Diagnostic Reassessment of a Historical Case of Atypical Heparin-Induced Thrombocytopenia: Between Spontaneous Heparin-Induced Thrombocytopenia and a Vaccine-Induced Immune Thrombotic Thrombocytopenia-Like Syndrome
Abstract
1. Introduction
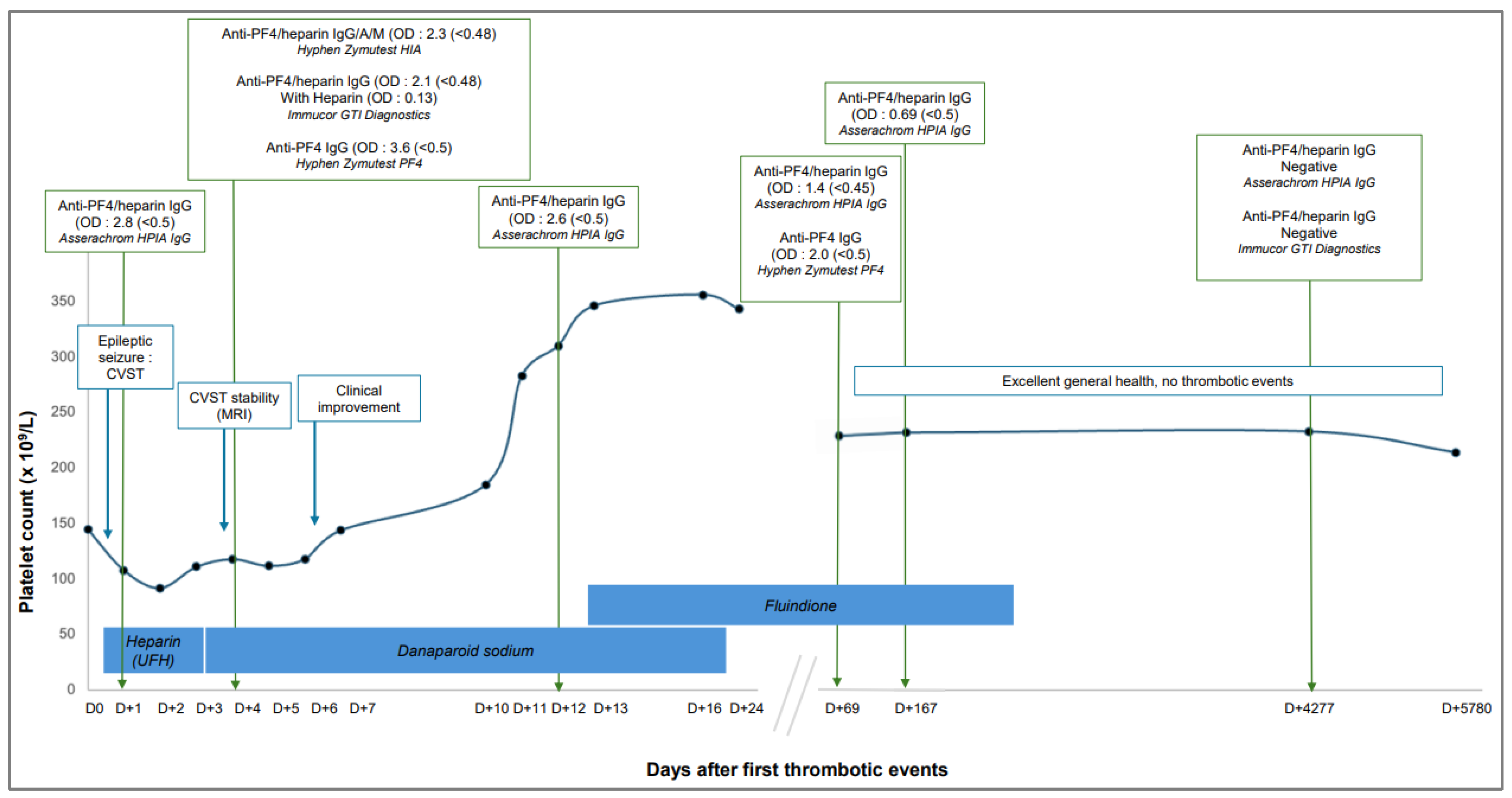
2. Case Presentation
3. Discussion
- Autoimmune HIT (aHIT):
- Refractory HIT, defined by the persistence of thrombocytopenia for several weeks after heparin withdrawal, and appears to be associated with the continued presence of antibodies capable of heparin-independent platelet activation [11, 12]. This form typically resolves following intravenous administration of polyvalent immunoglobulins [13, 14].
