Machine Learning-Based Insights into Environmental Determinants of Morchella importuna Growth in Muğla, Türkiye
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Study Area
2.2. Machine Learning Algorithms
2.3. Goodness of Fit Measures
2.4. Feature Importance
3. Results and Discussion
3.1. Descriptive Statistics
3.2. Selection of Important Features
4. Conclusions
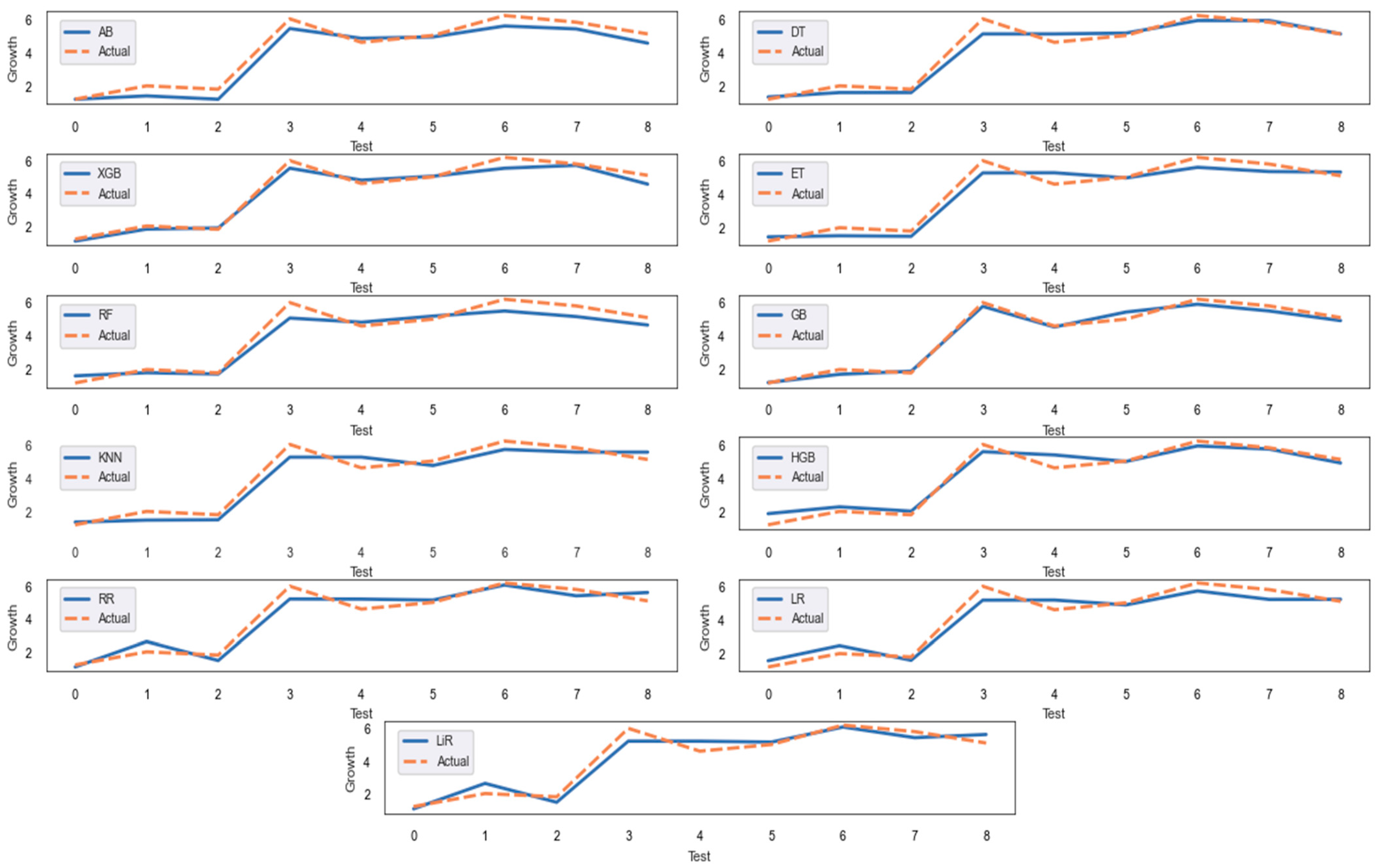
- It is observed that the model that best explained the relationship between the 20 features measured from air and soil and the growth of the M. importuna is GB, according to the test set.
