Arthrospira platensis as Protein-Rich Source for Human Nutrition
Abstract
1. Introduction
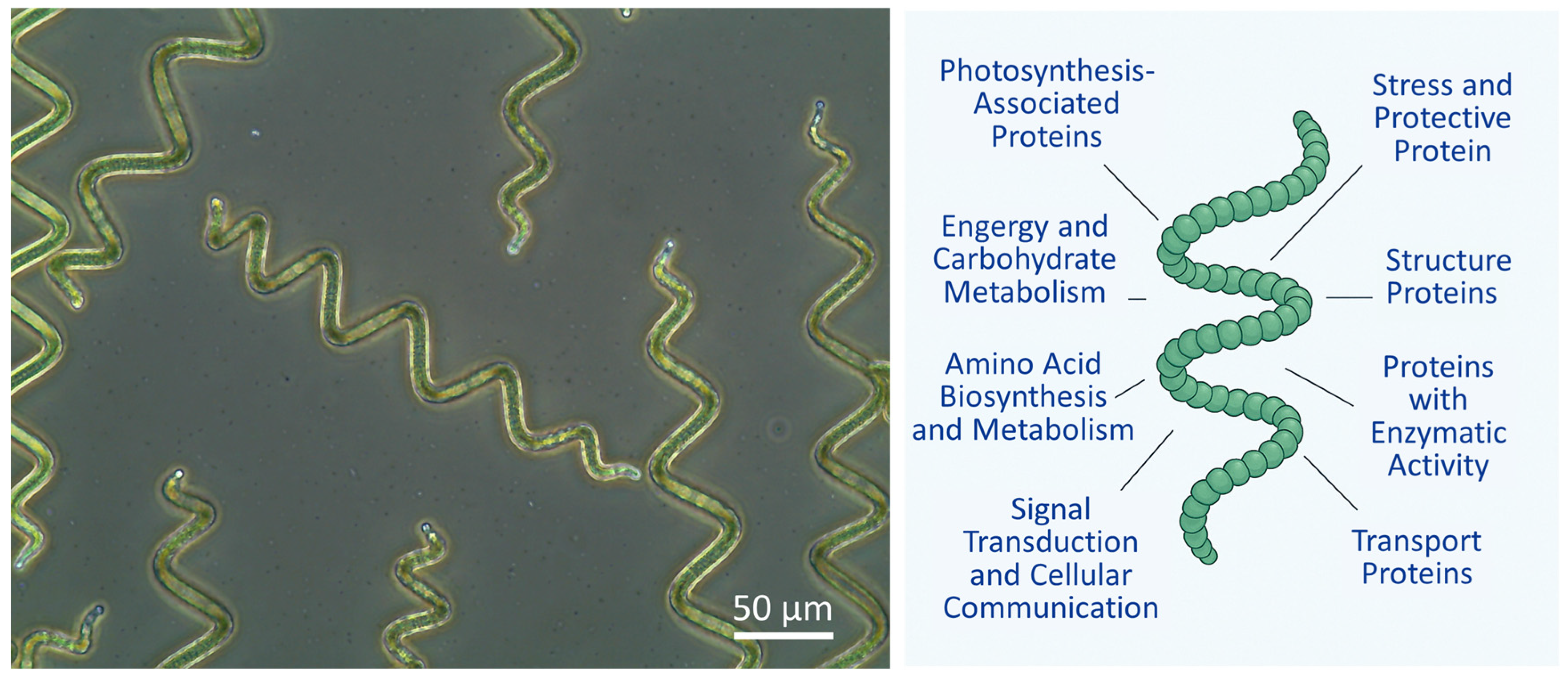
2. Protein Quantity
3. Protein Quality
3.1. Amino Acid Profile
3.2. Quality Scoring of Dietary Protein
3.3. In Vivo Studies
4. Technological Challenges in Production and Processing
4.1. Cultivation Strategies
4.2. Biotic and Abiotic Contamination
4.3. Functional and Sensory Characteristics
5. Environmental Considerations
6. Regulatory Aspects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations (Ed.) Global Population Growth and Sustainable Development; United Nations publication; United Nations: New York, NY, USA, 2021; ISBN 978-92-1-148350-5. [Google Scholar]
- Semba, R.D. The Rise and Fall of Protein Malnutrition in Global Health. Ann. Nutr. Metab. 2016, 69, 79–88. [Google Scholar] [CrossRef]
- Wu, G. Dietary Protein Intake and Human Health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Rizvi, S.; Pagnutti, C.; Fraser, E.; Bauch, C.T.; Anand, M. Global Land Use Implications of Dietary Trends. PLoS ONE 2018, 13, e0200781. [Google Scholar] [CrossRef]
- Lan, Y.; Xu, B.; Huan, Y.; Guo, J.; Liu, X.; Han, J.; Li, K. Food Security and Land Use under Sustainable Development Goals: Insights from Food Supply to Demand Side and Limited Arable Land in China. Foods 2023, 12, 4168. [Google Scholar] [CrossRef]
- Intergovernmental Panel On Climate Change. Climate Change and Land: IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems, 1st ed.; Cambridge University Press: Cambridge, UK, 2022; ISBN 978-1-009-15798-8. [Google Scholar]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in Functional Food Products with the Incorporation of Some Microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Effect of Cumulative Spirulina Intake on Broiler Meat Quality, Nutritional and Health-Related Attributes. Foods 2024, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in Bread Formulated with Wheat Flours of Different Alveograph Strength. Foods 2023, 12, 3724. [Google Scholar] [CrossRef]
- Ruszkowska, M.; Tańska, M.; Miedzianka, J.; Kowalczewski, P.Ł. Field Cricket (Gryllus Bimaculatus) and Spirulina (Arthrospira Platensis) Powders as Environmentally Friendly Protein Enrichment Ingredients in Corn Snacks. Foods 2024, 13, 2390. [Google Scholar] [CrossRef]
- Lambiase, C.; Braghieri, A.; Barone, C.M.A.; Di Francia, A.; Pacelli, C.; Serrapica, F.; Lorenzo, J.M.; De Rosa, G. Use of Cyanobacterium Spirulina (Arthrospira Platensis) in Buffalo Feeding: Effect on Mozzarella Cheese Quality. Foods 2023, 12, 4095. [Google Scholar] [CrossRef]
- Prandi, B.; Boukid, F.; Van De Walle, S.; Cutroneo, S.; Comaposada, J.; Van Royen, G.; Sforza, S.; Tedeschi, T.; Castellari, M. Protein Quality and Protein Digestibility of Vegetable Creams Reformulated with Microalgae Inclusion. Foods 2023, 12, 2395. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Demarinis, C.; Rizzello, C.G.; Pontonio, E. Bioprocessing to Preserve and Improve Microalgae Nutritional and Functional Potential: Novel Insight and Perspectives. Foods 2023, 12, 983. [Google Scholar] [CrossRef]
- Rotter, A.; Giannakourou, A.; Argente García, J.E.; Quero, G.M.; Auregan, C.; Triantaphyllidis, G.; Venetsanopoulou, A.; De Carolis, R.; Efstratiou, C.; Aboal, M.; et al. Identification of Marine Biotechnology Value Chains with High Potential in the Northern Mediterranean Region. Mar. Drugs 2023, 21, 416. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Fais, G.; Manca, A.; Bolognesi, F.; Borselli, M.; Concas, A.; Busutti, M.; Broggi, G.; Sanna, P.; Castillo-Aleman, Y.M.; Rivero-Jiménez, R.A.; et al. Wide Range Applications of Spirulina: From Earth to Space Missions. Mar. Drugs 2022, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Sinetova, M.A.; Kupriyanova, E.V.; Los, D.A. Spirulina/Arthrospira/Limnospira—Three Names of the Single Organism. Foods 2024, 13, 2762. [Google Scholar] [CrossRef]
