Putrescine Mitigates the Biomass–β-Carotene Conflict in Dunaliella salina Under Thermal Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Strain and Culture Conditions
2.2. Experimental Design and Put Treatment
2.3. Analytical Methods
2.3.1. Biomass Quantification
2.3.2. Chlorophyll Quantification
2.3.3. β-Carotene Quantification
2.3.4. Photosynthetic Activity
2.3.5. Chlorophyll Fluorescence
2.3.6. Intracellular NO Analysis
2.3.7. Antioxidative Responses
2.3.8. Statistical Analysis
3. Results
3.1. Put Modulates the Biomass–β-Carotene Trade-Off Through Temperature-Dependent Mechanisms
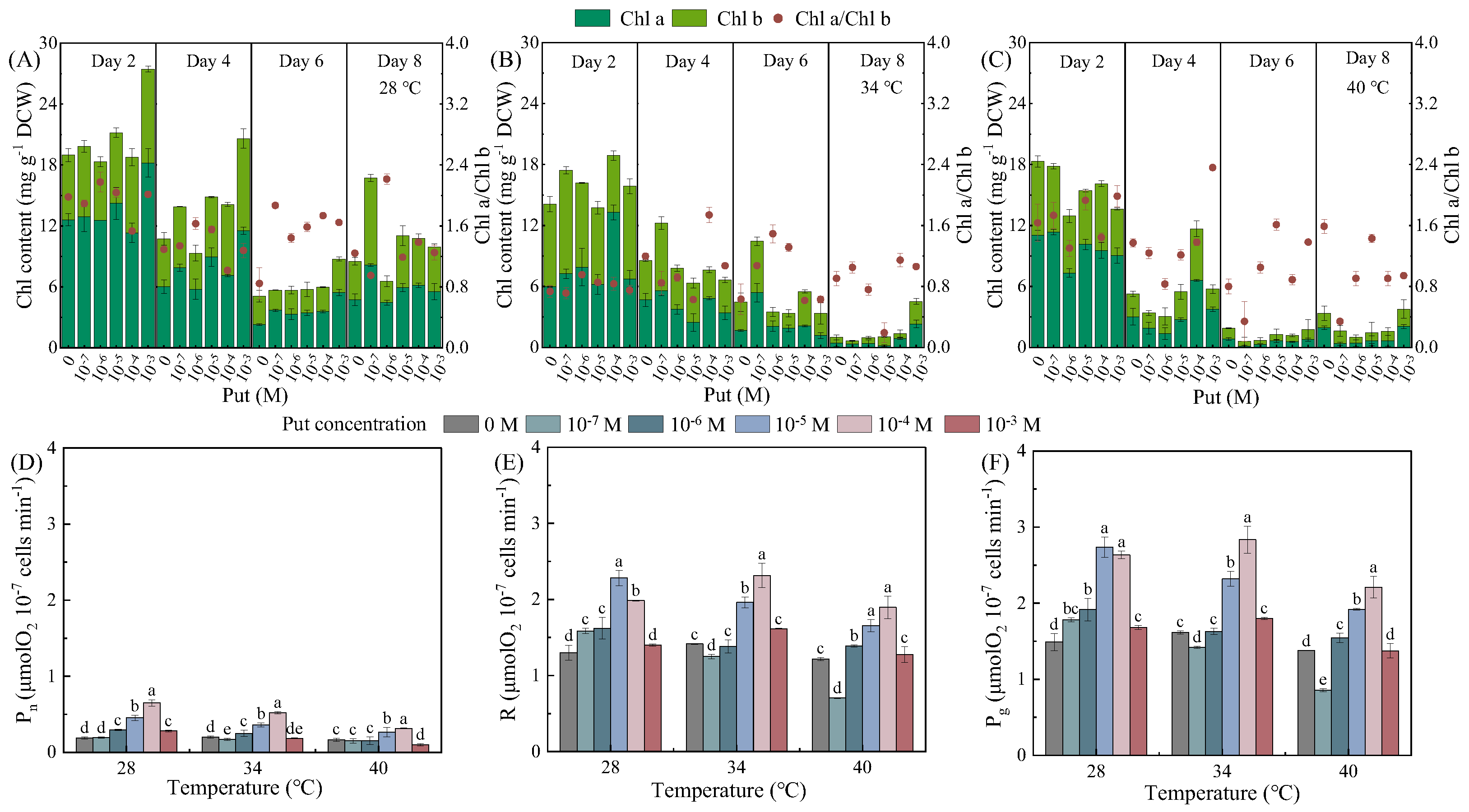
3.2. Temperature- and Concentration-Dependent Regulation of Photosynthetic Pigments and Carbon Fixation by Put
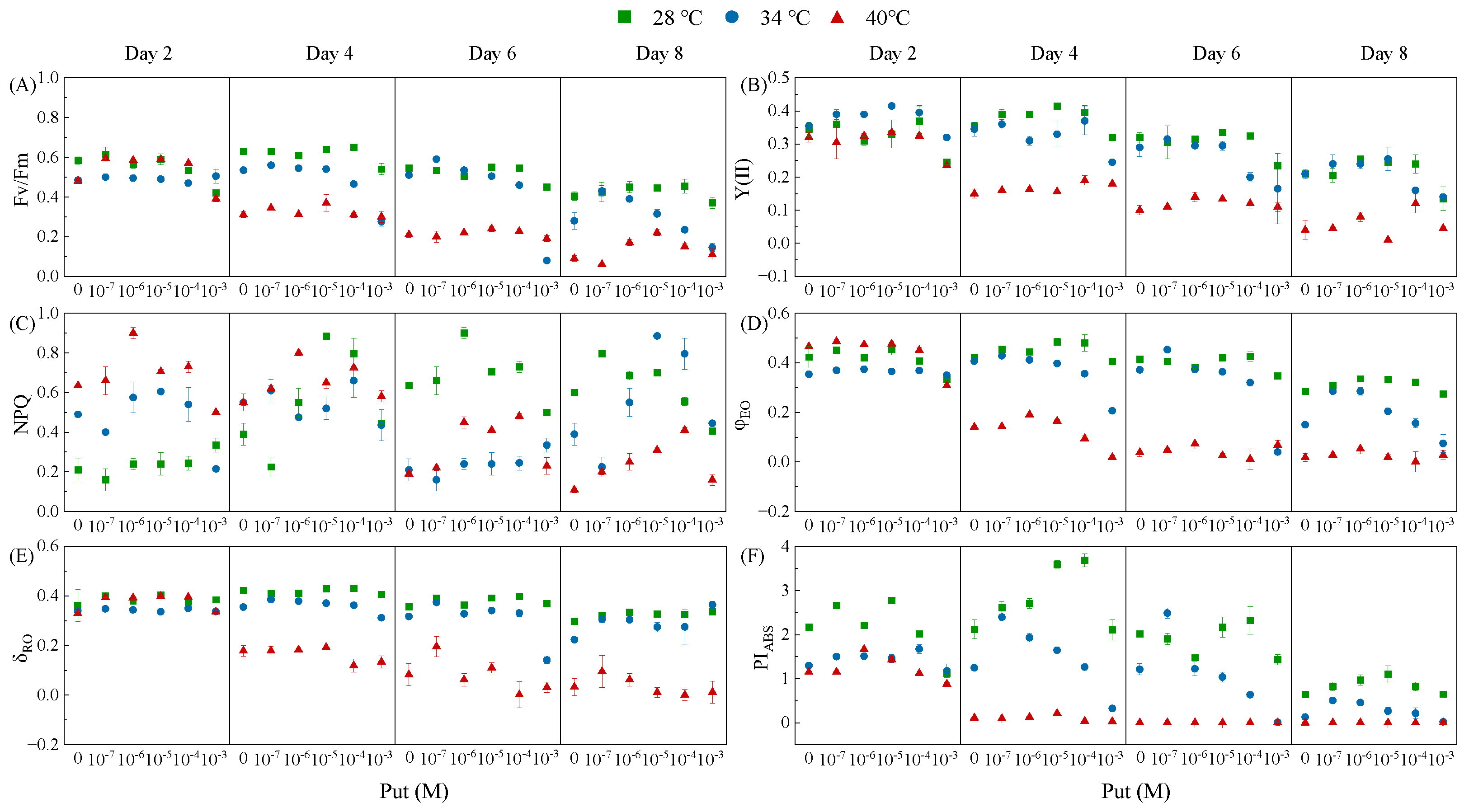
3.3. Temperature-Specific Modulation of Photochemical Efficiency by Put
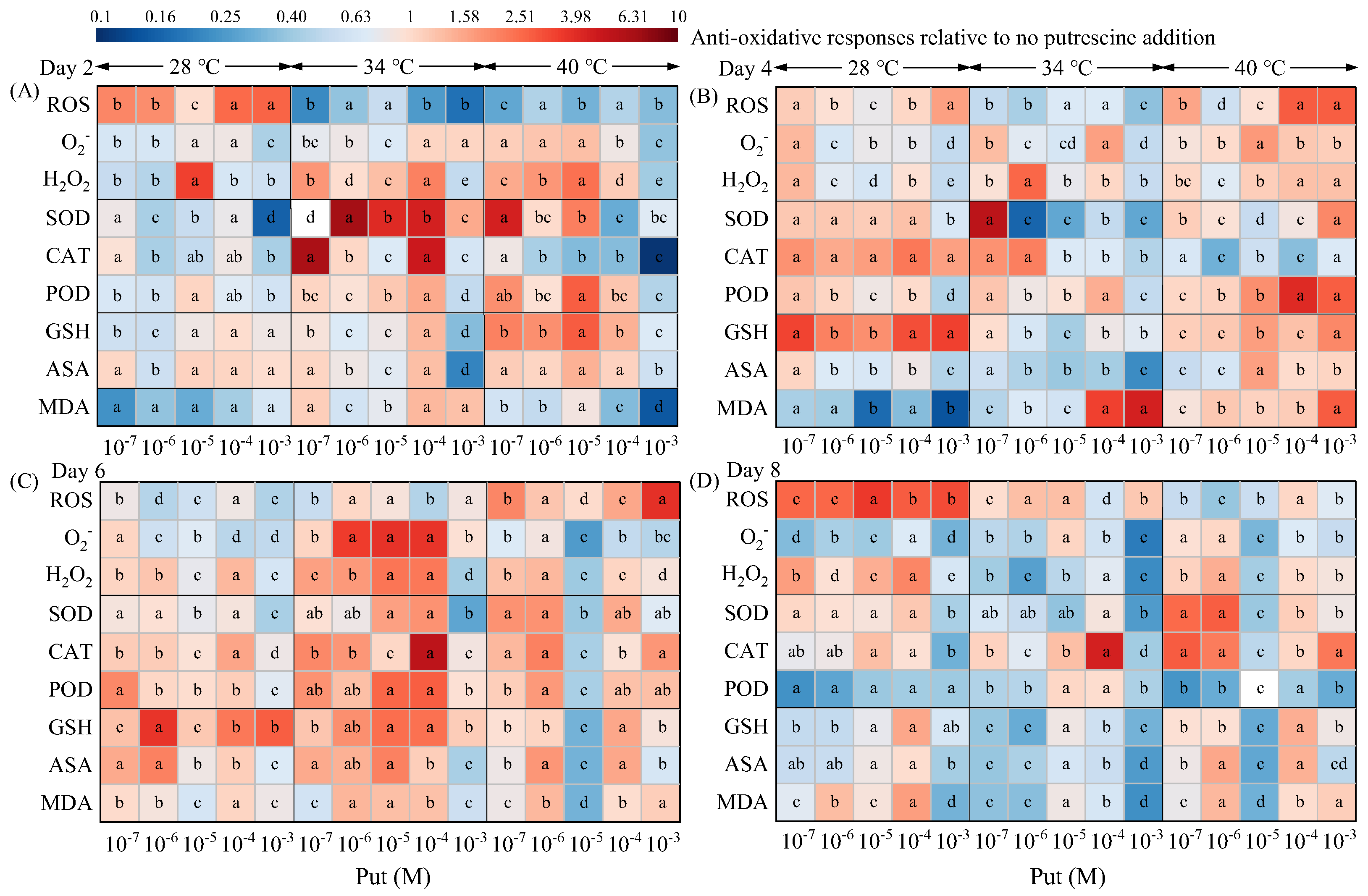
3.4. Temperature-Stratified Modulation of Antioxidant Systems by Put
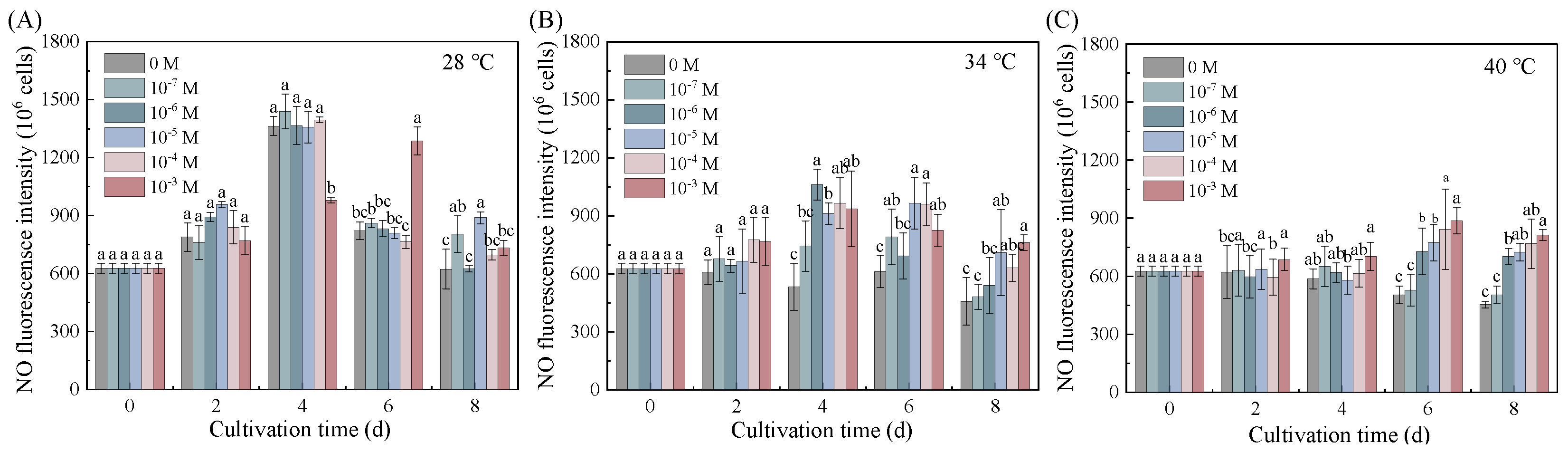
3.5. NO as a Central Mediator of Put-Induced Thermal Adaptation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Put | Putrescine |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| NPQ | Non-photochemical quenching |
| PGRs | Plant growth regulators |
| PAs | Polyamines |
| DCW | Dry cell weight |
| DO | Dissolved oxygen |
| Pn | Net photosynthetic rate |
| R | Respiratory rate |
| Pg | Gross photosynthetic rate |
| O2− | Superoxide anion |
| H2O2 | Hydrogen peroxide |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| GSH | Glutathione |
| ASA | Ascorbic acid |
| MDA | Malondialdehyde |
| PAO | Polyamine oxidase |
References
- Khuntong, S.; Samranrit, T.; Koedprasong, P.; Teeka, J.; Chiu, C.H.; Srila, W.; Areesirisuk, A. Synergistic effects of Tween 20 and ethephon on yeast oil and β-carotene co-production by Rhodosporidium toruloides using purified biodiesel-derived crude glycerol as an alternative carbon source. Bioresour. Technol. 2025, 422, 132211. [Google Scholar] [CrossRef]
