Myotonometric and Postural Analysis in Patients with Post-Stroke Hemiparesis Included in a Rehabilitation Program: A Study Protocol
Abstract
1. Introduction
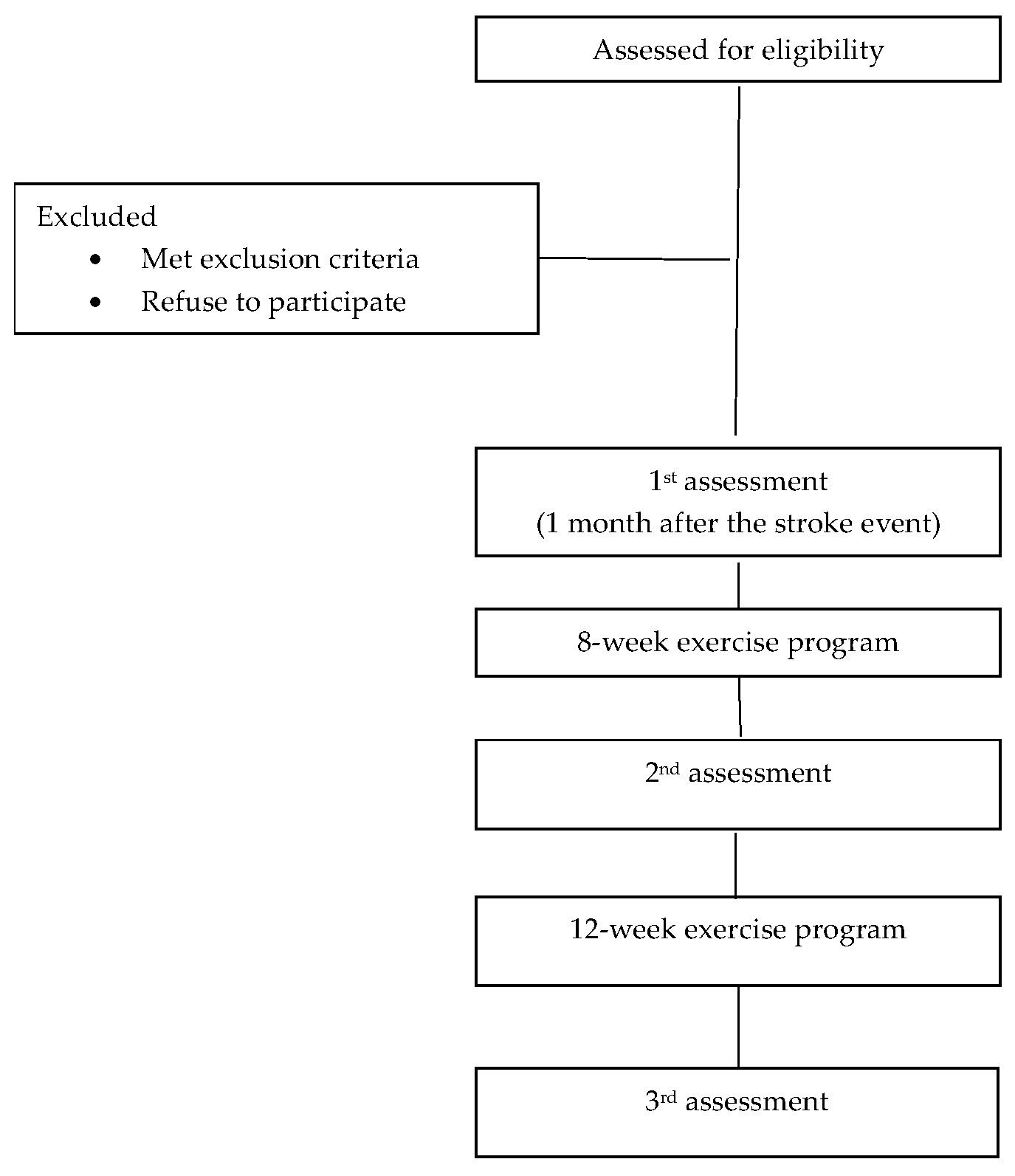
2. Materials and Methods
- Upper limb: biceps brachii and triceps brachii.
- Lower limb: tibialis anterior and gastrocnemius muscles (medial and lateral heads).