- Roussel, T.; Halary, S.; Duval, C.; Piquet, B.; Cadoret, J.-P.; Vernès, L.; Bernard, C.; Marie, B. Monospecific Renaming within the Cyanobacterial Genus Limnospira (Spirulina) and Consequences for Food Authorization. J. Appl. Microbiol. 2023, 134, lxad159. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S.; Roytrakul, S.; Settachaimongkon, S.; Wattanasiritham, L.S.; Boonbumrung, S.; Mookdasanit, J.; Sithtisarn, S. Arthrospira Platensis Mutagenesis for Protein and C-Phycocyanin Improvement and Proteomics Approaches. Life 2022, 12, 911. [Google Scholar] [CrossRef]
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.-H. Spirulina platensis, a Super Food? J. Cell. Biotechnol. 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Meat Analog as Future Food: A Review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef]
- Barbosa, M.; Inácio, L.G.; Afonso, C.; Maranhão, P. The Microalga Dunaliella and Its Applications: A Review. Appl. Phycol. 2023, 4, 99–120. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, W.; Wang, J.; He, Y.; Yang, S.; Sun, H. A Comprehensive Review on the Heterotrophic Production of Bioactive Compounds by Microalgae. World J. Microbiol. Biotechnol. 2024, 40, 210. [Google Scholar] [CrossRef]
- Manirafasha, E.; Murwanashyaka, T.; Ndikubwimana, T.; Rashid Ahmed, N.; Liu, J.; Lu, Y.; Zeng, X.; Ling, X.; Jing, K. Enhancement of Cell Growth and Phycocyanin Production in Arthrospira (Spirulina) Platensis by Metabolic Stress and Nitrate Fed-Batch. Bioresour. Technol. 2018, 255, 293–301. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.; McGinn, P. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Nosratimovafagh, A.; Fereidouni, A.E.; Krujatz, F. Modeling and Optimizing the Effect of Light Color, Sodium Chloride and Glucose Concentration on Biomass Production and the Quality of Arthrospira Platensis Using Response Surface Methodology (RSM). Life 2022, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira Platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef]
- Ciferri, O. Spirulina, the Edible Microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Minkova, K.M.; Tchernov, A.A.; Tchorbadjieva, M.I.; Fournadjieva, S.T.; Antova, R.E.; Busheva, M.C. Purification of C-Phycocyanin from Spirulina (Arthrospira) Fusiformis. J. Biotechnol. 2003, 102, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Belal, E.B.; Khalafalla, M.M.E.; El-Hais, A.M.A. Use of Spirulina (Arthrospira Fusiformis) for Promoting Growth of Nile Tilapia Fingerlings. Afr. J. Microbiol. Res. 2012, 6, 6423–6431. [Google Scholar] [CrossRef]
- Milledge, J.J. Commercial Application of Microalgae Other than as Biofuels: A Brief Review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Aaronson, S.; Berner, T.; Dubinsky, Z. Microalgae as a Source of Chemicals and Natural Products. In Algae Biomass: Production and Use; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherland, 1980; pp. 595–601. [Google Scholar]
- Ferreira, L.S.; Rodrigues, M.S.; Converti, A.; Sato, S.; Carvalho, J.C.M. Arthrospira (Spirulina) Platensis Cultivation in Tubular Photobioreactor: Use of No-Cost CO2 from Ethanol Fermentation. Appl. Energy 2012, 92, 379–385. [Google Scholar] [CrossRef]
- Jung, C.G.H.; Nghinaunye, T.; Waldeck, P.; Braune, S.; Petrick, I.; Küpper, J.-H.; Jung, F. Decarbonization of Arthrospira Platensis Production by Using Atmospheric CO2 as an Exclusive Carbon Source: Proof of Principle. Int. J. Environ. Sci. Technol. 2024, 21, 4635–4644. [Google Scholar] [CrossRef]
- Jung, F.; Jung, C.G.H.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.-H. Factors Influencing the Growth of Spirulina Platensis in Closed Photobioreactors under CO2—O2 Conversion. J. Cell. Biotechnol. 2019, 5, 125–134. [Google Scholar] [CrossRef]
- Nghinaunye, T.; Waldeck, P.; Jung, C.G.H.; Küpper, J.-H.; Jung, F.; Braune, S. Response of Arthrospira Platensis to Different Temperatures Regarding Growth and Biochemical Composition. Clin. Hemorheol. Microcirc. 2024, 86, 205–211. [Google Scholar] [CrossRef]
- Tredici, M.R.; Zittelli, G.C. Efficiency of Sunlight Utilization: Tubular versus Flat Photobioreactors. Biotechnol. Bioeng. 1998, 57, 187–197. [Google Scholar] [CrossRef]