- Alagawany, M.; Rudayni, H.A.; El-Kholy, M.S.; Alawam, A.S.; Allam, A.A.; Elnesr, S.S.; Abass, K.S.; Farag, M.R. Promising effects and therapeutic applications of Dunaliella salina algae as a functional food in humans and animals: Toward sustainable animal production and food security. Food Biosci. 2025, 71, 107280. [Google Scholar] [CrossRef]
- Ang, W.S.; Kee, P.E.; Lan, J.C.W.; Chen, W.H.; Chang, J.S.; Khoo, K.S. Unveiling the rise of microalgae-based foods in the global market: Perspective views and way forward. Food Biosci. 2025, 66, 105390. [Google Scholar] [CrossRef]
- Dai, J.L.; He, Y.J.; Chen, H.H.; Jiang, J.G. Dual roles of two malic enzymes in lipid biosynthesis and salt stress response in Dunaliella salina. J. Agric. Food Chem. 2023, 71, 17067–17079. [Google Scholar] [CrossRef] [PubMed]
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A.; Golzary, A. Factors affecting production of beta-carotene from Dunaliella salina microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.; Maurya, P.; Mohanta, A.; Dubey, H.; Khadim, S.R.; Singh, A.K.; Pandey, A.K.; Singh, A.K.; Asthana, R.K. Bioaccumulation of selenium in halotolerant microalga Dunaliella salina and its impact on photosynthesis, reactive oxygen species, antioxidative enzymes, and neutral lipids. Mar. Pollut. Bull. 2023, 190, 114842. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, Z.; Wang, J.; Kong, F.; Chi, Z. The interplay between Ca2+ and ROS in regulating β-carotene biosynthesis in Dunaliella salina under high temperature stress. Algal Res. 2025, 88, 104023. [Google Scholar] [CrossRef]