2.1. Sample Size Calculation
2.2. Recruitment and Informed Consent
2.2.1. The Inclusion Criteria for Imaging-Confirmed Stroke Diagnosis (CT or MRI)
2.2.2. Exclusion Criteria
2.2.3. Discontinuation Criteria
2.3. Interventions: Exercise Program
- Improve muscle tone.
- Increase range of motion.
- Prevent postural deformities and joint contractures.
- Supine Position:
- Passive ankle mobilization (flexion and extension): 2 sets of 10 repetitions per leg.
- Passive ankle rotation (circular movements): 2 sets of 10 reps in each direction.
- Passive knee mobilization (flexion and extension): 2 sets of 10 repetitions per leg.
- Passive foot mobilization (pushing the ankle toward the floor): 2 sets of 10 reps per leg.
- Passive arm mobilization (raising the arm above the head): 2 sets of 10 repetitions.
- 2.
- Side-Lying Position (on the non-affected side):
- Lifting the affected leg sideways: 2 sets of 10 repetitions.
- Knee flexion sideways: 2 sets of 10 repetitions per leg.
- Arm stretch (sideways): 2 sets of 10 repetitions.
- 3.
- Seated Position:
- Knee flexion and extension: 2 sets of 10 repetitions per leg.
- Toe standing for balance: 2 sets of 5 repetitions per leg.
- Arm mobilization (external rotation while seated or at bedside): 2 sets of 10 repetitions per arm.
- Finger stretching: 2 sets of 10 repetitions.
- 4.
- Standing Position:
- Assisted wall squats or frame-supported squats: 2 sets of 5 repetitions.
- Tiptoe standing (assisted): 2 sets of 5 reps per leg.
- Assisted single-leg stance: 2 sets of 3 repetitions per leg.
- Stretching of the posterior leg muscles.
- Strengthening of the anterolateral muscles.
- Enhancing joint mobility.
- Increasing muscle strength and endurance.
- Supine Position:
- Active ankle flexion and extension: 2 sets of 10 repetitions.
- Active ankle rotation (clockwise and counter-clockwise): 2 sets of 10 reps in each direction.
- Active knee flexion and extension: 2 sets of 10 repetitions.
- Active ankle pushing toward the floor: 2 sets of 10 repetitions per leg.
- Active arm movement above the head: 2 sets of 10 repetitions.
- 2.
- Side-Lying Position:
- Active leg lift (sideways): 2 sets of 10 repetitions.
- Active knee flexion (sideways): 2 sets of 10 repetitions per leg.
- Active arm stretching (sideways): 2 sets of 10 repetitions.
- 3.
- Seated Position:
- Active knee flexion and extension: 2 sets of 10 repetitions per leg.
- Active toe lifts: 2 sets of 10 repetitions per leg.
- Active external arm rotation: 2 sets of 10 repetitions per arm.
- Active finger extension: 2 sets of 10 repetitions.
- 4.
- Standing Position:
- Active wall squats: 2 sets of 5 repetitions.
- Active tiptoe standing: 2 sets of 5 repetitions per leg.
- Active single-leg stance: 2 sets, maintaining balance for 2 s per leg.
- Improve coordination of the affected upper and lower limbs.
- Enhance balance during functional tasks.
- Supine Position:
- Active leg lifts (hip and knee flexion): 2 sets of 10 repetitions per leg.
- Active ankle flexion and extension with resistance: 2 sets of 10 repetitions.
- Active leg abduction (sideways lifting): 2 sets of 10 repetitions per leg.
- Active overhead arm movement (shoulder flexion): 2 sets of 10 repetitions.
- 2.
- Side-Lying Position (for toning and coordination):
- Lateral leg raises (active, with pelvic stability control): 2 sets of 10 repetitions.
- Active arm abduction (side-lying): 2 sets of 10 repetitions per arm.
- Active knee flexion (side-lying): 2 sets of 10 repetitions.
- 3.
- Seated Position (for balance and coordination):
- Active knee flexion and extension (simulated walking in place): 2 sets of 10 repetitions per leg.
- Tiptoe raises with weight shifting: 2 sets of 10 repetitions per leg.
- Active external arm rotation (arm-leg coordination): 2 sets of 10 repetitions per arm.
- Active finger stretching while seated: 2 sets of 10 repetitions.