- Niangoran, N.U.F.; Buso, D.; Zissis, G.; Prudhomme, T. Influence of Light Intensity and Photoperiod on Energy Efficiency of Biomass and Pigment Production of Spirulina (Arthrospira Platensis). OCL 2021, 28, 37. [Google Scholar] [CrossRef]
- Bezerra, R.P.; Matsudo, M.C.; Converti, A.; Sato, S.; De Carvalho, J.C.M. Influence of Ammonium Chloride Feeding Time and Light Intensity on the Cultivation of Spirulina (Arthrospira) Platensis. Biotechnol. Bioeng. 2008, 100, 297–305. [Google Scholar] [CrossRef]
- Jung, C.H.G.; Waldeck, P.; Sykora, S.; Braune, S.; Petrick, I.; Küpper, J.-H.; Jung, F. Influence of Different Light-Emitting Diode Colors on Growth and Phycobiliprotein Generation of Arthrospira Platensis. Life 2022, 12, 895. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge Studies in Biotechnology; Cambridge University Press: Cambridge, UK; New York, NY, USA; Melbourne, Australia, 1994; ISBN 978-0-521-35020-4. [Google Scholar]
- Saito, Y.; Itakura, K.; Kuramoto, M.; Kaho, T.; Ohtake, N.; Hasegawa, H.; Suzuki, T.; Kondo, N. Prediction of Protein and Oil Contents in Soybeans Using Fluorescence Excitation Emission Matrix. Food Chem. 2021, 365, 130403. [Google Scholar] [CrossRef]
- Adeyi, A.M.; Osunde, Z.D.; Ogunjirin, O.A.; Awu, J.I.; Ibrahim, A. Effect of Drying on Nutritional Quality of Dried Meat (Kilishi). J. Agric. Eng. Technol. (JAET) 2015, 23, 14–34. Available online: https://jaet.niae.net/index.php/jaet/issue/view/8 (accessed on 28 October 2025).
- Arahou, F.; Hassikou, R.; Arahou, M.; Rhazi, L.; Wahby, I. Influence of Culture Conditions on Arthrospira Platensis Growth and Valorization of Biomass as Input for Sustainable Agriculture. Aquac. Int. 2021, 29, 2009–2020. [Google Scholar] [CrossRef]
- Fagiri, Y.M.A.; Salleh, A.; El-Nagerabi, S.A.F. Impact of Physico-Chemical Parameters on the Physiological Growth of Arthrospira (Spirulina Platensis) Exogenous Strain UTEXLB2340. Afr. J. Biotechnol. 2013, 12, 5458–5465. [Google Scholar] [CrossRef]
- Gómez, C.; Guzmán-Carrasco, A.; Lafarga, T.; Acién-Fernández, F.G. Optimization of a New Culture Medium for the Large-Scale Production of Protein-Rich Arthrospira Platensis (Oscillatoriales, Cyanophyceae). J. Phycol. 2021, 57, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Mejia-da-Silva, L.D.C.; Matsudo, M.C.; Morocho-Jacome, A.L.; de Carvalho, J.C.M. Application of Physicochemical Treatment Allows Reutilization of Arthrospira Platensis Exhausted Medium. Appl. Biochem. Biotechnol. 2018, 186, 40–53. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Sato, S.; de Carvalho, J.C.M. Ferric Sulfate Coagulation and Powdered Activated Carbon Adsorption as Simultaneous Treatment to Reuse the Medium in Arthrospira Platensis Cultivation. J. Chem. Technol. Biotechnol. 2016, 91, 901–910. [Google Scholar] [CrossRef]
- Coca, M.; Barrocal, V.M.; Lucas, S.; González-Benito, G.; García-Cubero, M.T. Protein Production in Spirulina Platensis Biomass Using Beet Vinasse-Supplemented Culture Media. Food Bioprod. Process. 2015, 94, 306–312. [Google Scholar] [CrossRef]
- Avila-Leon, I.; Chuei Matsudo, M.; Sato, S.; de Carvalho, J.C.M. Arthrospira Platensis Biomass with High Protein Content Cultivated in Continuous Process Using Urea as Nitrogen Source. J. Appl. Microbiol. 2012, 112, 1086–1094. [Google Scholar] [CrossRef]
- Colla, L.M.; Oliveira Reinehr, C.; Reichert, C.; Costa, J.A.V. Production of Biomass and Nutraceutical Compounds by Spirulina Platensis under Different Temperature and Nitrogen Regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Tokuşoglu, Ö.; üUnal, M.K. Biomass Nutrient Profiles of Three Microalgae: Spirulina Platensis, Chlorella Vulgaris, and Isochrisis Galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Matsudo, M.C.; Bezerra, R.P.; Sato, S.; Perego, P.; Converti, A.; Carvalho, J.C.M. Repeated Fed-Batch Cultivation of Arthrospira (Spirulina) Platensis Using Urea as Nitrogen Source. Biochem. Eng. J. 2009, 43, 52–57. [Google Scholar] [CrossRef]
- Phang, S.M.; Miah, M.S.; Yeoh, B.G.; Hashim, M.A. Spirulina Cultivation in Digested Sago Starch Factory Wastewater. J. Appl. Phycol. 2000, 12, 395–400. [Google Scholar] [CrossRef]