- Liang, S.; Li, L.; Ling, M.; Ruan, L.; Huang, L.; Shang, C. 24-Epibrassinolide promoted growth and organic compounds accumulation in Dunaliella parva by enhancing photosynthesis. Algal Res. 2024, 84, 103753. [Google Scholar] [CrossRef]
- Dai, J.L.; Yan, M.M.; Wu, F.C.; Chen, H.H.; Liang, M.H.; Jiang, J.G. Enhancing carotenoid accumulation in Dunaliella bardawil by combined treatments with fulvic acid and optimized culture conditions. Plant Physiol. Biochem. 2024, 217, 109206. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Prasad, N.; Singh, S.; Asthana, R.K. Phytohormone augmented biomass and lipid production in Dunaliella salina under two-stage cultivation strategy: A comprehensive characterization of biomass and lipid using DLS, FTIR, CHNS and NMR for bioenergy prospects. J. Environ. Chem. Eng. 2024, 12, 114942. [Google Scholar] [CrossRef]
- Xie, S.R.; Li, Y.; Chen, H.H.; Liang, M.H.; Jiang, J.G. A strategy to promote carotenoids production in Dunaliella bardawil by melatonin combined with photoinduction. Enzyme Microb. Technol. 2022, 161, 110115. [Google Scholar] [CrossRef]
- Seemashree, M.H.; Chauhan, V.S.; Sarada, R. Phytohormone supplementation mediated enhanced biomass production, lipid accumulation, and modulation of fatty acid profile in Porphyridium purpureum and Dunaliella salina cultures. Biocatal. Agric. Biotechnol. 2022, 39, 102253. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Collado, J.; López-Marín, J.; Del Amor, F.M. Foliar application of putrescine before a short-term heat stress improves the quality of melon fruits (Cucumis melo L.). J. Sci. Food Agric. 2021, 101, 1428–1435. [Google Scholar] [CrossRef]
- Sharma, P.; Lakra, N.; Ahlawat, Y.; Zaid, A.; Abd-ElGawad, A.M.; Elansary, H.O.; Gupta, A. Putrescine mitigates high temperature effects by modulating morpho-physiological and biochemical attributes in Brassica juncea seedlings. Agronomy 2023, 13, 1879. [Google Scholar] [CrossRef]