- 4.
- Standing Position (for functional balance and coordination):
- Deep squats (supported by wall or walker): 2 sets of 8 repetitions.
- Tiptoe raises with weight shifting: 2 sets of 5 repetitions per leg.
- Single-leg stance with weight transfer: 2 sets of 10 s per leg.
- Supine Position:
- Active leg lifts (controlled hip and knee flexion): 2 sets of 10 repetitions per leg.
- Circular foot slides on the floor (to tone leg and hip muscles): 2 sets of 10 repetitions per leg.
- Active arm movement (shoulder flexion and extension): 2 sets of 10 repetitions.
- Side-to-side leg movements (abduction and adduction): 2 sets of 10 repetitions per leg.
- 2.
- Side-Lying Position (for lateral mobility and stability):
- Controlled lower limb abduction with brief hold: 2 sets of 10 repetitions per leg.
- Simultaneous active abduction of arm and lower limb: 2 sets of 10 repetitions per limb.
- Active knee flexion and extension: 2 sets of 10 repetitions.
- 3.
- Seated Position (for functional coordination and balance):
- Active knee flexion and extension: 2 sets of 10 repetitions per leg.
- Tiptoe raises with weight shifting (stability training): 2 sets of 10 repetitions per leg.
- Marching in place with arm movements (interlimb coordination): 2 sets of 2 min.
- 4.
- Standing Position (for dynamic balance and functional movement):
- Marching in place with single-leg support: 2 sets of 1–2 min.
- Deep squats with weight transfer (stability training): 2 sets of 8 repetitions.
- Tiptoe walking (to mobilize the ankle and improve balance): 2 sets of 2 min.
2.4. Measurements
- Myotonometric Evaluation
- Biceps brachii: Patient in supine position, arm relaxed alongside the trunk, forearm in neutral position. A towel is placed under the wrist for comfort. Measurement point: long head, lateral side of the muscle, mid-belly.
- Triceps brachii: Patient in side-lying position, arm relaxed. Measurement point: medial head of the triceps, mid-belly.
- Tibialis anterior: Patient in supine position, relaxed. Measurement taken on the anterior compartment, at the upper third of the line connecting the tibial tuberosity and lateral malleolus.
- Gastrocnemius (medial and lateral): Patient in prone position, relaxed.
- Lateral head: measured at the upper third of the line from the popliteal fold to the lateral malleolus.
- Medial head: measured at the upper third of the line from the popliteal fold to the medial malleolus.
- 2.
- Postural Analysis
- 3.
- Completion of clinical scales: Barthel Index, IADL, Modified Ashworth Scale, and Modified Rankin Scale (all validated in Romanian).
2.5. Statistical Analysis
3. Expected Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ALIA|Asociatia Pentru Lupta Impotriva AVC. Available online: https://alia.org.ro/ (accessed on 9 August 2025).
- Taş, S.; Aktaş, A.; Tüfek, M.T.; Dağ, F. MyotonPRO is a reliable and repeatable tool for measuring mechanical properties of the upper limp muscles in patients with chronic stroke. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Harb, A.; Margetis, K.; Kishner, M. Modified Ashworth Scale. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Gao, J. Quantitative Ultrasound to Assess Skeletal Muscles in Post Stroke Spasticity. J. Cent. Nerv. Syst. Dis. 2021, 13, 1179573521996141. [Google Scholar] [CrossRef] [PubMed]
- Myoton—Muscle Tone, Stiffness, Elasticity Measurement Device. Available online: https://www.myoton.com/ (accessed on 9 August 2025).