- Ranganathan, P.; Amal, J.C.; Savithri, S.; Haridas, A. Experimental and Modelling of Arthrospira Platensis Cultivation in Open Raceway Ponds. Bioresour. Technol. 2017, 242, 197–205. [Google Scholar] [CrossRef]
- Dewi, E.N.; Amalia, U.; Mel, M. The Effect of Different Treatments to the Amino Acid Contents of Micro Algae Spirulina sp. Aquat. Procedia 2016, 7, 59–65. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed Proteins: Biochemical, Nutritional Aspects and Potential Uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation, Bethesda, MD, USA, 4–8 December 1989; FAO food and nutrition paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1991; ISBN 978-92-5-103097-4. [Google Scholar]
- U.S. Department of Agriculture. Agricultural Research Service Seaweed, Spirulina, Dried. Available online: https://fdc.nal.usda.gov/food-details/170495/nutrients (accessed on 10 March 2025).
- Panel on Macronutrients; Panel on the Definition of Dietary Fiber; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board; Institute of Medicine. Chapter 10: Protein and Amino Acids. In Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005; p. 10490. ISBN 978-0-309-08525-0. [Google Scholar]
- Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation [on Protein Quality Evaluation in Human Nutrition], 31 March–2 April 2011, Auckland, New Zealand; FAO food and nutrition paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107417-6.
- Brestenský, M.; Nitrayová, S.; Patráš, P.; Nitray, J. Dietary Requirements for Proteins and Amino Acids in Human Nutrition. Curr. Nutr. Food Sci. 2019, 15, 638–645. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Agricultural Research Service Eggs, Grade A, Large, Egg White. Available online: https://fdc.nal.usda.gov/food-details/747997/nutrients (accessed on 10 March 2025).
- Mróz, M.; Parchem, K.; Jóźwik, J.; Domingues, M.R.; Kusznierewicz, B. The Impact of Different Drying Methods on the Metabolomic and Lipidomic Profiles of Arthrospira Platensis. Molecules 2024, 29, 1747. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Kim, Y.-K. Drying Techniques of Microalgal Biomass: A Review. Appl. Chem. Eng. 2022, 33, 145–150. [Google Scholar] [CrossRef]
- Vermorel, M.; Toullec, G.; Dumond, D.; Pion, R. Valeur protĕique et energĕtique des algues bleues spirulines supplĕmentĕes en acides aminĕs: Utizisation digestive et mĕtabolique par le rat en croissance. Ann. Nutr. Aliment. 1975, 29, 535–552. [Google Scholar]
- Schaafsma, G. The Protein Digestibility-Corrected Amino Acid Score. J. Nutr. 2000, 130, 1865S–1867S. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falvo, M.J. Protein—Which Is Best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design Future Foods Using Plant Protein Blends for Best Nutritional and Technological Functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Moughan, P.J.; Lim, W.X.J. Digestible Indispensable Amino Acid Score (DIAAS): 10 Years On. Front. Nutr. 2024, 11, 1389719. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; McKenna, C.F.; Salvador, A.F.; Paulussen, K.J.M.; Moore, D.R. Dietary Protein Quantity, Quality, and Exercise Are Key to Healthy Living: A Muscle-Centric Perspective Across the Lifespan. Front. Nutr. 2019, 6, 83. [Google Scholar] [CrossRef]
- Lamb, M.W.; Harden, M.L. Protein as a Source of Amino Acids. In The Meaning of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 1973; pp. 153–191. ISBN 978-0-08-017079-4. [Google Scholar]
- Mansilla, W.D.; Marinangeli, C.P.F.; Cargo-Froom, C.; Franczyk, A.; House, J.D.; Elango, R.; Columbus, D.A.; Kiarie, E.; Rogers, M.; Shoveller, A.K. Comparison of Methodologies Used to Define the Protein Quality of Human Foods and Support Regulatory Claims. Appl. Physiol. Nutr. Metab. 2020, 45, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Ad Hoc Expert Committee on Energy and Protein Requirements; World Health Organization; Food and Agriculture Organization of the United Nations. Energy and Protein Requirements: Report of a Joint FAO/WHO ad hoc Expert Committee, Rome, Italy, 22 March–2 April 1971. 1973. Available online: https://iris.who.int/items/7243fc9d-a5df-44d8-80fb-b732c34e1e54 (accessed on 28 October 2025).
