverse event profile, categorized by nature and frequency.
- -
- The duration of the therapeutic effect.
- -
- Changes in hemodynamic parameters as assessed by penile duplex ultrasonography.
2.6. Data Synthesis and Risk of Bias Assessment
3. Results
3.1. Characteristics of Included Studies
3.2. Efficacy Outcomes
3.3. Safety and Adverse Events
3.4. Predictors of Response and Durability
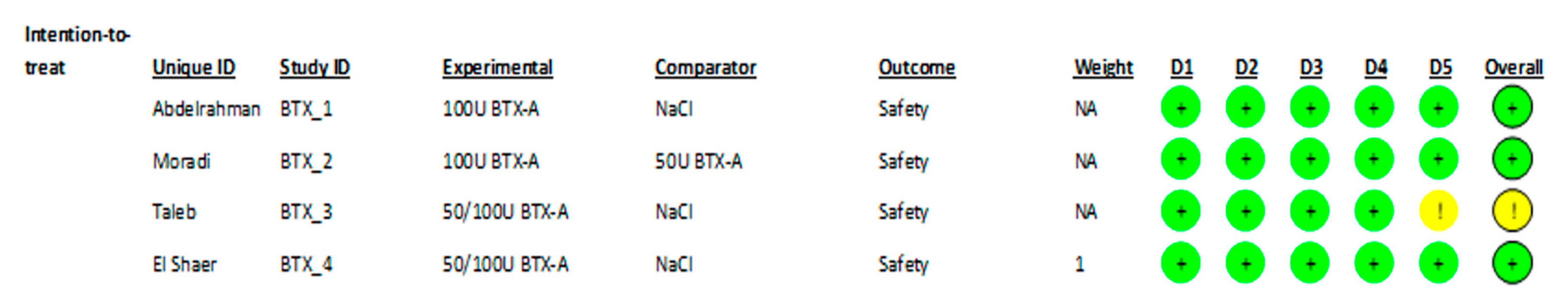
3.5. Risk of Bias Assessment
4. Discussion
4.1. Limitations
4.2. Unresolved Questions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ED | Erectile Dysfunction |
| PDE5-Is | Phosphodiesterase Type 5 Inhibitors |
| BoNT-A | Botulinum Toxin Type A |
| FDA | U.S. Food and Drug Administration |
| IIEF | International Index of Erectile Function |
| SHIM | Sexual Health Inventory for Men |
| EHS | Erection Hardness Score |
| CIs | Confidence Intervals |
| RCT | Randomized Controlled Trial |
| NO | Nitric Oxide |
References
- Pang, K.H. The effectiveness and safety of intracavernosal botulinum toxin injections in the management of erectile dysfunction: A systematic review and meta-analysis of clinical studies. Sex. Med. 2025, 13, qfaf034. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.; Denys, P.; Joussain, C. Safety and Effectiveness of Repeated Botulinum Toxin A Intracavernosal Injections in Men with Erectile Dysfunction Unresponsive to Approved Pharmacological Treatments: Real-World Observational Data. Toxins 2023, 15, 382. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.; Joussain, C.; Denys, P. Safety and Efficacy of Intracavernosal Injections of AbobotulinumtoxinA (Dysport®) as Add on Therapy to Phosphosdiesterase Type 5 Inhibitors or Prostaglandin E1 for Erectile Dysfunction—Case Studies. Toxins 2019, 11, 283. [Google Scholar] [CrossRef]
- Abdelrahman, I.F.S.; Raheem, A.A.; Elkhiat, Y.; Aburahma, A.A.; Abdel-Raheem, T.; Ghanem, H. Safety and efficacy of botulinum neurotoxin in the treatment of erectile dysfunction refractory to phosphodiesterase inhibitors: Results of a randomized controlled trial. Andrology 2022, 10, 254–261. [Google Scholar] [CrossRef]
- Chung, E. A review of current and emerging therapeutic options for erectile dysfunction. Med. Sci. 2019, 7, 91. [Google Scholar] [CrossRef]
- Duncan, C.; Omran, G.; Teh, J.; Davis, N.; Bolton, D.; Lawrentschuk, N. Erectile dysfunction: A global review of intracavernosal injectables. World J. Urol. 2019, 37, 1007–1014. [Google Scholar] [CrossRef]
- Moradi, S.; Khazaeli, D.; Dadfar, M.; Bakhtiari, N. Efficacy of Intracavernosal Injections of 50-Unit versus 100-Unit Doses of AbobotulinumtoxinA (Masport®) in Vasculogenic Erectile Dysfunction with Phosphodiesterase Type 5 Inhibitors Resistant. Nephrourol. Mon. 2022, 14, e119131. [Google Scholar] [CrossRef]
- Zahr, R.A.; Kheir, G.B.; Mjaess, G.; Jabbour, T.; Chalhoub, K.; Diamand, R.; Roumeguère, T. Intra-Cavernosal injection of botulinum toxin in the treatment of erectile dysfunction: A systematic review and meta-analysis. Urology 2022, 170, 5–13. [Google Scholar] [CrossRef]
- Ragab, M.W.; Ramzy, D.; Zidane, A.; El-Bohy, A.; Bendary, A.Z.; A Shawky, K.; El Shorbagy, G. (335) A Novel Ultrasound Technique Can Predict Efficacy of Botox Injection in Patients with Erectile Dysfunction. J. Sex. Med. 2024, 21, qdae041-012. [Google Scholar] [CrossRef]
