Cardiac Hemangioma in the Right Atrium: Diagnostic Challenges, Imaging Clues, and a Novel Algorithm for Differential Diagnosis
Abstract
1. Introduction
2. Case Presentation
3. Discussion
3.1. Diagnostic Challenges and Imaging Clues
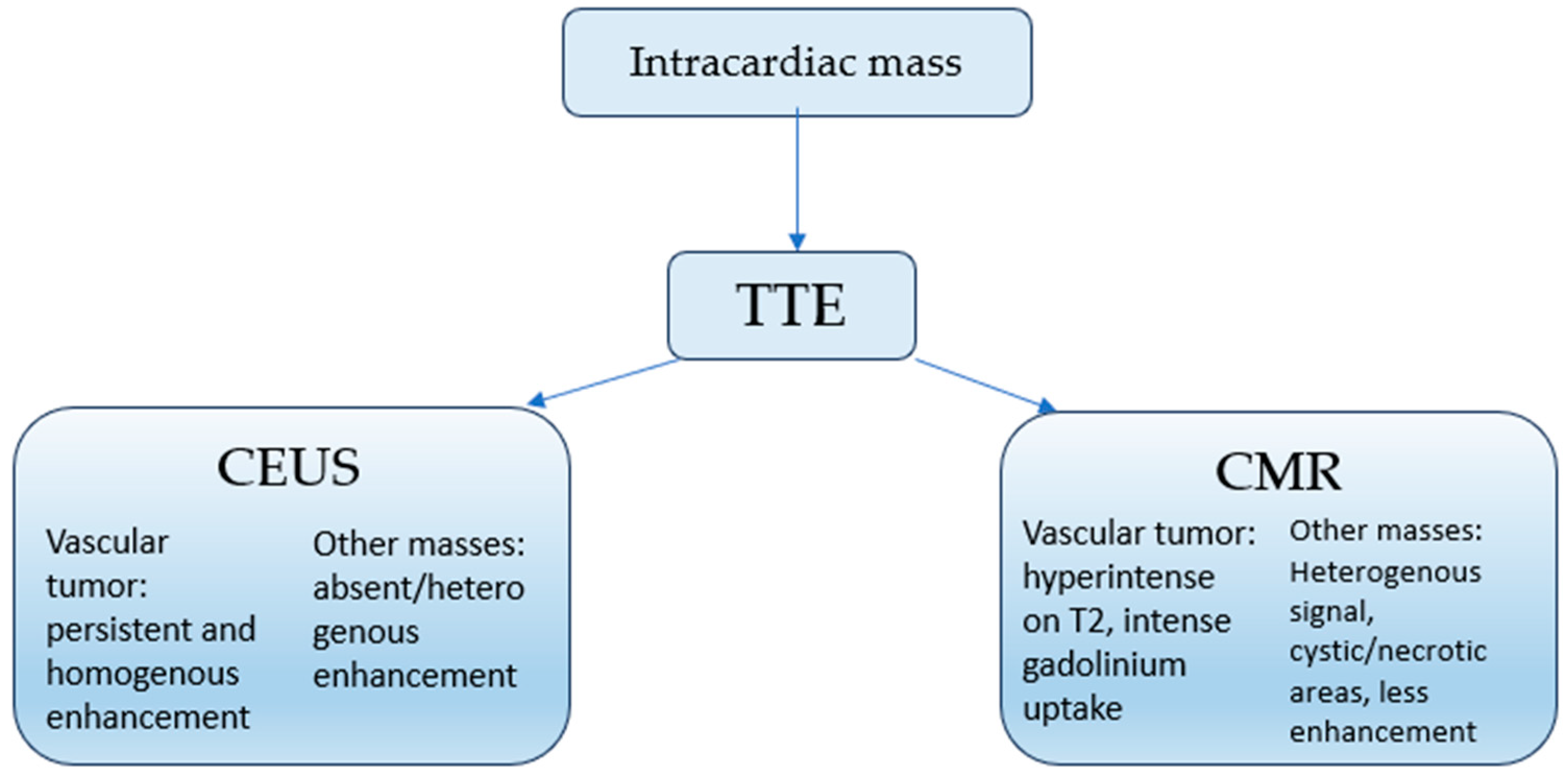
3.2. Proposed Diagnostic Algorithm
3.3. Subdiagnosis and Implications
3.4. Surgical Management and Prognosis
3.5. Limitations
3.6. Future Perspectives
4. Conclusions
- Cardiac hemangiomas are likely underdiagnosed, frequently misclassified as other benign tumors.