- A recent history of orthopedic surgery, especially knee arthroplasty, in which patients develop a clinical HIT-like syndrome sometimes even while receiving alternative, non-heparin anticoagulation, such as vitamin K antagonists [21, 22]. These cases frequently exhibit a strong female predominance, a high rate of thrombotic events, and often hemorrhagic adrenal gland necrosis [19].
- A recent infectious syndrome (such respiratory, skin or oral infections, of either bacterial or viral origin), with patients typically presenting with thrombosis and thrombocytopenia, or with a rapid decline in platelet count following heparin administration. The thrombotic profile in these situations is generally marked by venous thromboses, particularly CVST [19].
- VITT and VITT-like syndromes:
| Classic HIT | Auto-Immune HIT | Spontaneous HIT | VITT | VITT-Like Syndrome | |
|---|---|---|---|---|---|
| Trigger | Heparin | Heparin | No proximate heparin Knee surgery or Infectious syndrome | No proximate heparin COVID-19 AAV | No proximate heparin or COVID-19 AAV Infectious syndrome, non-AAV, other trigger? |
| Onset time | 5–10 days after starting heparin | 5–10 days after immunizing exposure to heparin (sometimes persists after stopping) | 5–10 days after the triggering events occur | 5–30 days after COVID-19 AAV administration | A few days to a few weeks after the triggering events occur |
| Antibody type | Low-avidity, polyclonal anti-PF4/heparin IgG | High-avidity, polyclonal anti-PF4 IgG AND Low-avidity, polyclonal anti-PF4/heparin IgG | High-avidity, polyclonal anti-PF4 IgG | High-avidity, mono-oligoclonal anti-PF4 IgG | High-avidity, mono-oligoclonal anti-PF4 IgG |
| Thrombocytopenia | Often mild to moderate (50–100 G/L) | Often severe (<50 G/L) | Often severe (<50 G/L) | Often severe (<50 G/L) | Often severe (<50 G/L) |