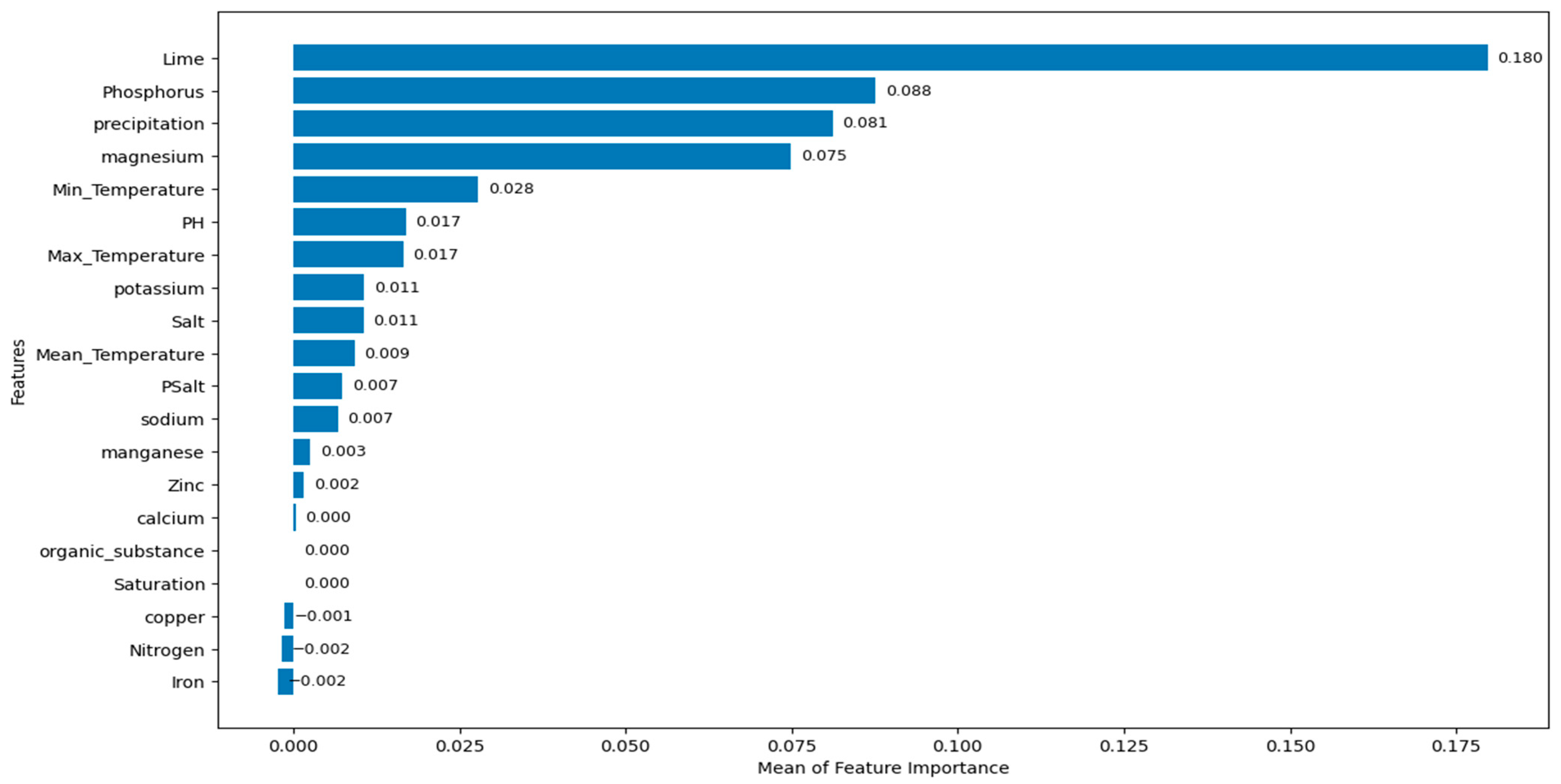
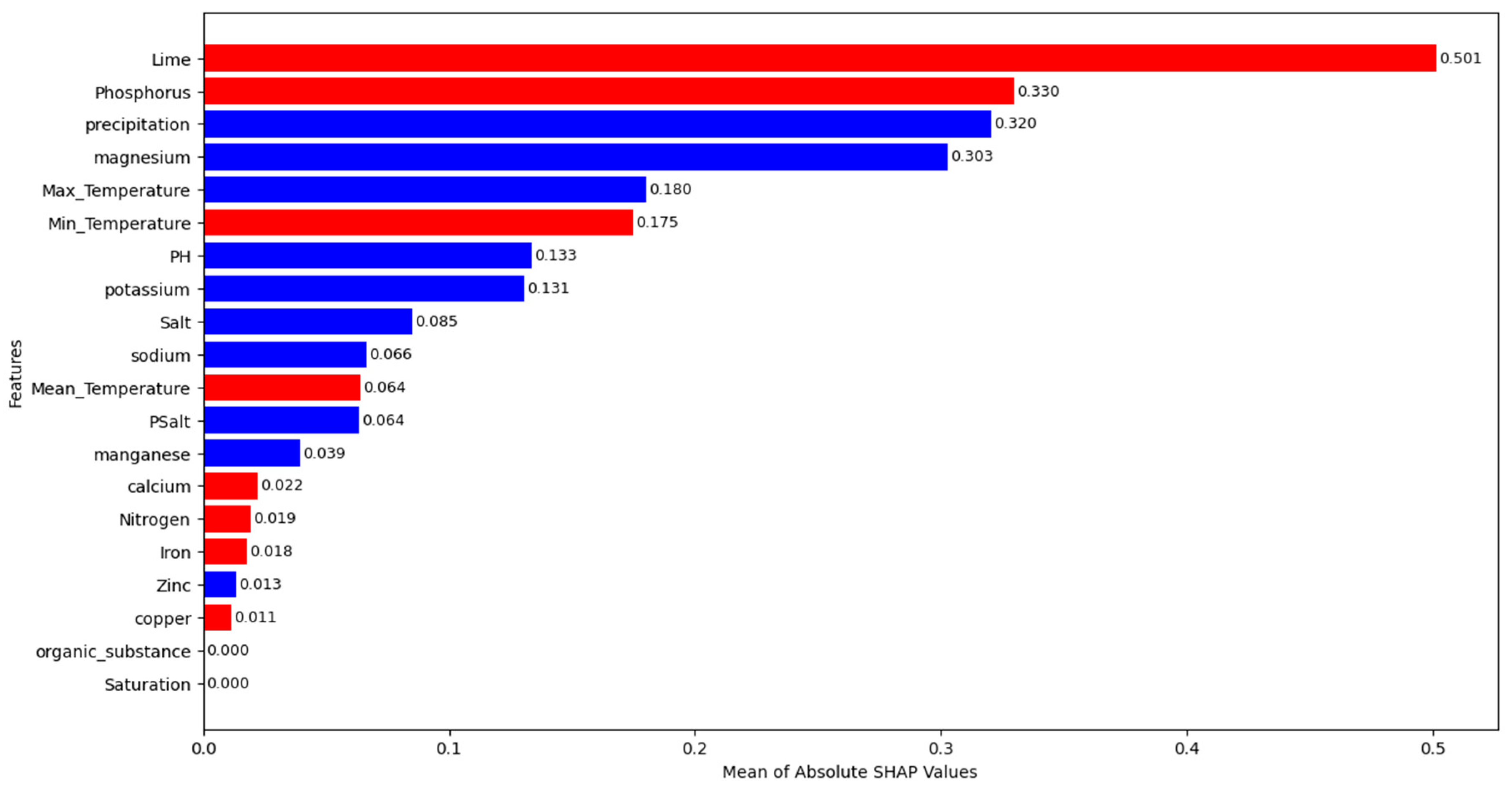
- Among the 20 evaluated features, lime (%) is found to be the most critical factor according to both permutation importance and SHAP methods.
- According to both feature importance methods, lime (%) is followed by phosphorus, precipitation, and magnesium in terms of its contribution to predictions. These findings are consistent with ecological observations and highlight the importance of both soil chemistry and climatic conditions in morel cultivation.
- There are no significant effects of the organic substance and the saturation on the predictions. Manganese, zinc, and calcium are features that have small effects on growth predictions. According to permutation importance scores, copper, nitrogen and iron increase the prediction error.
- According to SHAP values, an increase in lime, phosphorus, and minimum temperature decreases growth predictions for M. importuna, while an increase in precipitation, magnesium, maximum temperature, PH, and potassium increases them.