- Multimodal imaging—including contrast echocardiography, cardiac MRI, and coronary angiography—should be incorporated into the diagnostic pathway for atrial masses as it may provide valuable preoperative clues and reduce misdiagnosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Atrial Septal Defect |
| CEUS | Contrast-Enhanced Echocardiography |
| CMR | Cardiac Magnetic Resonance |
| CPB | Cardiopulmonary Bypass |
| H&E | Hematoxylin–Eosin |
| LVEF | Left Ventricular Ejection Fraction |
| MR | Mitral Regurgitation |
| NYHA | New York Heart Association |
| PASP | Pulmonary Artery Systolic Pressure |
| RA | Right Atrium |
| RCA | Right Coronary Artery |
| RV | Right Ventricle |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TTE | Transthoracic Echocardiography |
| TR | Tricuspid Regurgitation |
References
- Li, W.; Teng, P.; Xu, H.; Ma, L.; Ni, Y. Cardiac Hemangioma: A Comprehensive Analysis of 200 Cases. Ann. Thorac. Surg. 2015, 99, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Brizard, C.; Latremouille, C.; Jebara, V.A.; Acar, C.; Fabiani, J.N.; Deloche, A.; Carpentier, A.F. Cardiac Hemangiomas. Ann. Thorac. Surg. 1993, 56, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Reynen, K. Frequency of Primary Tumors of the Haart. Am. J. Cardiol. 1996, 77, 107. [Google Scholar] [CrossRef]
- McAllister, H.A.; Fenoglio, J.J. Tumors of the Cardiovascular System. Hartmann 1978, 2, 81. [Google Scholar]
- Munteanu, I.R.; Novaconi, R.C.; Merce, A.P.; Dima, C.N.; Falnita, L.S.; Manzur, A.R.; Streian, C.G.; Feier, H.B. Cardiac Hemangiomas: A Five-Year Systematic Review of Diagnosis, Treatment, and Outcomes. Cancers 2025, 17, 1532. [Google Scholar] [CrossRef]
- Okongwu, C.C.; Olaofe, O.O. Cardiac Myxoma: A Comprehensive Review. J. Cardiothorac. Surg. 2025, 20, 151. [Google Scholar] [CrossRef]
- Ashinze, P.; Banerjee, S.; Egbunu, E.; Salawu, W.; Idris-Agbabiaka, A.; Obafemi, E.; Olajuwon, T.J.; Chukwu, B.; Aremu, S.A.; David, O.-O.O.; et al. Cardiac Myxomas: A Review of Current Treatment Approaches and Emerging Molecular Therapies. Cardiothorac. Surg. 2024, 32, 22. [Google Scholar] [CrossRef]
- Mendyka, D.; Płonek, T.; Jędrasek, T.; Korman, A.; Złotowska, A.; Jędrasek, A.; Skalik, R.; Kustrzycki, W. The Therapeutic Potential of Different Surgical Approaches in the Management of Cardiac Myxoma: A Systematic Review. J. Clin. Med. 2025, 14, 121. [Google Scholar] [CrossRef]
- Richter, G.T.; Friedman, A.B. Hemangiomas and Vascular Malformations: Current Theory and Management. Int. J. Pediatr. 2012, 2012, 645678. [Google Scholar] [CrossRef]
- Fletcher, C.D.M. Diagnostic Histopathology of Tumors; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Mosby. WHO Classification of Soft Tissue Tumors. In Anderson’s Pathology, 10th ed.; Mosby: Maryland Heights, MO, USA, 1996. [Google Scholar]