- Xing, H.; Zhao, Y.; Li, T.; Han, B.; Zhao, P.; Yu, X. Enhancing astaxanthin and lipid coproduction in Haematococcus pluvialis by the combined induction of plant growth regulators and multiple stresses. Bioresour. Technol. 2022, 344, 126225. [Google Scholar] [CrossRef]
- Jahan, M.S.; Hasan, M.M.; Alotaibi, F.S.; Alabdallah, N.M.; Alharbi, B.M.; Ramadan, K.M.A.; Bendary, E.S.A.; Alshehri, D.; Jabborova, D.; Al-Balawi, D.A.; et al. Exogenous putrescine increases heat tolerance in tomato seedlings by regulating chlorophyll metabolism and enhancing antioxidant defense efficiency. Plants 2022, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Karwa, S.; Taunk, J.; Bahuguna, R.N.; Chaturvedi, A.K.; Kumar, P.; Chinnusamy, V.; Pal, M. Putrescine exogenous application alleviates oxidative stress in reproductive tissue under high temperature in rice. Plant Physiol. Rep. 2021, 26, 381–391. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Sheng, X.J.L.; Li, B.Z.; Wang, W.; Xue, Y.Z.; Zhang, J.; Li, W.Q.; Wang, X.; Wang, F.; et al. Exogenous addition of putrescine enhanced lipid accumulation in Tetradesmus obliquus for increased biodiesel productivity. Renew. Energy 2023, 206, 263–273. [Google Scholar] [CrossRef]
- Kusano, T.; Yamaguchi, K.; Berberich, T.; Takahashi, Y. Advances in polyamine research in 2007. J. Plant Res. 2007, 120, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Kawano, T.; Kawano, N.; Muto, S.; Lapeyrie, F. Cation-induced superoxide generation in tobacco cell suspension culture is dependent on ion valence. Plant Cell Environ. 2001, 24, 1235–1241. [Google Scholar] [CrossRef]
- Tang, C.F.; Liu, Y.G.; Zeng, G.M.; Li, X.; Xu, W.H.; Li, C.F.; Yuan, X.Z. Effects of exogenous spermidine on antioxidant system responses of Typha latifolia L. under Cd2+ stress. J. Integr. Plant Biol. 2005, 47, 428–434. [Google Scholar] [CrossRef]