- The study has shown that an increase in maximum temperature increases growth predictions. This result may not be consistent with the literature [15]. However, the highest temperature observed during our measurement period was 22. Therefore, it will not be correct to say that growth predictions will increase at high temperatures.
- It has been determined that increases in minimum and mean temperature values will decrease the growth predictions. Again, the maximum values of minimum and mean temperatures obtained during the measurement period were measured as 16.2 and 18.5, respectively. This part of the study is consistent with the literature [15].
- Correlation analysis (Table 2) found a moderate negative correlation (−0.57) between pH and calcium with the growth, a high negative correlation (−0.85) between zinc and the growth, and a moderate positive correlation (0.57) between copper and manganese with the growth. These correlations contradict the SHAP results. However, the correlation analysis assumed the independence of the features and investigated their linear correlation.
- Practically, the results provide quantitative guidance for optimizing cultivation strategies. For example, monitoring lime, phosphorus, magnesium concentration, and temperature regimes could significantly enhance growth outcomes. Additionally, these insights may inform conservation efforts by identifying habitats most favorable for sustainable harvesting. The integration of machine learning into fungal cultivation studies could open new opportunities for precision agriculture and biotechnology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dennis, R.W.G. British Ascomycetes; J. Cramer (Lubrecht & Cramer Ltd.): Vaduz, Liechtenstein, 1978; 585p. [Google Scholar]
- Breitenbach, J.; Kranzlin, J. Fungi of Switzerland. Volume 1: Ascomycetes; Verlag Mykologia: Luzern, Switzerland, 1984; 364p. [Google Scholar]
- Taşkin, H.; Doğan, H.H.; Büyükalaca, S. Morchella galilaea, an autumn species from Türkiye. Mycotaxon 2015, 130, 215–221. [Google Scholar] [CrossRef]
- Dahlstrom, J.L.; Smith, J.E.; Weber, N.S. Mycorrhiza-like interaction by Morchella with species of the Pinaceae in pure culture synthesis. Mycorrhiza 2000, 9, 279–285. [Google Scholar] [CrossRef]
- Isiloglu, M.; Alli, H.; Spooner, B.M.; Solak, M.H. Morchella anatolica (Ascomycota), a new species from southwestern Anatolia, Türkiye. Mycologia 2010, 102, 455–458. [Google Scholar] [CrossRef]
- Loizides, M.; Alvarado, P.; Clowez, P.; Moreau, P.A.; de la Osa, L.R.; Palazón, A. Morchella tridentina, M. rufobrunnea, and M. kakiicolor: A study of three poorly known Mediterranean morels, with nomenclatural updates in section Distantes. Mycol. Prog. 2015, 14, 13. [Google Scholar] [CrossRef]
- Yatsiuk, I.; Saar, I.; Kalamees, K.; Sulaymonov, S.; Gafforov, Y.; O’ Donnell, K. Epitypification of Morchella steppicola (Morchellaceae, Pezizales), a morphologically, phylogenetically and biogeographically distinct member of the Esculenta Clade from central Eurasia. Phytotaxa 2016, 284, 31–40. [Google Scholar] [CrossRef]
- Loizides, M. Morels: The story so far. Field Mycol. 2017, 18, 42–53. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; He, P.X. Morel Biology and Cultivation; Jilin Science and Technology Press: Changchun, China; Royal Botanic Gardens Kew: London, UK, 2017; pp. 1–340. [Google Scholar]
- Zhang, C.; Shi, X.; Zhang, J.; Zhang, Y.; Wang, W. Dynamics of soil microbiome throughout the cultivation life cycle of morel (Morchella sextelata). Front. Microbiol. 2023, 14, 979835. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl. Microbiol. Biotechnol. 2018, 102, 1639–1650. [Google Scholar] [CrossRef]
- He, P.; Wang, K.; Cai, Y.; Hu, X.; Zheng, Y.; Zhang, J.; Liu, W. Involvement of autophagy and apoptosis and lipid accumulation in sclerotial morphogenesis of Morchella importuna. Micron 2018, 109, 34–40. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, V.P.; Kumar, S. Studies on domestication of the true morel Morchella importuna in India. Indian Phytopaghol. 2024, 77, 301–310. [Google Scholar] [CrossRef]
- Sambyal, K.; Singh, R.V. A comprehensive review on Morchella importuna: Cultivation aspects, phytochemistry, and other significant applications. Folia Microbiol. 2021, 66, 147–157. [Google Scholar] [CrossRef]