- Gourmelon, J.; Loobuyck, V.; Silvestri, V.; Chaput, A.; Altes, A. Right Atrial Cardiac Hemangioma: A Multidisciplinary Pathway From Symptoms to Surgery. JACC Case Rep. 2024, 29, 102920. [Google Scholar] [CrossRef] [PubMed]
- Qamar, F.; Kharsa, C.; Letham, P.; Aoun, J.; Goel, S.S.; Kleiman, N.S.; Reardon, M.J.; Atkins, M.D. Cardiac Cavernous Hemangioma. JACC Case Rep. 2025, 30, 102956. [Google Scholar] [CrossRef]
- Kondo, Y.; Yasutsune, T.; Kado, Y.; Jinzai, Y.; Takigawa, T.; Kishigami, T.; Inaba, Y.; Nishimura, Y. Giant Cardiac Hemangioma in the Right Atrium: An Asymptomatic Surgical Case. General. Thorac. Cardiovasc. Surg. Cases 2023, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Shashikanth, M.; Nicola, S.; Yi, C.; Julian, S. Right Atrial Cavernous Hemangioma. Ann. Card. Anaesth. 2020, 23, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Thilak, R.; Sivanesan, A.; Munuswamy, H.; Toi, P.C. A Giant Right Atrial Hemangioma–Case Report. Cureus 2022, 14, e24622. [Google Scholar] [CrossRef] [PubMed]
- Kesieme, E.B.; Buchan, K.G. Multiple Right Ventricular Haemangiomas. Cureus 2023, 15, e36570. [Google Scholar] [CrossRef]
- Cattapan, C.; Testolin, L.; Caprioglio, F.; Cerrito, L.F.; Basso, C.; Rizzo, S.; Gerosa, G. Preoperative Transcatheter Diagnosis of Right Atrial Hemangioma. JACC Case Rep. 2023, 15, 101857. [Google Scholar] [CrossRef]
- Fu, Y.; Ma, H.; Guo, Y. Giant Cavernous Hemangioma in the Aortic Root and Right Atrioventricular Groove. J. Thorac. Cardiovasc. Surg. 2020, 159, e291–e293. [Google Scholar] [CrossRef]
- Dobritoiu, F.; Moldovan, H.; Oncica, R.; Vasile, G.; Nechifor, E.; Copaescu, C. Giant Cavernous Hemangioma of the Right Atrium—A Rare Case and Literature Review. Chirurgia 2020, 115, 267–273. [Google Scholar] [CrossRef]
- Parato, V.M.; Nocco, S.; Alunni, G.; Becherini, F.; Conti, S.; Cucchini, U.; Di Giannuario, G.; Di Nora, C.; Fabiani, D.; La Carrubba, S.; et al. Alunni Imaging of Cardiac Masses: An Updated Overview. J. Cardiovasc. Echogr. 2022, 32, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ku, L.; Chen, Y.; Wang, Y.; Liu, Z.; Ma, X. IMAGE OF THE MONTH Multimodality Imaging for the Diagnosis of Giant Cavernous Hemangioma of the Right Ventricle. Hell. J. Cardiol. 2024; in press. [Google Scholar] [CrossRef]
- Jin, Y.N.; Cheng, J.L.; Zhang, Y.; Shao, X.N.; Zhang, X.P.; Zhang, W.B. An Mri Image Analysis of Primary Cardiac Neoplasms. Int. J. Gen. Med. 2021, 14, 2943–2951. [Google Scholar] [CrossRef]