- Devi, M.A.; Venkataraman, L.V. Supplementary Value of the Proteins of Blue Green Algae Spirulina Platensis to Rice and Wheat Proteins. Nutr. Rep. Int. 1983, 28, 1029–1035. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S.; Baredar, P. Food Supplements Formulated with Spirulina. In Algae; Mandotra, S.K., Upadhyay, A.K., Ahluwalia, A.S., Eds.; Springer: Singapore, 2021; pp. 201–226. ISBN 978-981-15-7517-4. [Google Scholar]
- Furst, P.T. Spirulina a Nutricious Alga, Once a Staple of Aztec Diet, Could Feed Many of the World Hungry People. Hum. Nat. 1978, 3, 60–65. [Google Scholar]
- Devi, S.; Varkey, A.; Sheshshayee, M.S.; Preston, T.; Kurpad, A.V. Measurement of Protein Digestibility in Humans by a Dual-Tracer Method. Am. J. Clin. Nutr. 2018, 107, 984–991. [Google Scholar] [CrossRef]
- De La Jara, A.; Ruano-Rodriguez, C.; Polifrone, M.; Assunçao, P.; Brito-Casillas, Y.; Wägner, A.M.; Serra-Majem, L. Impact of Dietary Arthrospira (Spirulina) Biomass Consumption on Human Health: Main Health Targets and Systematic Review. J. Appl. Phycol. 2018, 30, 2403–2423. [Google Scholar] [CrossRef]
- Yang, S.; Xu, H.; Chen, J.-H.; Liu, B.; Cheng, K.-W. Microalgae as a Sustainable Protein Source: Key Issues Related to Their Production, Application, and the Way Forward. Food Bioprocess Technol. 2024, 17, 1–33. [Google Scholar] [CrossRef]
- Safdar, W.; Qazi, A.S.; Ahmed, S.; Tariq, M.R.; Ahmed, H. Nonconventional and Novel Strategies to Produce Spirulina Biomass. In Pharmaceutical and Nutraceutical Potential of Cyanobacteria; Mehmood, M.A., Verma, P., Shah, M.P., Betenbaugh, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 161–194. ISBN 978-3-031-45522-3. [Google Scholar]
- Chojnacka, K.; Zielińska, A. Evaluation of Growth Yield of Spirulina (Arthrospira) Sp. in Photoautotrophic, Heterotrophic and Mixotrophic Cultures. World J. Microbiol. Biotechnol. 2012, 28, 437–445. [Google Scholar] [CrossRef]
- Smetana, S.; Sandmann, M.; Rohn, S.; Pleissner, D.; Heinz, V. Autotrophic and Heterotrophic Microalgae and Cyanobacteria Cultivation for Food and Feed: Life Cycle Assessment. Bioresour. Technol. 2017, 245, 162–170. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A Review on Microalgae Cultivation and Harvesting, and Their Biomass Extraction Processing Using Ionic Liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chang, J. Heterotrophic Microalgal Cultivation. In Bioreactors for Microbial Biomass and Energy Conversion; Liao, Q., Chang, J., Herrmann, C., Xia, A., Eds.; Green Energy and Technology; Springer: Singapore, 2018; pp. 117–160. ISBN 978-981-10-7676-3. [Google Scholar]
- Mühling, M.; Belay, A.; Whitton, B.A. Screening Arthrospira (Spirulina) Strains for Heterotrophy. J. Appl. Phycol. 2005, 17, 129–135. [Google Scholar] [CrossRef]
- Marquez, F.J.; Sasaki, K.; Kakizono, T.; Nishio, N.; Nagai, S. Growth Characteristics of Spirulina Platensis in Mixotrophic and Heterotrophic Conditions. J. Ferment. Bioeng. 1993, 76, 408–410. [Google Scholar] [CrossRef]
- Pereira, M.I.B.; Chagas, B.M.E.; Sassi, R.; Medeiros, G.F.; Aguiar, E.M.; Borba, L.H.F.; Silva, E.P.E.; Neto, J.C.A.; Rangel, A.H.N. Mixotrophic Cultivation of Spirulina Platensis in Dairy Wastewater: Effects on the Production of Biomass, Biochemical Composition and Antioxidant Capacity. PLoS ONE 2019, 14, e0224294. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.P.; Ballotta, M.; Usai, L.; Torre, S.; Giordano, M.; Fais, G.; Casula, M.; Dessì, D.; Nieri, P.; Damergi, E.; et al. Mixotrophic Cultivation of Arthrospira Platensis (Spirulina) under Salt Stress: Effect on Biomass Composition, FAME Profile and Phycocyanin Content. Mar. Drugs 2024, 22, 381. [Google Scholar] [CrossRef]
- Amha Belay Mass Culture of Spirulina Outdoors—The Earthrise Farms Experience. In Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology; CRC Press: Boca Raton, FL, USA; Taylor and Francis: Abingdon, UK, 1997; pp. 131–158. ISBN 978-0-429-07994-8.