- Liu, W.; He, P.; Shi, X.; Zhang, Y.; Perez-Moreno, J.; Yu, F. Large-scale field cultivation of Morchella and relevance of basic knowledge for its steady production. J. Fungi 2023, 9, 855. [Google Scholar] [CrossRef]
- Larson, A.J.; Cansler, C.A.; Cowdery, S.G.; Hiebert, S.; Furniss, T.J.; Swanson, M.E.; Lutz, J.A. Post-fire morel (Morchella) mushroom abundance, spatial structure, and harvest sustainability. For. Ecol. Manag. 2016, 377, 16–25. [Google Scholar] [CrossRef]
- Soylu, M.K. Isolation and molecular identification of the pure culture of Morchella collected from Türkiye and its characteristics. Horticulturae 2024, 10, 1020. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. True morels (Morchella)—Nutritional and phytochemical composition, health benefits and flavor: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef]
- Tan, H.; Kohler, A.; Miao, R.; Liu, T.; Zhang, Q.; Zhang, B.; Jiang, L.; Wang, Y.; Xie, L.; Tang, J.; et al. Multi-omic analyses of exogenous nutrient bag decomposition by the black morel Morchella importuna reveal sustained carbon acquisition and transferring. Environ. Microbiol. 2019, 21, 3909–3926. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. Aroma-volatile profile of black morel (Morchella importuna) grown in Israel. J. Sci. Food Agric. 2018, 98, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Bellanger, J.M.; Clowez, P.; Hansen, K.; O’Donnell, K.; Urban, A.; Sauve, M.; Courtecuisse, R.; Moreau, P.A. True morels (Morchella, Pezizales) of Europe and North America: Evolutionary relationships inferred from multilocus data and a unified taxonomy. Mycologia 2015, 107, 359–382. [Google Scholar] [CrossRef]
- Pilz, C.; Wegensteiner, R.; Keller, S. Selection of entomopathogenic fungi for the control of the Western Corn Rootworm Diabrotica virgifera virgifera. J. Appl. Entomol. 2007, 131, 426–431. [Google Scholar] [CrossRef]
- Kuo, M.; Dewsbury, D.R.; O’Donnell, K.; Carter, M.C.; Rehner, S.A.; Moore, J.D.; Moncalvo, J.M.; Canfield, S.A.; Stephenson, S.L.; Methven, A.S.; et al. Taxonomic revision of true morels (Morchella) in Canada and the United States. Mycologia 2012, 104, 1159–1177. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.F.; Khan, M.; Fawad, M.; Alabduljabbar, H.; Najeh, T.; Gamil, Y. Comparative analysis of various machine learning algorithms to predict strength properties of sustainable green concrete containing waste foundry sand. Sci. Rep. 2024, 14, 14617. [Google Scholar] [CrossRef]
- Gupta, R.; Yadav, A.K.; Jha, S.K.; Pathak, P.K. Predicting global horizontal irradiance of north central region of India via machine learning regressor algorithms. Eng. Appl. Artif. Intell. 2024, 133Pt E, 108426. [Google Scholar] [CrossRef]
- Chung, Y.W.; Khaki, B.; Li, T.; Chu, C.; Gadh, R. Ensemble machine learning-based algorithm for electric vehicle user behavior prediction. Appl. Energy 2019, 254, 113732. [Google Scholar] [CrossRef]
- Abdelhedi, M.; Gabtni, H. Performance comparison of various machine learning models for predicting water quality parameters in the Chebika Zone of Central Tunisia. Earth Sci. Inform. 2024, 17, 4245–4259. [Google Scholar] [CrossRef]
- Akyüz, İ.; Polat, K.; Bardak, S.; Ersen, N. Prediction of values, Borsa İstanbul Forest, paper and printing index using, machine learning methods. BioResources 2024, 19, 5141–5157. [Google Scholar] [CrossRef]
- Chen, L.; Xu, C.; Lim, W.H.; Sharma, A.; Tiang, S.S.; Chong, K.S.; El-kenawy, E.M.; Alhussan, A.A.; Eid, M.M.; Khafaga, D.S. Transparent and reliable construction cost prediction using advanced machine learning and explainable AI. Eng. Sci. Technol. Int. 2025, 70, 102159. [Google Scholar] [CrossRef]
- Chiadighikaobi, P.C.; Hematibahar, M.; Kharun, M.; Stashevskaya, N.A.; Camara, K. Predicting mechanical properties of self-healing concrete with Trichoderma Reesei Fungus using machine learning. Cogent Eng. 2024, 11, 2307193. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4766–4777. [Google Scholar]
- Scikit-Learn. Available online: https://scikit-learn.org/stable/modules/permutation_importance.html (accessed on 5 June 2024).