- Angeli, F.; Bodega, F.; Bergamaschi, L.; Armillotta, M.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; Cavallo, D.; Foà, A.; et al. Multimodality Imaging in the Diagnostic Work-Up of Patients with Cardiac Masses: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 847–862. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.; Zhang, X.; Zhong, X.; Chang, S.; Yang, J.; Liang, J.; You, Q.; Zhou, H.; Zhang, J. The Usefulness of Contrast Echocardiography in the Evaluation of Cardiac Masses: A Multicenter Study. BMC Cardiovasc. Disord. 2024, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Benign or Malignant Cardiac Mass: Refining the Role of Cardiac Magnetic Resonance. Circ. Cardiovasc. Imaging 2024, 17, E016574. [Google Scholar] [CrossRef]
- Barison, A.; Timoteo, A.T.; El Messaoudi, S.; Borodzicz-Jazdzyk, S.; Moscatelli, S.; Mandoli, G.E.; Luong, C.; Levelt, E.; Ramkisoensing, A.A.; Raisi-Estabragh, Z.; et al. Cardiovascular Imaging in 2024: Review of Current Research and Innovations. Eur. Heart J.—Imaging Methods Pract. 2025, 3, qyaf066. [Google Scholar] [CrossRef] [PubMed]
- Rattenni, F.; Arlati, F.G.; Galanti, A.; Sansone, F.; Clerici, A.; Triggiani, M.; Muneretto, C. Advanced Presentation of Cardiac Hemangioma. J. Cardiothorac. Surg. 2024, 19, 620. [Google Scholar] [CrossRef]
- Bernal-Gallego, B.; Hernández-Jiménez, V.; Castillo, L.; González-Davia, R.; De Antonio-Antón, N.; Reyes-Copa, G. Unexpected Diagnosis: Large Hemangioma in the Interatrial Septum. J. Cardiothorac. Surg. 2024, 19, 305. [Google Scholar] [CrossRef] [PubMed]
- Dema, A.; Taban, S.; Cornianu, M. Morfopatologie Generala; Victor Babes: Timisoara, Romania, 2019. [Google Scholar]
- Wang, P.; Chapman, D.; Siddiqui, F. A Rare Cardiac Cavernous Hemangioma Treated with Radiotherapy. Case Rep. Vasc. Med. 2022, 2022, 2698475. [Google Scholar] [CrossRef]
- Fernandez-Flores, A.; Cassarino, D.; Colmenero, I. Vascular Malformations: A Histopathologic and Conceptual Appraisal. Actas Dermosifiliogr. 2023, 114, 213–228. [Google Scholar] [CrossRef]
- Ilcheva, L.; Cholubek, M.; Loiero, D.; Dzemali, O. Cardiac Hemangioma in the Left Ventricular Septum. Thorac. Cardiovasc. Surg. Rep. 2024, 13, e4–e7. [Google Scholar] [CrossRef]
- Shah, M.; Russo, L.P.; Haddad, D.; Chang, J.; Okere, A. Cardiac Hemangioma: A Rare Tumor Presenting as Postpartum Chest Pain. Cureus 2023, 15, e44407. [Google Scholar] [CrossRef]
- Berdica, L.; Kola, E.; Nakuci, D.; Horjeti, E.; Alimehmeti, M. Cardiac Hemangioma Presenting as a Primary Cardiac Tumor. Cardio-Oncology 2023, 9, 3. [Google Scholar] [CrossRef]
- Park, O.; Ying, G.W.; Ram, A.; Vemireddy, L.P.; Zahra, F. A Rare Case of Cavernous Hemangioma of the Mitral Valve Presenting As Multifocal Embolic Brain Infarcts. Cureus 2021, 13, e17721. [Google Scholar] [CrossRef] [PubMed]