| Thrombotic events | Usually macrovascular (venous > arterial) thrombosis Low frequency of UST | Usually macrovascular (venous > arterial) thrombosis Higher frequency of UST | Usually macrovascular (venous > arterial) thrombosis Higher frequency of UST (CVT, MVT, BAH) | High frequency of UST (CVT, MVT) sometimes followed by hemorrhagic transformation | High frequency of UST (CVT, MVT) sometimes followed by hemorrhagic transformation |
| PF4-dependant ELISA | Solid-phase: High sensitivity for both anti-PF4/heparin and anti-PF4 tests Fluid-phase: Anti-PF4/heparin > Anti-PF4 | Solid-phase: High sensitivity for both anti-PF4/heparin and anti-PF4 tests Fluid-phase: Anti-PF4/heparin > Anti-PF4 | Solid-phase: High sensitivity for both anti-PF4/heparin and anti-PF4 tests Fluid-phase: Anti-PF4/heparin > Anti-PF4 test | Solid-phase: High sensitivity for both anti-PF4/heparin and anti-PF4 tests Fluid-phase: Anti-PF4 > Anti-PF4/heparin | Solid-phase: High sensitivity for both anti-PF4/heparin and anti-PF4 tests Fluid-phase: Anti-PF4 > Anti-PF4/heparin |
| PF4-dependant RIA | High-sensitivity | High-sensitivity | High to moderate-sensitivity | Low-sensitivity | Low-sensitivity |
| Platelet activation test | Heparin-dependent activation SRA: Higher sensitivity with heparin and/or PF4 HIPA/PIPA: High sensitivity | Heparin-independent activation SRA: Higher sensitivity with heparin and/or PF4 HIPA/PIPA: High sensitivity | Heparin-independent activation SRA: Higher sensitivity with PF4 > Heparin HIPA/PIPA: High sensitivity | Heparin-independent activation SRA: Higher sensitivity with PF4 Inhibition in presence of heparin PIPA > HIPA | Heparin-independent activation SRA: Higher sensitivity with PF4 Inhibition in presence of heparin PIPA > HIPA |
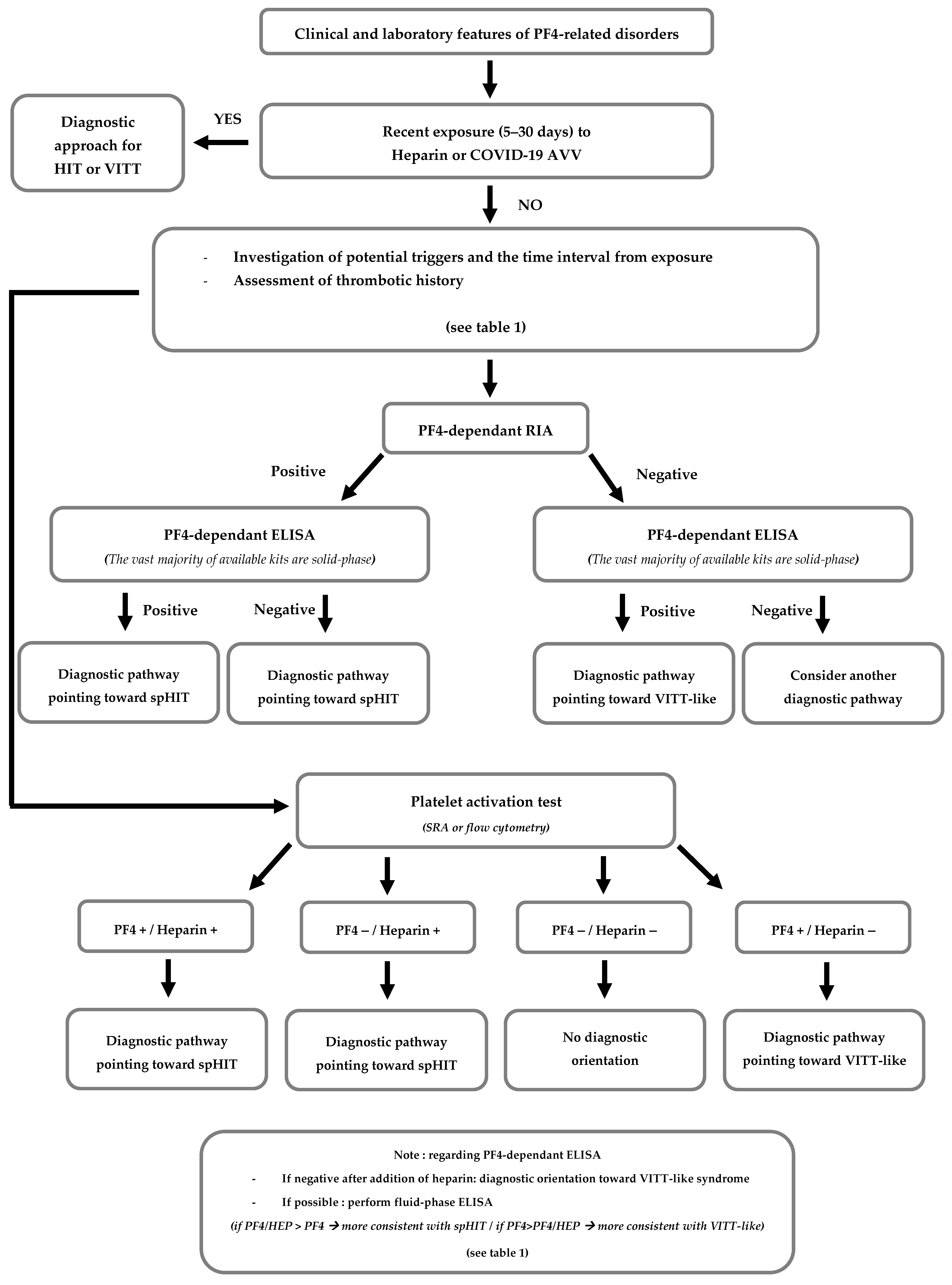
- About the case: spHIT or VITT-like syndrome?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aHIT | Autoimmune Heparin-Induced Thrombocytopenia |
| cHIT | Classical Heparin-Induced Thrombocytopenia |
| CMV | Cytomegalovirus |
| CRP | C-Reactive Protein |
| CVST | Cerebral Venous Sinus Thrombosis |
| ELISA | Enzyme-linked immunosorbent assay |
| HIPA | Heparin-induced Platelet Activation Assay |
| HIT | Heparin-induced Thrombocytopenia |