- Besford, R.T.; Richardson, C.M.; Campos, J.L.; Tiburcio, A.F. Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta 1993, 189, 201–206. [Google Scholar] [CrossRef]
- Kotakis, C.; Theodoropoulou, E.; Tassis, K.; Oustamanolakis, C.; Ioannidis, N.E.; Kotzabasis, K. Putrescine, a fast-acting switch for tolerance against osmotic stress. J. Plant Physiol. 2014, 171, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Bian, J.; Luo, G.; Kong, F.; Chi, Z. Enhanced β-carotene production in Dunaliella salina under relative high flashing light. Algal Res. 2022, 67, 102857. [Google Scholar] [CrossRef]
- Asthir, B.; Koundal, A.; Bains, N.S. Putrescine modulates antioxidant defense response in wheat under high temperature stress. Biol. Plant. 2012, 56, 757–761. [Google Scholar] [CrossRef]
- Sharma, P.; Lakra, N.; Luhach, A.; Zaid, A.; Siddique, K.H.M. Putrescine attenuates heat stress by modulating membrane stability, antioxidant activity, and gaseous exchange in Brassica juncea L. Plant Sci. 2025, 359, 112609. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zhao, Y.; Yu, X. Cross-talk between gama-aminobutyric acid and calcium ion regulates lipid biosynthesis in Monoraphidium sp. QLY-1 in response to combined treatment of fulvic acid and salinity stress. Bioresour. Technol. 2020, 315, 123833. [Google Scholar] [CrossRef]
- Groppa, M.D.; Ianuzzo, M.P.; Tomaro, M.L.; Benavides, M.P. Polyamine metabolism in sunflower plants under long-term cadmium or copper stress. Amino Acids 2007, 32, 265–275. [Google Scholar] [CrossRef]
- Pottosin, I.; Shabala, S. Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.X.; Liu, Y.; Zhang, J.T.; Wang, J.H.; Chi, Z.Y. Effects of environmental microplastic exposure on Chlorella sp. biofilm characteristics and its interaction with nitric oxide signaling. Sci. Total Environ. 2024, 912, 169659. [Google Scholar] [CrossRef] [PubMed]