- Karaağaç, E.; Yeşilkaya, N.; Tellioğlu, T.M.; Ünay, F.Ç.; Beşir, Y. A Rare Cardiac Tumor Presenting with Myxoma: Primary Cardiac Hemangioendothelioma. Turk. J. Thorac. Cardiovasc. Surg. 2021, 29, 110–113. [Google Scholar] [CrossRef]
- Alsaloum, M.; Lee, C.; Dudorova, E.; Szabolcs, M.J.; Shetty, M.; Navot, B.; Ravalli, S. A Right Atrial Mass Discovered Postpartum: A Diagnostic Challenge. CASE 2023, 7, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, P.; Bordonaro, V.; Curione, D.; Perazzolo, A.; Ciancarella, P.; Santangelo, T.; Napolitano, C.; Natale, L.; Galletti, L.; Secinaro, A. Additional Value of Cardiac Magnetic Resonance Parametric Mapping in Tissue Characterization of Common Benign Paediatric Cardiac Tumours. Eur. Heart J. Cardiovasc. Imaging 2024, 26, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Numata, S.; Hohri, Y.; Kawajiri, H.; Yaku, H. Cardiac Cavernous Hemangioma with High Fluorodeoxyglucose Uptake on Preoperative Positron Emission Tomography/Computed Tomography: A Case Report. General. Thorac. Cardiovasc. Surg. Cases 2023, 2, 39. [Google Scholar] [CrossRef]
- Dursun, A.; Hakgor, A.; Kenger, M.Z.; Karaca, O. Concomitant Right Atrial Hemangioma Resection with LVAD Implantation: First-in-Human Experience. JACC Case Rep. 2024, 29, 24. [Google Scholar] [CrossRef]
- Bayfield, N.; Bibo, L.; Wang, E.; Passage, J. Cavernous Haemangioma of Anterior Mitral Valve Leaflet: Diagnostic Utility of 3D Echocardiography. BMJ Case Rep. 2022, 15, e247352. [Google Scholar] [CrossRef]
- Singh, H.; Dasagrandhi, V.; Kumar, R.; Kumar, R.; Mittal, B.R. Impact of 18F-Fluorodeoxyglucose Positron Emission Tomography Computed Tomography Imaging in a Case of Pericardial Cavernous Hemangioma. Indian J. Nucl. Med. 2020, 35, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Kalinic, N.; Tomanic, T.C.; Redzek, A.; Sobot, N.; Skrbic, R.; Radomir, B.; Bjeljac, I.; Jonjev, Z.; Maric, S.; Miljevic, I.B.; et al. Pericardial Hemangioma: An Extremely Rare Cardiac Tumor. Kardiol. Pol. 2024, 82, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Bangal, K.; Keshav, M.; Joshi, S.S.; Murthy, K. Hemangioma (Arteriovenous Type) Forming an Interventricular Septal Mass. Ann. Card. Anaesth. 2023, 26, 237–238. [Google Scholar] [CrossRef]
- Caicedo, D.; Oshiro, K.; Glickstein, J.S.; Krishnan, U.; DiLorenzo, M.P. Cardiac Hemangioma in an Asymptomatic Teenager with a History of Congenital Heart Disease. CASE 2020, 4, 362–364. [Google Scholar] [CrossRef]
- Yildirim, S.; Işik, M.; Tanyeli, Ö.; Görmüş, N. Giant Left Atrial Capillary Haemangioma Invading Left-Main Coronary Artery. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 631–633. [Google Scholar] [CrossRef]