- Wang, L.; Yuan, D.; Li, Y.; Ma, M.; Hu, Q.; Gong, Y. Contaminating Microzooplankton in Outdoor Microalgal Mass Culture Systems: An Ecological Viewpoint. Algal Res. 2016, 20, 258–266. [Google Scholar] [CrossRef]
- Yuan, D.; Zhan, X.; Wang, M.; Wang, X.; Feng, W.; Gong, Y.; Hu, Q. Biodiversity and Distribution of Microzooplankton in Spirulina (Arthrospira) Platensis Mass Cultures throughout China. Algal Res. 2018, 30, 38–49. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F. Tendencies Affecting the Growth and Cultivation of Genus Spirulina: An Investigative Review on Current Trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef]
- Vardaka, E.; Kormas, K.A.; Katsiapi, M.; Genitsaris, S.; Moustaka-Gouni, M. Molecular Diversity of Bacteria in Commercially Available “Spirulina” Food Supplements. PeerJ 2016, 4, e1610. [Google Scholar] [CrossRef]
- Furmaniak, M.A.; Misztak, A.E.; Franczuk, M.D.; Wilmotte, A.; Waleron, M.; Waleron, K.F. Edible Cyanobacterial Genus Arthrospira: Actual State of the Art in Cultivation Methods, Genetics, and Application in Medicine. Front. Microbiol. 2017, 8, 2541. [Google Scholar] [CrossRef]
- Rhoades, J.; Fotiadou, S.; Paschalidou, G.; Papadimitriou, T.; Ordóñez, A.Á.; Kormas, K.; Vardaka, E.; Likotrafiti, E. Microbiota and Cyanotoxin Content of Retail Spirulina Supplements and Spirulina Supplemented Foods. Microorganisms 2023, 11, 1175. [Google Scholar] [CrossRef]
- Pinchart, P.-E.; Leruste, A.; Pasqualini, V.; Mastroleo, F. Microcystins and Cyanobacterial Contaminants in the French Small-Scale Productions of Spirulina (Limnospira Sp.). Toxins 2023, 15, 354. [Google Scholar] [CrossRef]
- Belay, A. Spirulina (Arthrospira): Production and Quality Assurance. In Spirulina in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–25. [Google Scholar]
- Joseph, C. Dott Elements of Pond Design and Construction. In Handbook of Microalgal Mass Culture; CRC Press: Boca Raton, FL, USA, 1986; pp. 265–283. [Google Scholar]
- Jung, C.H.G.; Braune, S.; Waldeck, P.; Küpper, J.-H.; Petrick, I.; Jung, F. Morphology and Growth of Arthrospira Platensis during Cultivation in a Flat-Type Bioreactor. Life 2021, 11, 536. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chew, K.W.; Teo, M.Y.M.; Ling, T.C.; Alharthi, S.; Alsanie, W.F.; Show, P.L. Evaluation of Real-Time Monitoring on the Growth of Spirulina Microalgae: Internet of Things and Microalgae Technologies. IEEE Internet Things J. 2024, 11, 3274–3281. [Google Scholar] [CrossRef]
- Havlik, I.; Lindner, P.; Scheper, T.; Reardon, K.F. On-Line Monitoring of Large Cultivations of Microalgae and Cyanobacteria. Trends Biotechnol. 2013, 31, 406–414. [Google Scholar] [CrossRef]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of pH on the Functional Properties of Arthrospira (Spirulina) Platensis Protein Isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Chronakis, I.S. Gelation of Edible Blue-Green Algae Protein Isolate (Spirulina platensis Strain Pacifica): Thermal Transitions, Rheological Properties, and Molecular Forces Involved. J. Agric. Food Chem. 2001, 49, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Q.; Yang, Q.; Gong, Z.; Wu, Y.; Liu, X. Effects of Molecular Structure and Charge State on the Foaming and Emulsifying Properties of Spirulina Protein Isolates. Food Res. Int. Ott. Ont 2024, 187, 114407. [Google Scholar] [CrossRef]
- Ang, W.S.; Kee, P.E.; Lan, J.C.-W.; Chen, W.-H.; Chang, J.-S.; Khoo, K.S. Unveiling the Rise of Microalgae-Based Foods in the Global Market: Perspective Views and Way Forward. Food Biosci. 2025, 66, 105390. [Google Scholar] [CrossRef]
- Tagliamonte, S.; Oliviero, V.; Vitaglione, P. Food Bioactive Peptides: Functionality beyond Bitterness. Nutr. Rev. 2025, 83, 369–381. [Google Scholar] [CrossRef]
- Begum, N.; Qi, F.; Yang, F.; Khan, Q.U.; Khan, Q.U.; Faizan, F.Q.; Li, J.; Wang, X.; Wang, X.; Wang, J.; et al. Nutritional Composition and Functional Properties of A. Platensis-Derived Peptides: A Green and Sustainable Protein-Rich Supplement. Processes 2024, 12, 2608. [Google Scholar] [CrossRef]
- Ughetti, A.; D’Eusanio, V.; Strani, L.; Russo, A.L.; Roncaglia, F. Influence of Drying and Storage Conditions on the Volatile Organic Compounds Profile of Spirulina Platensis. Separations 2024, 11, 180. [Google Scholar] [CrossRef]
- Shahid, A.; Fan, Z.; Su, K.; Zhao, A.; Mehmood, M.A.; Chang, J.-S.; Solovchenko, A.E.; Alam, M.A.; Xu, J. Sensory Chemistry of Spirulina: Unveiling Trends and Innovations in Aromatic Volatile Organic Compound Biosynthesis in off-Flavors and Odor Mitigation Strategies. Trends Food Sci. Technol. 2025, 156, 104886. [Google Scholar] [CrossRef]
- Koli, D.; Rudra, S.; Bhowmik, A.; Pabbi, S. Nutritional, Functional, Textural and Sensory Evaluation of Spirulina Enriched Green Pasta: A Potential Dietary and Health Supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef]