- Tavallali, V.; Alhavi, N.; Gholami, H.; Mirazimi Abarghuei, F. Developmental and phytochemical changes in pot marigold (Calendula officinalis L.) using exogenous application of polyamines. Plant Physiol. Biochem. 2022, 183, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, N.; Yuasa, A.; Sakai, M.; Fujihara, S.; Nishio, S. Free polyamine concentrations in coastal seawater during phytoplankton bloom. Fish. Sci. 2001, 67, 79–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, M.Z.; Wang, J.X.; Wang, J.H.; Chi, Z.Y. Less toxic combined microplastics exposure towards attached Chlorella sorokiniana in the presence of sulfamethoxazole while massive microalgal nitrous oxide emission under multiple stresses. J. Hazard. Mater. 2025, 487, 137223. [Google Scholar] [CrossRef]
- Sun, W.; Hao, J.; Fan, S.; Liu, C.; Han, Y. Transcriptome and metabolome analysis revealed that exogenous spermidine-modulated flavone enhances the heat tolerance of lettuce. Antioxidants 2022, 11, 2332. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Kong, F.; Chi, Z. Putrescine Mitigates the Biomass–β-Carotene Conflict in Dunaliella salina Under Thermal Stress. Life 2025, 15, 1807. https://doi.org/10.3390/life15121807
Tang J, Kong F, Chi Z. Putrescine Mitigates the Biomass–β-Carotene Conflict in Dunaliella salina Under Thermal Stress. Life. 2025; 15(12):1807. https://doi.org/10.3390/life15121807
Chicago/Turabian StyleTang, Jianxin, Fantao Kong, and Zhanyou Chi. 2025. "Putrescine Mitigates the Biomass–β-Carotene Conflict in Dunaliella salina Under Thermal Stress" Life 15, no. 12: 1807. https://doi.org/10.3390/life15121807
APA StyleTang, J., Kong, F., & Chi, Z. (2025). Putrescine Mitigates the Biomass–β-Carotene Conflict in Dunaliella salina Under Thermal Stress. Life, 15(12), 1807. https://doi.org/10.3390/life15121807