- Cheaban, R.; Piran, M.; Opacic, D.; Gummert, J.F.; Rojas, S.V. Epicardial Cavernous Haemangioma; A Case Report of a Unique Incidental Finding. Eur. Heart J. Case Rep. 2024, 8, ytae146. [Google Scholar] [CrossRef]
- Harrison, S. Rare Case of Cavernous Haemangioma of the Right Atrium with Probable Hepatic Haemangioma. Case Rep. Cardiol. 2022, 2022, 9214196. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, D.G.; Hajisaeed, S.R.; Othman, Y.N.; Anwar, E.O.; Majeed, Z.S.; Ali, R.K.; Muhamad, H.N.; Qadir, O.O. Patient with Myelodysplastic Syndrome Presented with Recurrent Pericardial Effusion Diagnosed as Epicardial Hemangioma; Case Report of a Rare Diagnosis with Rare Presentation. Radiol. Case Rep. 2023, 18, 2253–2258. [Google Scholar] [CrossRef]
- Xie, T.; Masroor, M.; Chen, X.; Liu, F.; Zhang, J.; Yang, D.; Liu, C.; Xiang, M. Rheumatism as a Cause of Cardiac Hemangioma: A Rare Case Report and Review of Literature with Special Focus on Etiology. BMC Cardiovasc. Disord. 2023, 23, 203. [Google Scholar] [CrossRef]
- Batko, J.; Rams, D.J.; Bartuś, K.; Bartoszcze, A.; Litwinowicz, R.A. Cardiac Hemangioma in the Atrioventricular Node Localization. Kardiochirurgia I Torakochirurgia Pol. 2023, 20, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.Y.; Silva, D.R.d.; Cardoso, A.P.T.; Stolf, N.A.G.; Pozzan, G. Cardiac Papillary Muscle Hemangioma. Autops. Case Rep. 2020, 10, e2020169. [Google Scholar] [CrossRef]
- Yang, C.M.; Hu, Y.N. Cardiac Hemangioma Mimicking Infective Endocarditis. Diagnostics 2024, 14, 2109. [Google Scholar] [CrossRef]
- Osada, H.; Yamazaki, K.; Suzuki, T.; Tomotsuka, S.; Sugimoto, A.; Fujimoto, M.; Minatoya, K. Cardiac Capillary Hemangioma Originating from the Mitral Valve. JTCVS Tech. 2023, 20, 127–129. [Google Scholar] [CrossRef]
- Fan, J.; Guo, L.; Teng, P.; Dai, X.; Zheng, Q.; Wu, S.; Ni, Y. Diagnostic Mystery—A Rare Right Ventricular Cardiac Hemangioma: A Case Report. J. Cardiothorac. Surg. 2021, 16, 362. [Google Scholar] [CrossRef]
- Anbardar, M.H.; Soleimani, N.; Mohammadzadeh, S. Two Cases of Cardiac Hemangioma in Different Anatomical Locations Presenting with Chest Pain and Palpitation. Clin. Case Rep. 2022, 10, e05495. [Google Scholar] [CrossRef]
- Toscano, M.; Alves, A.R.; Matias, C.; Carvalho, M.; Marques, M. Hemangioma of the Mitral Valve: Following the Murmur. Rev. Port. De Cardiol. 2022, 41, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Kaewboonlert, N.; Chunharas, P.; Pluthikarmpae, N.; Poontananggul, J.; Wongthep, A.; Pongsuwan, N.; Lerssuttipon, U. Right Ventricular Outflow Tract Obstruction by Cardiac Hemangioma in Asymptomatic Patient. J. Surg. Case Rep. 2024, 2024, rjae321. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dong, M.; Li, Q. Lobulated Hemangioma as a Rare Cause of Tricuspid Regurgitation. Clin. Med. Insights Case Rep. 2024, 17, 1–3. [Google Scholar] [CrossRef]