- Ng, S.S.M.; Chen, P.; Choi, S.H.; Lam, T.J.; Lau, H.Y.; Lau, H.K.; Law, H.Y.; Lam, D.Y.W.; Lai, C.Y.Y. Muscle strength and stiffness of elbow muscles: Correlation with upper limb motor functions in people with chronic stroke. J. Rehabil. Med. 2025, 57, jrm44075. [Google Scholar] [CrossRef] [PubMed]
- Francisco, G.E.; Wissel, J.; Platz, T.; Li, S. Post-Stroke Spasticity. In Clinical Pathways in Stroke Rehabilitation: Evidence-Based Clinical Practice Recommendations; Platz, T., Ed.; Springer: Cham, Switzerland, 2021; pp. 149–173. [Google Scholar]
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.H.; Mueller, J. Early development of spasticity following stroke: A prospective, observational trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Lee, S.M.; Kim, J.H. Therapeutic effects of reaching with forward bending of trunk on postural stability, dynamic balance, and gait in individuals with chronic hemiparetic stroke. J. Phys. Ther. Sci. 2015, 27, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Park, S.K.; Kim, J.H.; Heo, J.W.; Lee, Y.S.; Uhm, Y.H. Effect of changes in postural alignment on foot pressure and walking ability of stroke patients. J. Phys. Ther. Sci. 2015, 27, 2943–2945. [Google Scholar] [CrossRef] [PubMed]
- Halmi, Z.; Stone, T.W.; Dinya, E.; Málly, J. Postural instability years after stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105038. [Google Scholar] [CrossRef] [PubMed]
- Postural Assessment Scale for Stroke. Available online: https://www.physio-pedia.com/Postural_Assessment_Scale_for_Stroke (accessed on 9 August 2025).
- Alam, M.F.; Zaki, S.; Sharma, S.; Nuhmani, S. Establishing the Reliability of the GaitON® Motion Analysis System: A Foundational Study for Gait and Posture Analysis in a Healthy Population. Sensors 2024, 24, 6884. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Zieliński, G. Effect Size Guidelines for Individual and Group Differences in Physiotherapy. Arch. Phys. Med. Rehabil. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Amaricai, E.; Sandu, L. Posture analysis and dynamic balance in adolescents with idiopathic scoliosis. Gait Posture 2023, 106 (Suppl. 1), S8. [Google Scholar] [CrossRef]
- Williams, G. Barthel Index. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 345–346. [Google Scholar] [CrossRef]
- Mahoney, F.L.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Lawton Scale: CGA Toolkit Plus. Available online: https://www.cgakit.com/f-1-lawton-scale (accessed on 9 August 2025).
- Lawton Instrumental Activities of Daily Living Scale. Available online: https://www.msdmanuals.com/professional/multimedia/table/lawton-instrumental-activities-of-daily-living-scale (accessed on 9 August 2025).
- Graf, C. The Lawton Instrumental Activities of Daily Living (IADL) Scale; The Hartford Institute for Geriatric Nursing, New York University College of Nursing: New York, NY, USA, 2007; Available online: https://geriatrictoolkit.missouri.edu/funct/Lawton_IADL.pdf (accessed on 9 August 2025).
- Edemekong, P.F.; Bomgaars, D.L.; Sukumaran, S.; Schoo, C. Activities of Daily Living; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Hugos, C.L.; Cameron, M.H. Assessment and Measurement of Spasticity in MS: State of the Evidence. Curr. Neurol. Neurosci. Rep. 2019, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Modified Ashworth Scale—Physiopedia. Available online: https://www.physio-pedia.com/Modified_Ashworth_Scale (accessed on 9 August 2025).
- LOINC Modified Rankin Scale. Available online: https://loinc.org/75859-9 (accessed on 9 August 2025).
- Bolovan, A.D.; Onofrei, R.R.; Hogea, G.B.; Abu-Awwad, A.; Lazarescu, E.A.; Abu-Awwad, S.A.; Tapardea, A.R.; Suba, M.I.; Amaricai, E.C. Comparison between Exercise Program-Foot Orthoses Treatment and Exercise Program Alone after Pilon Fracture Surgery: Study Protocol for a Randomized Controlled Trial. Life 2023, 13, 2187. [Google Scholar] [CrossRef] [PubMed]
- García-Bernal, M.I.; González-García, P.; Madeleine, P.; Casuso-Holgado, M.J.; Heredia-Rizo, A.M. Characterization of the Structural and Mechanical Changes of the Biceps Brachii and Gastrocnemius Muscles in the Subacute and Chronic Stage after Stroke. Int. J. Environ. Res. Public Health 2023, 20, 1405. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.L.; Wu, C.Y.; Lin, K.C.; Lur, S.Y. Quantitative Mechanical Properties of the Relaxed Biceps and Triceps Brachii Muscles in Patients with Subacute Stroke: A Reliability Study of the Myoton-3 Myometer. Stroke Res. Treat. 2012, 2012, 617694. [Google Scholar] [CrossRef] [PubMed]
- Biceps Brachii—Physiopedia. Available online: https://www.physio-pedia.com/Biceps_Brachii (accessed on 9 August 2025).