- Gray, G.M.; Ahumada, L.M.; Rehman, M.A.; Varughese, A.; Fernadez, A.M.; Fackler, J.; Yates, H.M.; Habre, W.; Disma, N.; Lonsdale, H. A machine-learning approach for decision support and risk stratification of pediatric perioperative patients based on the APRICOT dataset. Paediatr. Anaesth. 2023, 33, 683–775. [Google Scholar] [CrossRef]
- Ekanayake, I.U.; Meddage, D.P.P.; Rathnayake, U. A novel approach to explain the black-box nature of machine learning in compressive strength predictions of concrete using Shapley additive explanations(SHAP). Case Stud. Constr. Mater. 2022, 16, e01059. [Google Scholar] [CrossRef]
- Abekoon, T.; Sajindra, H.; Rathnayake, N.; Ekanayake, I.U.; Jayakody, A.; Rathnayake, U. A novel application with explainable machine learning (SHAP and LIME) to predict soil N, P, and K nutrient content in cabbage cultivation. Smart Agric. Technol. 2025, 11, 100879. [Google Scholar] [CrossRef]
- Scikit-Learn. Available online: https://scikit-learn.org/stable/modules/generated/sklearn.model_selection.KFold.html (accessed on 23 October 2025).

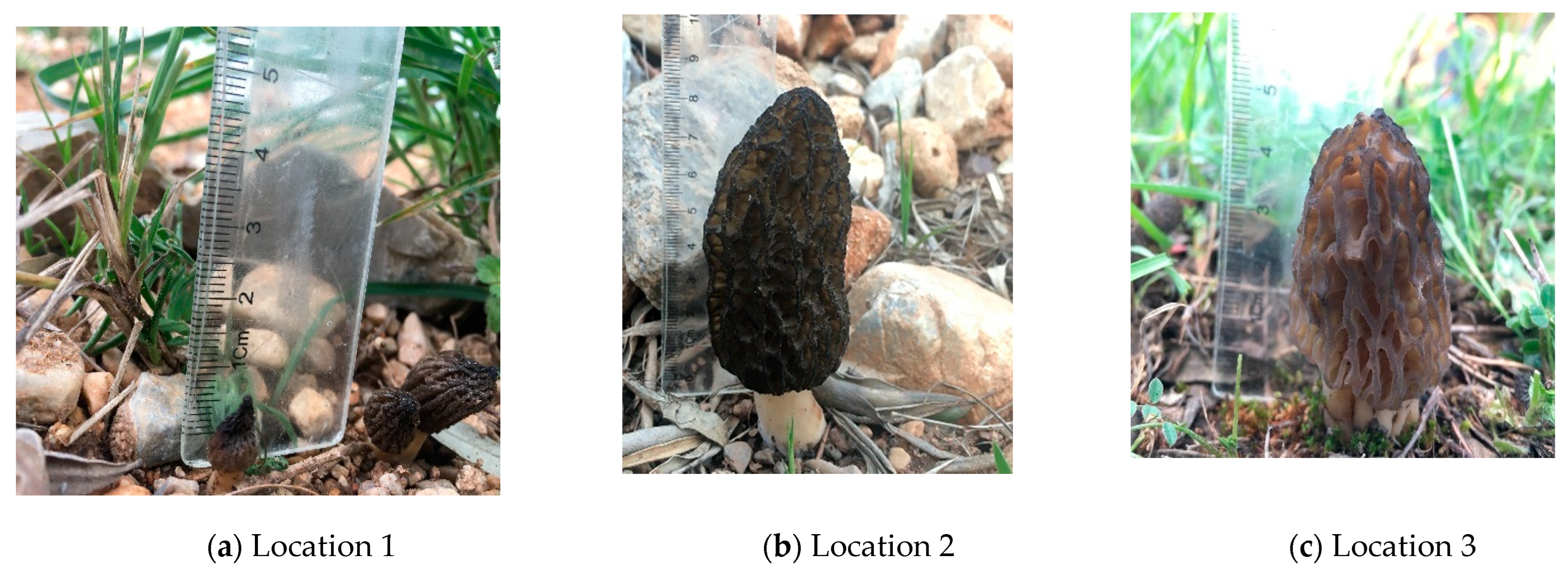
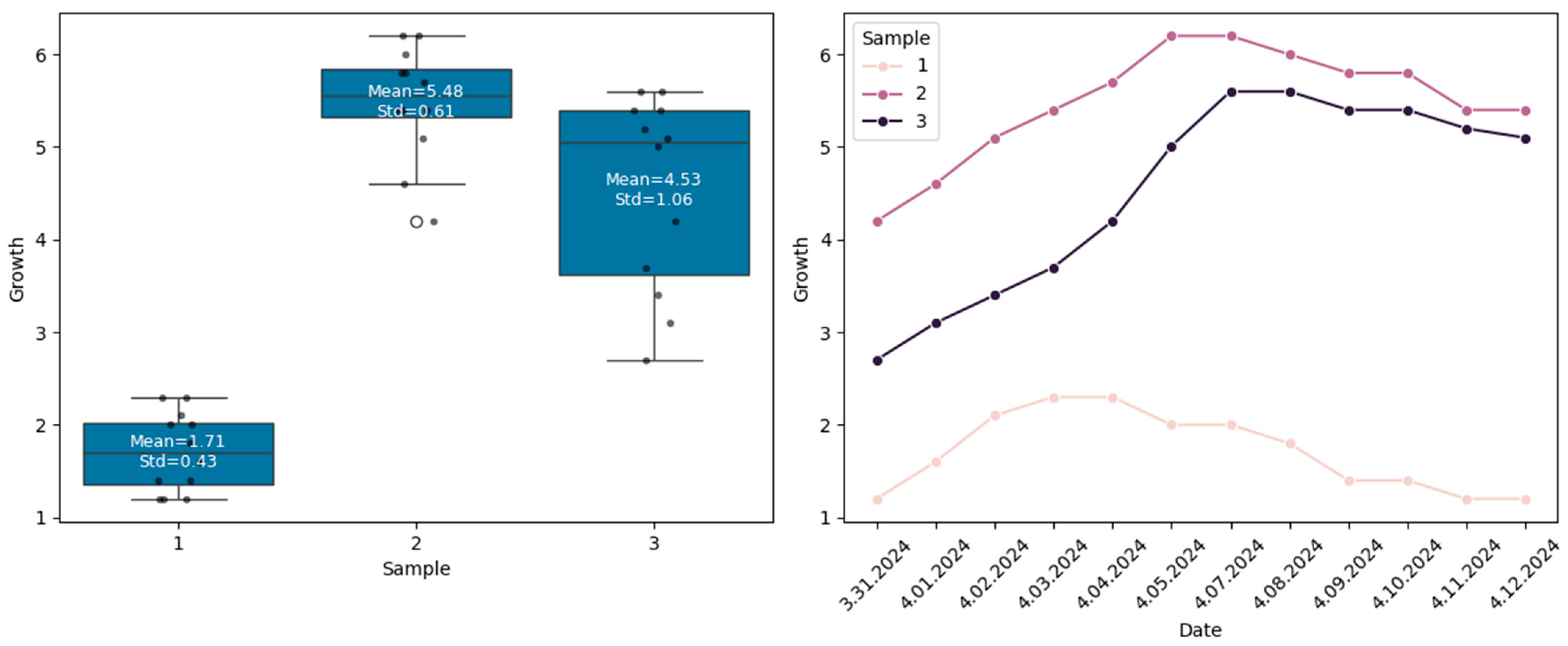
| Minimum | Maximum | Mean | Std. Deviation | ||
|---|---|---|---|---|---|
| X1 | Saturation (%) | 34.80 | 51.00 | 41.33 | 7.07 |
| X2 | Saltness (dS/m) | 0.28 | 0.38 | 0.32 | 0.04 |
| X3 | Salt (%) | 0.01 | 0.01 | 0.01 | 0.00 |
| X4 | pH | 7.19 | 8.13 | 7.77 | 0.42 |