- Aljobair, M.O.; Albaridi, N.A.; Alkuraieef, A.N.; AlKehayez, N.M. Physicochemical Properties, Nutritional Value, and Sensory Attributes of a Nectar Developed Using Date Palm Puree and Spirulina. Int. J. Food Prop. 2021, 24, 845–858. [Google Scholar] [CrossRef]
- Ebid, W.M.A.; Ali, G.S.; Elewa, N.A.H. Impact of Spirulina Platensis on Physicochemical, Antioxidant, Microbiological and Sensory Properties of Functional Labneh. Discov. Food 2022, 2, 29. [Google Scholar] [CrossRef]
- Ottombrino, A.; Cianciabella, M.; Medoro, C.; Picariello, A.; Oliviero, A.; De Sena, V.; Predieri, S.; Rossi, M. Sensory Analyses Driven Formulation of Fruit Cereal Bars Enriched With Arthrospira platensis Dried Powder. Food Sci. Nutr. 2025, 13, e70154. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of New Microalgae-Based Sourdough “Crostini”: Functional Effects of Arthrospira Platensis (Spirulina) Addition. Sci. Rep. 2019, 9, 19433. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I. Effect of Arthrospira Platensis (Spirulina) Fortification on Physicochemical, Nutritional, Bioactive, Textural, and Sensory Properties of Vegan Basil Pesto. Nutrients 2024, 16, 2825. [Google Scholar] [CrossRef] [PubMed]
- Mufidatun, A.; Koerniawan, M.D.; Siregar, U.J.; Suwanti, L.T.; Budiman, A.; Suyono, E.A. The Effect of pH on Contamination Reduction and Metabolite Contents in Mass Cultures of Spirulina (Arthrospira Platensis Gomont). Int. J. Adv. Sci. Eng. Inf. Technol. 2023, 13, 84–90. [Google Scholar] [CrossRef]
- McBride, R.C.; Lopez, S.; Meenach, C.; Burnett, M.; Lee, P.A.; Nohilly, F.; Behnke, C. Contamination Management in Low Cost Open Algae Ponds for Biofuels Production. Ind. Biotechnol. 2014, 10, 221–227. [Google Scholar] [CrossRef]
- Mary Leema, J.T.; Kirubagaran, R.; Vinithkumar, N.V.; Dheenan, P.S.; Karthikayulu, S. High Value Pigment Production from Arthrospira (Spirulina) Platensis Cultured in Seawater. Bioresour. Technol. 2010, 101, 9221–9227. [Google Scholar] [CrossRef]
- Franke, S.; Steingröwer, J.; Walther, T.; Krujatz, F. The Oxygen Paradigm—Quantitative Impact of High Concentrations of Dissolved Oxygen on Kinetics and Large-Scale Production of Arthrospira Platensis. ChemEngineering 2022, 6, 14. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, J.; Liu, G.; Gui, B. Selection of Microalgae for High CO2 Fixation Efficiency and Lipid Accumulation from Ten Chlorella Strains Using Municipal Wastewater. J. Environ. Sci. 2016, 46, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A.; Laorawat, S.; Bunnag, B.; Tanticharoen, M. The Effect of Light Availability on the Photosynthetic Activity and Productivity of Outdoor Cultures of Arthrospira Platensis (Spirulina). J. Appl. Phycol. 2014, 26, 1309–1315. [Google Scholar] [CrossRef]
- Sili, C.; Torzillo, G.; Vonshak, A. Arthrospira (Spirulina). In Ecology of Cyanobacteria II; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 677–705. ISBN 978-94-007-3854-6. [Google Scholar]
- Siva Kiran, R.R.; Madhu, G.; Satyanarayana, S.V. Spirulina in Combating Protein Energy Malnutrition (PEM) and Protein Energy Wasting (PEW)—A Review. J. Nutr. Res. 2015, 3, 62–79. [Google Scholar] [CrossRef]
- Melikoglu, M. Waste Valorization Strategies with Inputs for Microalgae Biorefineries: A Global Review. Clean. Water 2025, 4, 100125. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, W.-W.; Cui, K.-H.; Lau, B.P.-Y.; Lee, F.W.-F.; Xu, S.J.-L. Feasibility and Efficiency of Microalgae Cultivation for Nutrient Recycling and Energy Recovery from Food Waste Filtrate. PLoS ONE 2025, 20, e0315801. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods—Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. GRAS Notices, GRN No. 417, Dried Biomass of Arthrospira Platensis Also Known as Spirulina Platensis (Spirulina). 2012. Available online: https://hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=grasnotices&id=417 (accessed on 28 October 2025).
- Gromek, W.; Kołdej, N.; Kurowski, M.; Majsiak, E. Spirulina (Arthrospira Platensis): Antiallergic Agent or Hidden Allergen? A Literature Review. Foods Basel Switz. 2024, 13, 1052. [Google Scholar] [CrossRef]
- Le, T.-M.; Knulst, A.C.; Röckmann, H. Anaphylaxis to Spirulina Confirmed by Skin Prick Test with Ingredients of Spirulina Tablets. Food Chem. Toxicol. 2014, 74, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, E.; Yerly, D.; Caubet, J.-C.; Bergmann, M.M. Delayed IgE–Mediated Hypersensitivity to Arthrospira Platensis (Spirulina). Ann. Allergy. Asthma. Immunol. 2022, 129, 522–524. [Google Scholar] [CrossRef] [PubMed]
- French Agency for Food, Environmental and Occupational Health & Safety. ANSES Opinion Request No 2014-SA-0096: Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the “Risks Associated with the Consumption of Food Supplements Containing Spirulina”. ANSES Opin. 2017, 38. Available online: https://www.anses.fr/en/system/files/NUT2014SA0096EN.pdf (accessed on 28 October 2025).