- Langarizadeh, M.A.; Salary, A.; Tavakoli, M.R.; Nejad, B.G.; Fadaei, S.; Jahani, Z.; Forootanfar, H. An overview of the history, current strategies, and potential future treatment approaches in erectile dysfunction: A comprehensive review. Sex. Med. Rev. 2023, 11, 253–267. [Google Scholar] [CrossRef]
- Liu, M.; Chang, M.-L.; Wang, Y.-C.; Chen, W.-H.; Wu, C.; Yeh, S. Revisiting the regenerative therapeutic advances towards erectile dysfunction. Cells 2020, 9, 1250. [Google Scholar] [CrossRef]
- Giuliano, F.; Joussain, C.; Denys, P. Long Term Effectiveness and Safety of Intracavernosal Botulinum Toxin A as an Add-on Therapy to Phosphosdiesterase Type 5 Inhibitors or Prostaglandin E1 Injections for Erectile Dysfunction. J. Sex. Med. 2021, 19, 83–89. [Google Scholar] [CrossRef]
- Giuliano, F.; Denys, P.; Joussain, C. Effectiveness and Safety of Intracavernosal IncobotulinumtoxinA (Xeomin®) 100 U as an Add-on Therapy to Standard Pharmacological Treatment for Difficult-to-Treat Erectile Dysfunction: A Case Series. Toxins 2022, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Preto, M.; Keskin, H.; Vazquez, J.F.S.; Banthia, R.; Mahendran, T.; Deger, M.D.; Vinod, K.V.; Putra, R.; Sethupathy, T.; et al. Clinical effects and safety outcomes of platelet-rich plasma therapy in patients with vasculogenic erectile dysfunction: A systematic review and meta-analysis. World J. Men’s Health 2025, 43, e16. [Google Scholar] [CrossRef] [PubMed]
- Masterson, T.A.; Molina, M.; Ledesma, B.; Zucker, I.; Saltzman, R.; Ibrahim, E.; Han, S.; Reis, I.M.; Ramasamy, R. Platelet-rich plasma for the treatment of erectile dysfunction: A prospective, randomized, double-blind, placebo-controlled clinical trial. J. Urol. 2023, 210, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, H.; Raheem, A.; Abdelrahman, I.; Johnson, M.; Abdel-Raheem, T. Botulinum neurotoxin and its potential role in the treatment of erectile dysfunction. Sex. Med. Rev. 2018, 6, 135–142. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Cho, S.; Chung, H.; Rajasekaran, M. Novel emerging therapies for erectile dysfunction. World J. Men’s Health 2021, 39, 48–64. [Google Scholar] [CrossRef]
- Krivoborodov, G.G.; Kuzmin, I.V.; Slesarevskaya, M.N.; Efremov, N.S.; Gontar, A.A. Botulinum toxin therapy in urology: Historical aspect. Urol. Vedom. 2024, 14, 163–174. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Palea, S. Embracing the versatility of botulinum neurotoxins in conventional and new therapeutic applications. Toxins 2024, 16, 261. [Google Scholar] [CrossRef]
- Reddy, A.; Dick, B.; Natale, C.; Akula, K.; Yousif, A.; Hellstrom, W. Application of botulinum neurotoxin in male sexual dysfunction: Where are we now? Sex. Med. Rev. 2021, 9, 320–330. [Google Scholar] [CrossRef]
- Shaher, H.; Noah, K.; Abdelzaher, M.; Kandil, W.; Ahmed, I.S.; Nouh, I. Is bulbospongiosus muscle botox injection safe and effective in treating lifelong premature ejaculation? Randomised controlled study. World J. Urol. 2024, 42, 218. [Google Scholar] [CrossRef] [PubMed]
- Ragab, M.W.; Shawky, K.A.; Bendary, A.Z.E.F.; Albohy, A.-A.M. Penile shear-wave elastography predicts the outcome of botulinum neurotoxin (Botox) in the management of non-responders to phosphodiesterase-5-inhibitors: A pilot study. Arab. J. Urol. 2025, 23, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; EI-Tabey, M.; El-Shaer, W.; Ghanem, H.; Wahab, T. Comparative Study between Intracavernosal Injection of Botulinum Toxin type A [50 and 100 unit], Efficacy and Durability in the Treatment of Vascular Erectile Dysfunction. Benha J. Appl. Sci. 2019, 4, 31–35. [Google Scholar] [CrossRef]