| X5 | Lime (%) | 1.43 | 22.35 | 12.07 | 8.67 |
| X6 | Organic Substance (%) | 1.13 | 3.58 | 2.34 | 1.01 |
| X7 | Nitrogen (%) | 0.06 | 0.18 | 0.12 | 0.05 |
| X8 | Phosphorus (ppm) | 9.00 | 18.00 | 12.67 | 3.91 |
| X9 | Potassium (ppm) | 96.42 | 115.01 | 106.36 | 7.75 |
| X10 | Calcium (ppm) | 4750.99 | 8863.06 | 7473.53 | 1952.57 |
| X11 | Magnesium (ppm) | 85.46 | 163.97 | 120.92 | 32.96 |
| X12 | Sodium (ppm) | 41.84 | 49.73 | 46.81 | 3.58 |
| X13 | Iron (ppm) | 64.46 | 116.61 | 90.93 | 21.60 |
| X14 | Copper (ppm) | 0.92 | 1.03 | 0.97 | 0.05 |
| X15 | Manganese (ppm) | 85.90 | 121.60 | 104.16 | 14.79 |
| X16 | Zinc (ppm) | 0.37 | 0.77 | 0.59 | 0.17 |
| X17 | Mean Temperature | 12.70 | 18.50 | 15.00 | 1.77 |
| X18 | Max Temperature | 17.40 | 22.00 | 19.28 | 1.19 |
| X19 | Min Temperature | 5.80 | 16.20 | 11.15 | 3.13 |
| X20 | Precipitation | 0.00 | 12.10 | 2.38 | 3.84 |
| Y | M. Importuna Growth (cm) | 1.20 | 6.20 | 3.91 | 1.78 |
| Feature | Growth | Feature | Growth |
|---|---|---|---|
| Saturation | −0.28 | Magnesium (ppm) | 0.57 |
| Salt | −0.28 | Sodium (ppm) | 0.28 |
| Salt (%) | −0.28 | Iron (ppm) | 0.28 |
| pH | −0.57 | Copper (ppm) | 0.57 |
| Lime (%) | −0.57 | Manganese (ppm) | 0.57 |
| Organic Substance (%) | −0.28 | Zinc (ppm) | −0.85 |
| Nitrogen (%) | −0.28 | Mean Temperature | 0.01 |
| Phosphorus (ppm) | −0.85 | Max Temperature | −0.07 |
| Potassium (ppm) | 0.28 | Min Temperature | 0.03 |