- Vu, T.T.; Nguyen, V.T.; Tran, Q.T.; Ngo Thi, M.H.; Do, T.H.; Le, H.S.; Nguyen, N.T.; Nguyen, V.T.; Lam, K. A Case of a Small-Sized Cavernous Hemangioma in the Right Ventricle—An Incidental Finding. Radiol. Case Rep. 2022, 17, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Rocco, R.; Daly, R.; Arghami, A. Robotic-Assisted Resection of Rare Mitral Valve Hemangioma. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 249–252. [Google Scholar] [CrossRef]
- Takago, S.; Iino, K.; Yamamoto, Y.; Takemura, H. Cardiac Haemangioma Treated with Surgical Resection Involving Reconstruction of the Right Ventricle. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 153–155. [Google Scholar] [CrossRef]
- Piao, M.; Zhou, X.; Yan, M. Exploring the Causes of Newly Developed Mitral Valve Regurgitation after the Resection of a Giant Left Ventricular Tumor (Hemangioma). J. Cardiothorac. Vasc. Anesth. 2024, 39, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Zhang, C.; Fan, C.; Liu, L.; Wan, J. Case Report: A Primary Right Ventricular Vascular Malformation Presenting as a Mass. Front. Cardiovasc. Med. 2021, 8, 736199. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Jian, Z.; Yan, Y.; Guo, F. Right Ventricular Haemangioma as a Rare Cause of Chest Pain: A Case Report. Eur. Heart J. Case Rep. 2021, 5, ytab477. [Google Scholar] [CrossRef] [PubMed]
- Darbari, A.; Singh, D.; Gilbert, S.; Kumar, B.; Singh, N. Capillary Haemangioma of the Heart Presenting with Pericardial Effusion: A Case Report. J. Cardiovasc. Thorac. Res. 2021, 13, 250–253. [Google Scholar] [CrossRef]
- Nakajima, T.; Shibata, T.; Ogura, K.; Iba, Y.; Kawaharada, N. A Case of a Giant Hemangioma of a Primary Cardiac Tumor. Cureus 2023, 15, e43818. [Google Scholar] [CrossRef]
| Date/Interval | Clinical Events and Findings |
|---|---|
| 2021 | Total thyroidectomy for benign thyroid disease; levothyroxine replacement initiated. |
| Months prior to admission | Gradual onset of exertional dyspnea and fatigue, progressing to NYHA class II. |
| Admission (2025) | Physical examination: afebrile, hemodynamically stable, systolic murmur at the lower left sternal border. Electrocardiography: sinus tachycardia (102 bpm) with left axis deviation. Chest radiography unremarkable. Laboratory tests within normal range. |
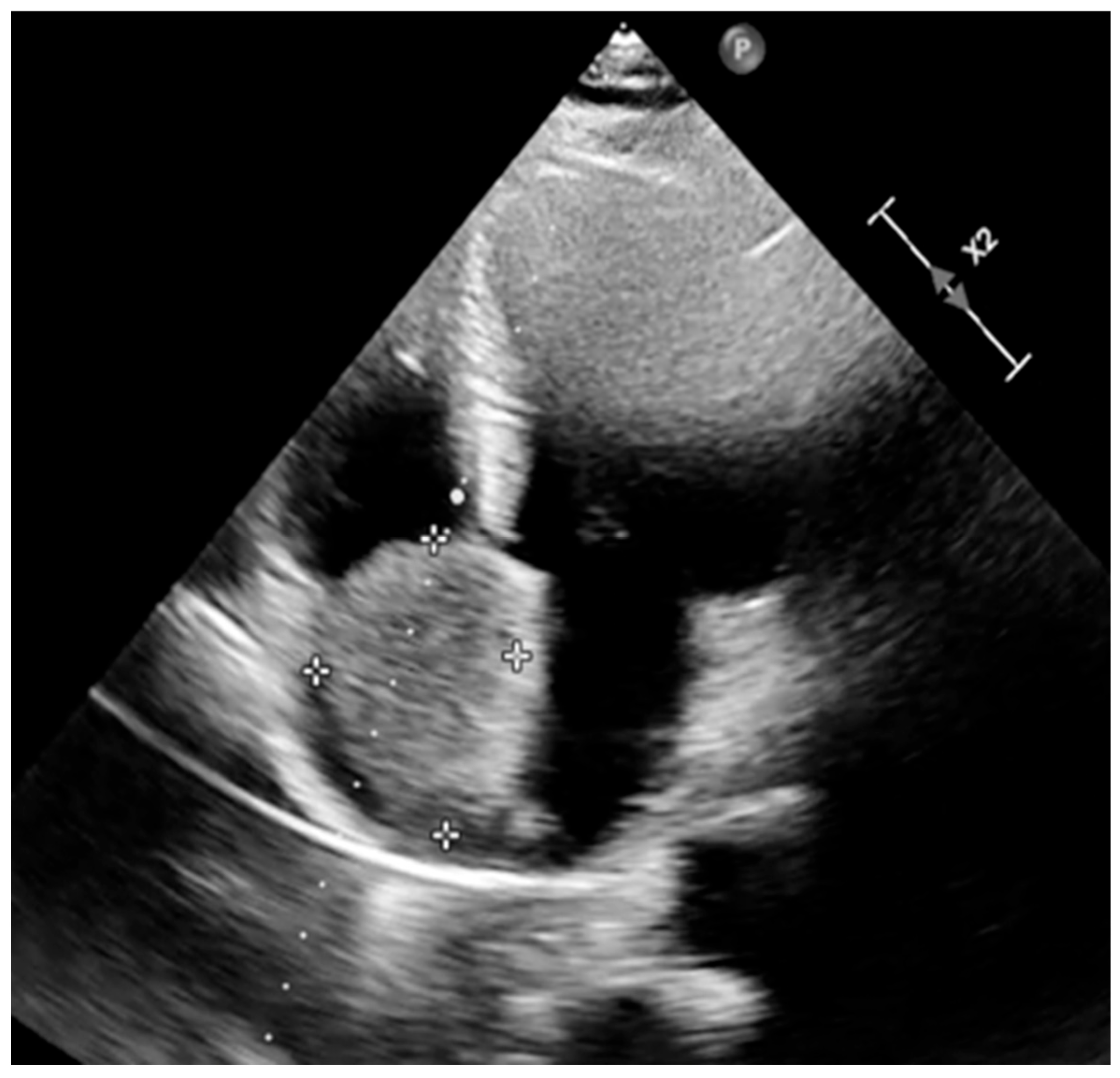
| Day 1 (hospital stay) | Transthoracic echocardiography: 5.5 × 3.5 cm right atrial mass attached at the fossa ovalis; functional tricuspid stenosis with moderate regurgitation; mild degenerative mitral regurgitation; no pericardial effusion. |