- Zhou, C.; Jepsen, C.S.; Rigby, N.; Lübeck, M.; Mackie, A.; Sancho, A.I.; Bøgh, K.L. Evaluation of Allergenicity of the Alternative Food Protein Sources Microalga Spirulina and Rapeseed Cake—A Study in Brown Norway Rats. Food Res. Int. 2025, 221, 117357. [Google Scholar] [CrossRef]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Discovery of Marker Peptides of Spirulina Microalga Proteins for Allergen Detection in Processed Foodstuffs. Food Chem. 2022, 393, 133319. [Google Scholar] [CrossRef]
- Mao, T.K.; Van de Water, J.; Gershwin, M.E. Effects of a Spirulina-Based Dietary Supplement on Cytokine Production from Allergic Rhinitis Patients. J. Med. Food 2005, 8, 27–30. [Google Scholar] [CrossRef]
- Cingi, C.; Conk-Dalay, M.; Cakli, H.; Bal, C. The Effects of Spirulina on Allergic Rhinitis. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol.—Head Neck Surg. 2008, 265, 1219–1223. [Google Scholar] [CrossRef]
- Nourollahian, M.; Rasoulian, B.; Gafari, A.; Anoushiravani, M.; Jabari, F.; Bakhshaee, M. Clinical Comparison of the Efficacy of Spirulina Platensis and Cetirizine for Treatment of Allergic Rhinitis. Acta Otorhinolaryngol. Ital. 2020, 40, 224–229. [Google Scholar] [CrossRef]
- Batista, I.G.; Person, O.C.; Bogar, P.; Angélico Júnior, F.V. Efficacy of Spirulina for Allergic Rhinitis. medRxiv 2021. [Google Scholar] [CrossRef]
- Cruz, J.D.; Vasconcelos, V. Legal Aspects of Microalgae in the European Food Sector. Foods Basel Switz. 2023, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Boisen, S.; Eggum, B.O. Critical Evaluation of in Vitro Methods for Estimating Digestibility in Simple-Stomach Animals. Nutr. Res. Rev. 1991, 4, 141–162. [Google Scholar] [CrossRef] [PubMed]
| Species | Protein Content [% Dry Matter] | Reference |
|---|---|---|
| Arthrospira fusiformis | 35–64 | [30,31] |
| Arthrospira maxima | 60–71 | [32,33,34] |
| Arthrospira platensis | 17–80 | [35,36] |
| Crop | Total Yield [Tons/(ha per Year)] | Protein Content [%] | Protein Yield [Tons/(ha per Year)] |
|---|---|---|---|
| Wheat | 6.7 | 9.5 | 0.64 |
| Maize | 14 | 7.4 | 1.04 |
| Rice (hulled) | 8 | 7.1 | 0.57 |
| Soybeans | 4 | 35 | 1.4 |
| Arthrospira platensis | 60–70 | 65 | 39–45 |
| Culture System | Culture Medium | Protein Content [%] | Reference |
|---|---|---|---|
| Erlenmeyer flask | Schlösser (+Beet vinasse) | 39–55 35–72 | [51] |
| Photobioreactor | Schlösser (urea as nitrogen source) | 18–71 | [52] |
| Photobioreactor | Zarrouk (sodium nitrate modification) | 56–70 | [53] |
| Photobioreactor | Zarrouk | 69 | [36] |
| Photobioreactor | FM-II | 58–63 | [48] |
| Semicontinuous culture system | Conwey | 61–64 | [54] |
| Elongated minitank (outdoor pond simulation) | Schlösser (urea as nitrogen source) | 45–62 | [55] |
| Open pond | Kosaric and digested sago starch factory wastewater | 53–68 | [56] |
| Open pond | Modified Zarrouk | 56 | [57] |
| Open pond | n.d. | 59 | [58] |
| Erlenmeyer flask | “Standard” with variations | 17–32 | [35] |
| n.d. | n.d. | 46–63 | [32] |
| Essential Amino Acids (EAAs) | Required [g/100 g Protein] [63,64] | Arthrospira [g/100 g Protein] [61] | Egg White [g/100 g Protein] [65] |
|---|---|---|---|
| Histidine | 1.6 | 1.08 | 0.257 |
| Isoleucine | 3.0 | 3.21 | 0.609 |
| Leucine | 6.1 | 4.95 | 1.02 |
| Lysine | 4.8 | 3.02 | 0.822 |
| Methionin + Cystine | 2.3 | 1.15 + 0.662 | 0.486 + 0.407 |
| Phenylalanin + Tyrosine | 4.1 | 2.78 + 2.58 | 0.726 + 0.466 |
| Threonine | 2.5 | 2.97 | 0.567 |
| Tryptophan | 0.66 | 0.929 | 0.188 |
| Valine | 4.0 | 3.51 | 0.779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braune, S.; Jung, C.G.H.; Küpper, J.-H.; Jung, F. Arthrospira platensis as Protein-Rich Source for Human Nutrition. Life 2025, 15, 1789. https://doi.org/10.3390/life15121789
Braune S, Jung CGH, Küpper J-H, Jung F. Arthrospira platensis as Protein-Rich Source for Human Nutrition. Life. 2025; 15(12):1789. https://doi.org/10.3390/life15121789
Chicago/Turabian StyleBraune, Steffen, Conrad G. H. Jung, Jan-Heiner Küpper, and Friedrich Jung. 2025. "Arthrospira platensis as Protein-Rich Source for Human Nutrition" Life 15, no. 12: 1789. https://doi.org/10.3390/life15121789
APA StyleBraune, S., Jung, C. G. H., Küpper, J.-H., & Jung, F. (2025). Arthrospira platensis as Protein-Rich Source for Human Nutrition. Life, 15(12), 1789. https://doi.org/10.3390/life15121789