| Calcium (ppm) | −0.57 | Precipitation | 0.26 |
| MAE | R2 | MAPE | |
|---|---|---|---|
| AB | 0.4085 | 0.9365 | 0.1196 |
| DT | 0.2970 | 0.9550 | 0.0861 |
| XGB | 0.2647 | 0.9661 | 0.0664 |
| ET | 0.4172 | 0.9359 | 0.1242 |
| RF | 0.4210 | 0.9275 | 0.1148 |
| GB | 0.2100 | 0.9827 | 0.0539 |
| KNN | 0.4278 | 0.9369 | 0.1213 |
| HGB | 0.3287 | 0.9523 | 0.1283 |
| RR | 0.4041 | 0.9373 | 0.1196 |
| LR | 0.4168 | 0.9349 | 0.1271 |
| LiR | 0.4064 | 0.9368 | 0.1215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allı, H.; Güler Dincer, N.; Pekmezci, A. Machine Learning-Based Insights into Environmental Determinants of Morchella importuna Growth in Muğla, Türkiye. Life 2025, 15, 1806. https://doi.org/10.3390/life15121806
Allı H, Güler Dincer N, Pekmezci A. Machine Learning-Based Insights into Environmental Determinants of Morchella importuna Growth in Muğla, Türkiye. Life. 2025; 15(12):1806. https://doi.org/10.3390/life15121806
Chicago/Turabian StyleAllı, Hakan, Nevin Güler Dincer, and Aytaç Pekmezci. 2025. "Machine Learning-Based Insights into Environmental Determinants of Morchella importuna Growth in Muğla, Türkiye" Life 15, no. 12: 1806. https://doi.org/10.3390/life15121806
APA StyleAllı, H., Güler Dincer, N., & Pekmezci, A. (2025). Machine Learning-Based Insights into Environmental Determinants of Morchella importuna Growth in Muğla, Türkiye. Life, 15(12), 1806. https://doi.org/10.3390/life15121806