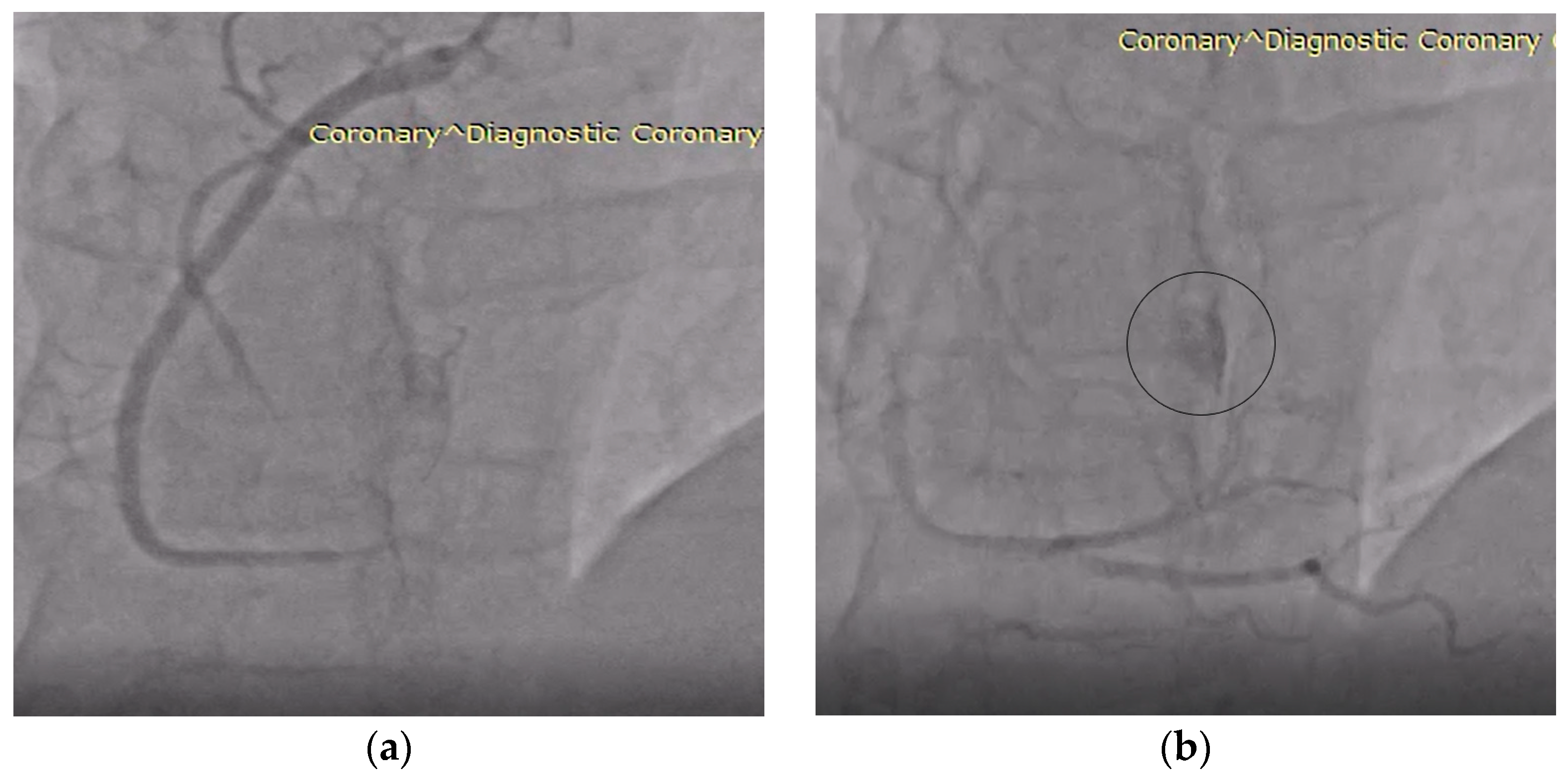
| Day 2 (hospital stay) | Coronary angiography: right-dominant system without stenoses; incidental fistulous connection draining into the right atrium, raising suspicion of a hypervascular lesion. |
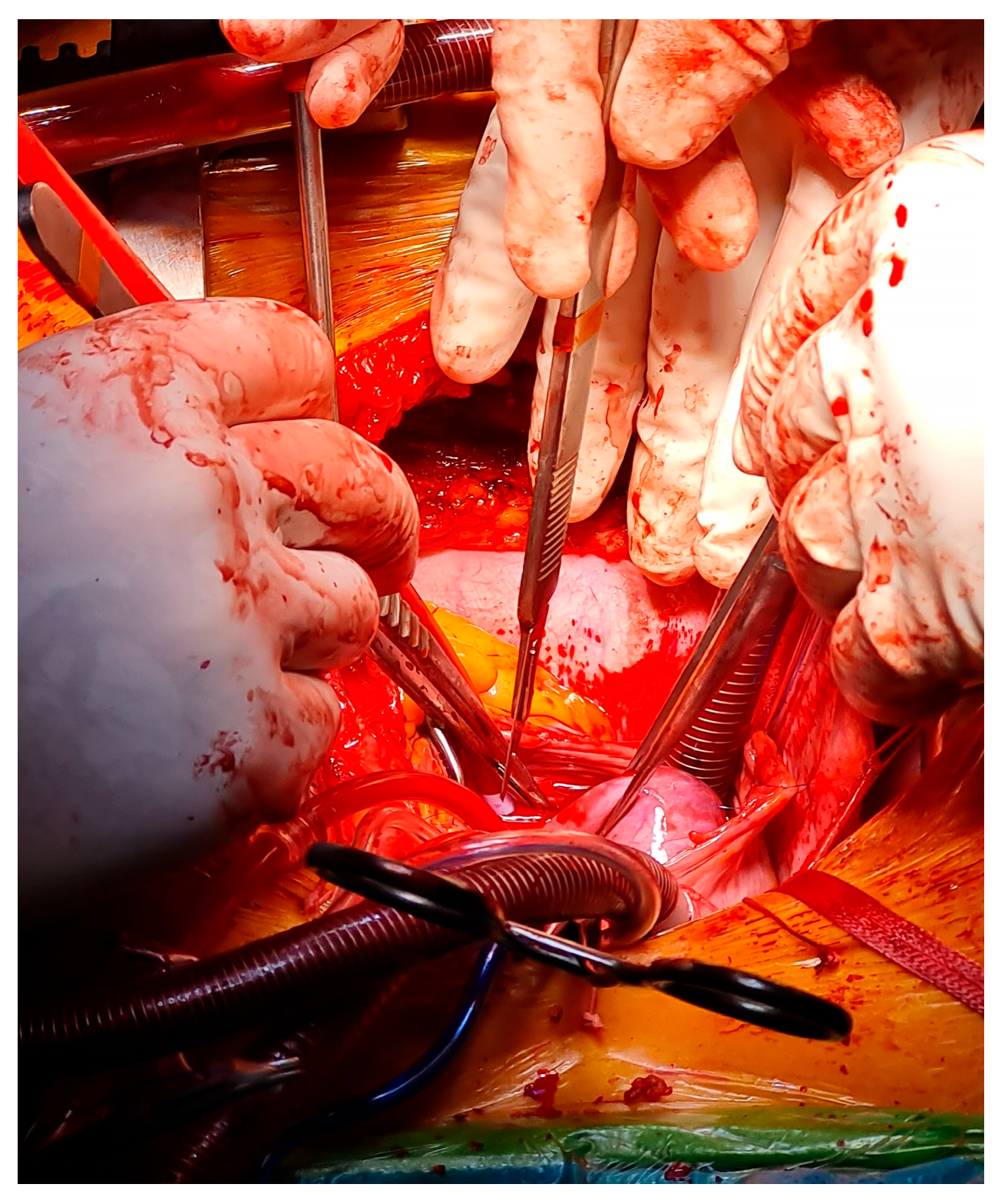
| Surgery (same admission) | Median sternotomy with cardiopulmonary bypass; en bloc excision of the right atrial mass and affected septal tissue; pericardial patch repair of atrial septal defect. |
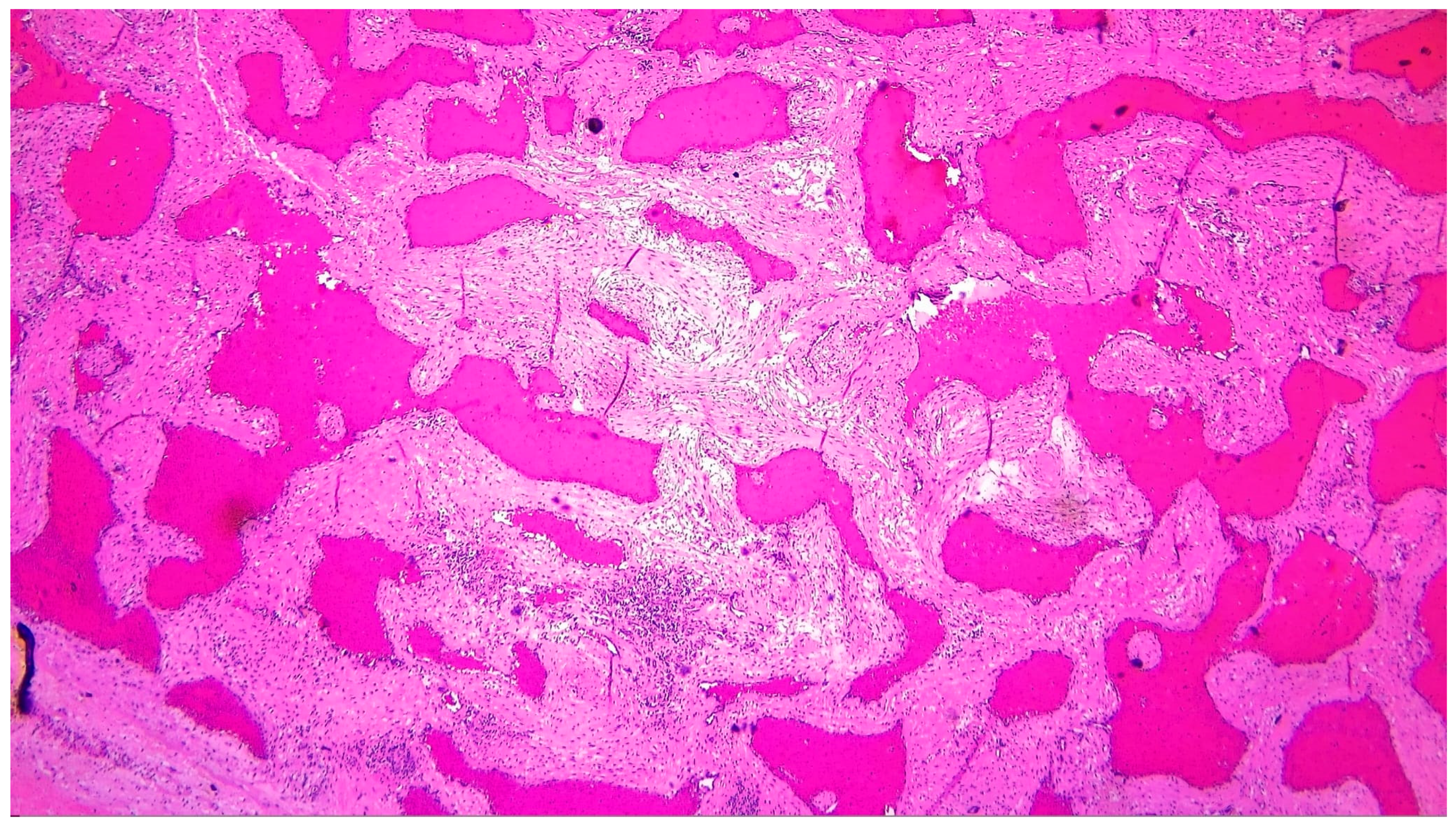
| Histopathology | Gross specimen: nodular, spongy mass (5.5 × 5 × 3 cm). Microscopy: cavernous hemangioma characterized by dilated vascular channels lined by flattened endothelial cells, with fibrous and focal myxoid stroma. |
| Early postoperative course | Transthoracic echocardiography: reduction of tricuspid regurgitation, preserved biventricular function. No residual mass. |
| Discharge (Day 7) | Patient clinically stable, hemodynamically normal, without pericardial effusion. Discharged on antiplatelet therapy, levothyroxine replacement, and lifestyle recommendations. |
| Follow-up (1, 3, 6, 12 months, then annually) | Sustained clinical improvement, no recurrence or residual mass on serial echocardiography. Excellent functional recovery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorescu, A.E.; Novaconi, R.C.; Munteanu, I.R.; Manzur, A.R.; Zus, A.S.; Lazar, M.-A.; Voinescu, O.R.; Crișan, S.; Feier, H.B. Cardiac Hemangioma in the Right Atrium: Diagnostic Challenges, Imaging Clues, and a Novel Algorithm for Differential Diagnosis. Life 2025, 15, 1816. https://doi.org/10.3390/life15121816
Grigorescu AE, Novaconi RC, Munteanu IR, Manzur AR, Zus AS, Lazar M-A, Voinescu OR, Crișan S, Feier HB. Cardiac Hemangioma in the Right Atrium: Diagnostic Challenges, Imaging Clues, and a Novel Algorithm for Differential Diagnosis. Life. 2025; 15(12):1816. https://doi.org/10.3390/life15121816
Chicago/Turabian StyleGrigorescu, Andrei Emanuel, Ramona Cristina Novaconi, Iulia Raluca Munteanu, Andrei Raul Manzur, Adrian Sebastian Zus, Mihai-Andrei Lazar, Oana Raluca Voinescu, Simina Crișan, and Horea Bogdan Feier. 2025. "Cardiac Hemangioma in the Right Atrium: Diagnostic Challenges, Imaging Clues, and a Novel Algorithm for Differential Diagnosis" Life 15, no. 12: 1816. https://doi.org/10.3390/life15121816
APA StyleGrigorescu, A. E., Novaconi, R. C., Munteanu, I. R., Manzur, A. R., Zus, A. S., Lazar, M.-A., Voinescu, O. R., Crișan, S., & Feier, H. B. (2025). Cardiac Hemangioma in the Right Atrium: Diagnostic Challenges, Imaging Clues, and a Novel Algorithm for Differential Diagnosis. Life, 15(12), 1816. https://doi.org/10.3390/life15121816