- El-Shaer, W.; Ghanem, H.; Diab, T.; Abo-Taleb, A.; Kandeel, W. Intra-cavernous injection of BOTOX® (50 and 100 Units) for treatment of vasculogenic erectile dysfunction: Randomized controlled trial. Andrology 2021, 9, 1166–1175. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Raheem, A.; Abdelrahman, I.; Abdel-Raheem, T.; Tawfik, A.; Elkhayat, Y.; Ghanem, H. Botulinum neurotoxin in the treatment of erectile dysfunction. J. Sex. Med. 2022, 19, S9–S10. [Google Scholar] [CrossRef]

| Key Papers | |||||
|---|---|---|---|---|---|
| Title | Author and Date | Study Design | Population | Key Result | Adverse Events |
| 1. The effectiveness and safety of intracavernosal botulinum toxin injections in the management of erectile dysfunction: a systematic review and meta-analysis of clinical studies | K.H. Pang (2025) [1] | Systematic review and meta-analysis | Men with ED, various severities | 40–77.5% response rate, significant IIEF/SHIM improvement | Transient penile pain, rare priapism |
| 2. Safety and Effectiveness of Repeated Botulinum Toxin A Intracavernosal Injections in Men with Erectile Dysfunction Unresponsive to Approved Pharmacological Treatments: Real-World Observational Data | F. Giuliano et al. (2023) [2] | Retrospective case series | ED refractory to PDE5-Is/PGE1 | 77.5% response, increased with repeated injections | Mild pain, rare burn |
| 3. Safety and Efficacy of Botulinum Neurotoxin in the Treatment of Erectile Dysfunction Refractory to Phosphodiesterase Inhibitors: Results of a Randomized Controlled Trial | I.F.S. Abdelrahman et al. (2022) [4] | RCT | ED refractory to PDE5-Is | Significant improvement in EHS, SHIM, SEP-2/3 | No severe adverse events |
| 4. Long Term Effectiveness and Safety of Intracavernosal Botulinum Toxin A as an Add-on Therapy to Phosphodiesterase Type 5 Inhibitors or Prostaglandin E1 Injections for Erectile Dysfunction | F. Giuliano et al. (2021) [12] | Retrospective, uncontrolled | ED non-responders to PDE5-Is/PGE1 | 41–50% response at 6 months | Mild pain only |
| 5. Efficacy of Intracavernosal Injections of 50-Unit versus 100-Unit Doses of AbobotulinumtoxinA (Masport®) in Vasculogenic Erectile Dysfunction with Phosphodiesterase Type 5 Inhibitors Resistant | S. Moradi et al. (2022) [7] | Double-blind RCT | Vasculogenic ED, PDE5i-resistant | 100 U more effective and durable than 50 U | Brief penile pain only |
| Author (Year) | Country | Toxin Type | Dose (U) | Sample Size (N) | Population | Study Design | Main Findings |
|---|---|---|---|---|---|---|---|
| Taleb et al. (2019) [23] | Egypt | Onabotulinum toxin A | 50–100 U | 45 | Refractory vasculogenic ED | Double-blind RCT | Dose-dependent improvement in erectile function. |
| El-Shaer et al. (2021) [24] | Egypt | Onabotulinum toxin A | 50–100 U | 176 | Refractory vasculogenic ED | Double-blind RCT | Improvement in IIEF and SHIM scores; effect maintained up to 6 months. |
| Moradi et al. (2022) [7] | Iran | Abobotulinum toxin A | 100 U | 40 | Refractory vasculogenic ED | Double-blind RCT | Improved penile rigidity and patient satisfaction. |
| Abdelrahman et al. (2022) [4]. | Egypt | Onabotulinum toxin A | 100 U | 70 | Refractory vasculogenic ED | Double-blind RCT | Significant improvement in IIEF and EHS. |