- Myhre, J.; Sifris, D. The Anatomy of the Biceps. Available online: https://www.verywellhealth.com/biceps-anatomy-4688616 (accessed on 12 August 2025).
- Karunaharamoorthy, A. Biceps Brachii Muscle. Available online: https://www.kenhub.com/en/library/anatomy/biceps-brachii-muscle (accessed on 12 August 2025).
- Nelson, C.M.; Murray, W.M.; Dewald, J.P.A. Motor Impairment–Related Alterations in Biceps and Triceps Brachii Fascicle Lengths in Chronic Hemiparetic Stroke. Neurorehabil. Neural Repair 2018, 32, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Juneja, P.; Hubbard, J.B. Anatomy, Bony Pelvis and Lower Limb: Tibialis Anterior Muscles; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Hardin, J.M.; Devendra, S. Anatomy, Bony Pelvis and Lower Limb: Calf Common Peroneal Nerve (Common Fibular Nerve). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Azam, M.; Wehrle, C.J.; Shaw, P.M. Anatomy, Bony Pelvis and Lower Limb: Tibial Artery. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Gastrocnemius Muscle|Radiology Reference Article|Radiopedia.org. Available online: https://radiopaedia.org/articles/third-head-of-gastrocnemius (accessed on 13 August 2025).
- Gastrocnemius Muscle—Britannica. Available online: https://www.britannica.com/science/gastrocnemius-muscle (accessed on 9 August 2025).
- Jambi, L.; Hamad, A.; Salah, H.; Suleiman, A. Stroke and Disability: Incidence, Risk Factors, Management, and Impact. J. Disabil. Res. 2024, 3, 20240094. [Google Scholar] [CrossRef]
- WHO EMRO|Stroke, Cerebrovascular Accident. Available online: https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/ (accessed on 9 August 2025).
- eClinicalMedicine. The rising global burden of stroke. EClinicalMedicine 2023, 59, 102028. [Google Scholar] [CrossRef] [PubMed]
- World Stroke Organization. Impact of Stroke. Available online: https://www.world-stroke.org/world-stroke-day-campaign/about-stroke/impact-of-stroke (accessed on 13 August 2025).
- Zeng, H.; Chen, J.; Guo, Y.; Tan, S. Prevalence and Risk Factors for Spasticity After Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 11, 616097. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covasala, C.I.; Amaricai, E.C.; Sacui, S.; Onciulenco, A.V.; Ianculescu, A.; Chifane, C.L.; Roman, N.F.; Hreniuc, C.N. Myotonometric and Postural Analysis in Patients with Post-Stroke Hemiparesis Included in a Rehabilitation Program: A Study Protocol. Life 2025, 15, 1791. https://doi.org/10.3390/life15121791
Covasala CI, Amaricai EC, Sacui S, Onciulenco AV, Ianculescu A, Chifane CL, Roman NF, Hreniuc CN. Myotonometric and Postural Analysis in Patients with Post-Stroke Hemiparesis Included in a Rehabilitation Program: A Study Protocol. Life. 2025; 15(12):1791. https://doi.org/10.3390/life15121791
Chicago/Turabian StyleCovasala, Constantin Ioan, Elena Constanta Amaricai, Sorana Sacui (Teaha), Anca Valentina Onciulenco, Alexandru Ianculescu, Cosmin Liviu Chifane, Nicoleta Flavia Roman, and Catalin Nicolae Hreniuc. 2025. "Myotonometric and Postural Analysis in Patients with Post-Stroke Hemiparesis Included in a Rehabilitation Program: A Study Protocol" Life 15, no. 12: 1791. https://doi.org/10.3390/life15121791
APA StyleCovasala, C. I., Amaricai, E. C., Sacui, S., Onciulenco, A. V., Ianculescu, A., Chifane, C. L., Roman, N. F., & Hreniuc, C. N. (2025). Myotonometric and Postural Analysis in Patients with Post-Stroke Hemiparesis Included in a Rehabilitation Program: A Study Protocol. Life, 15(12), 1791. https://doi.org/10.3390/life15121791