| Claim | Evidence Strength | Reasoning | Papers |
|---|---|---|---|
| BoNT-A penile injection improves erectile function in men with ED refractory to standard therapies | Strong | Multiple RCTs and meta-analyses show significant improvements in IIEF/SHIM/EHS vs. placebo | [1,2,3,4,7,8] |
| BoNT-A injection is safe, with only mild, transient adverse events | Strong | Consistent safety profile across studies, no systemic side effects, rare serious events | [1,2,3,12] |
| Higher doses (100 U) of BoNT-A are more effective and durable than lower doses (50 U) | Moderate | Dose-comparison RCTs and case series show greater and longer-lasting effects with 100 U | [7,23] |
| Repeated BoNT-A injections may enhance and prolong the therapeutic response | Moderate | Observational data show increased response rates with multiple injections | [2,13] |
| Imaging biomarkers (e.g., shear wave elastography) may predict response to BoNT-A | Moderate | Pilot studies suggest a correlation between tissue stiffness and treatment outcome | [9] |
| Long-term efficacy and safety of BoNT-A penile injection remain uncertain | Weak | Lack of large, long-term RCTs and standardized protocols | [1,8,20,26] |
| Question | Why |
|---|---|
| What is the long-term efficacy and safety of repeated BoNT-A penile injections for ED? | To determine durability and cumulative risk, informing clinical guidelines. |
| Which patient characteristics or biomarkers best predict response to BoNT-A injection? | To optimize patient selection and maximize treatment benefit. |
| What is the optimal dosing regimen and injection protocol for BoNT-A in ED? | To standardize practice and improve outcomes across diverse populations. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talavera Cobo, V.; Yanez Ruiz, C.A.; Tapia Tapia, M.D.; Calva Lopez, A.; Muñoz Bastidas, C.A.; Guillen-Grima, F.; Ancizu Marckert, F.J.; Labairu Huerta, L.; Torres Roca, M.; Diez-Caballero Alonso, F.J.; et al. Intracavernosal Botulinum Toxin Injection for Erectile Dysfunction: A Comprehensive Systematic Review. Life 2025, 15, 1826. https://doi.org/10.3390/life15121826
Talavera Cobo V, Yanez Ruiz CA, Tapia Tapia MD, Calva Lopez A, Muñoz Bastidas CA, Guillen-Grima F, Ancizu Marckert FJ, Labairu Huerta L, Torres Roca M, Diez-Caballero Alonso FJ, et al. Intracavernosal Botulinum Toxin Injection for Erectile Dysfunction: A Comprehensive Systematic Review. Life. 2025; 15(12):1826. https://doi.org/10.3390/life15121826
Chicago/Turabian StyleTalavera Cobo, Vanessa, Carlos Andres Yanez Ruiz, Mario Daniel Tapia Tapia, Andres Calva Lopez, Carmina Alejandra Muñoz Bastidas, Francisco Guillen-Grima, Francisco Javier Ancizu Marckert, Luis Labairu Huerta, Marcos Torres Roca, Fernando Jose Diez-Caballero Alonso, and et al. 2025. "Intracavernosal Botulinum Toxin Injection for Erectile Dysfunction: A Comprehensive Systematic Review" Life 15, no. 12: 1826. https://doi.org/10.3390/life15121826
APA StyleTalavera Cobo, V., Yanez Ruiz, C. A., Tapia Tapia, M. D., Calva Lopez, A., Muñoz Bastidas, C. A., Guillen-Grima, F., Ancizu Marckert, F. J., Labairu Huerta, L., Torres Roca, M., Diez-Caballero Alonso, F. J., Sanchez Zalabardo, D., Miñana Lopez, B., & Robles Garcia, J. E. (2025). Intracavernosal Botulinum Toxin Injection for Erectile Dysfunction: A Comprehensive Systematic Review. Life, 15(12), 1826. https://doi.org/10.3390/life15121826