| MRI | Magnetic Resonance Imaging |
| OD | Optical Density |
| PF4 | Platelet Factor-4 |
| PIPA | PF4-Induced Platelet Activation Assay |
| RSV | Respiratory Syncytial Virus |
| SpHIT | Spontaneous Heparin-Induced Thrombocytopenia |
| SRA | Serotonin-release assay |
| UFH | Unfractionated Heparin |
| VITT | Vaccine-induced Thrombocytopenia and Thrombosis |
References
- Warkentin, T.E. Classification of Platelet-Activating Anti-Platelet Factor 4 Disorders. Int. J. Lab. Hematol. 2025. [Google Scholar] [CrossRef]
- Muir, K.L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Markus, W.; Anne-Hege, A.; Thor, H.S.; Geir, E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Baskin-Miller, J.; Raybould, A.L.; Sheppard, J.A.I.; Daka, M.; Nazy, I.; Moll, S. Adenovirus-Associated Thrombocytopenia, Thrombosis, and VITT-like Antibodies. N. Engl. J. Med. 2023, 389, 574–577. [Google Scholar] [CrossRef]
- Wittstock, M.; Cantré, D.; Won, S.Y.; Jürs, A.V.; Wesche, J.; Greger, N.; Greinacher, A.; Thiele, T. An anti-PF4 antibody-related disorder with cerebral venous sinus thrombosis and thrombocytopenia initially presenting as intracranial hemorrhage. J. Neurol. 2024, 271, 4651–4654. [Google Scholar] [CrossRef]
- Desprez, D.; Desprez, P.; Tardy, B.; Amiral, J.; Droulle, C.; Ducassou, S.; Mauvieux, L.; Grunebaum, L. Anticorps anti-PF4 et thrombopénie chez un enfant avec thrombophlébite cérébrale. Ann. Biol. Clin. 2010, 68, 725–728. [Google Scholar]
- Müller, L.; Wang, J.J.; Dabbiru, V.A.S.; Thiele, T.; Schönborn, L. Anti-Platelet factor 4 immunothrombosis—Not just heparin and vaccine triggers. Res. Pract. Thromb. Haemost. 2025, 9, 102729. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Kelton, J.G. Delayed-Onset Heparin-Induced Thrombocytopenia and Thrombosis. Ann. Intern. Med. 2001, 135, 502–506. [Google Scholar] [CrossRef]
- Rice, L.; Attisha, W.K.; Drexler, A.; Francis, J.L. Delayed-Onset Heparin-Induced Thrombocytopenia. Ann. Intern. Med. 2002, 136, 210–215. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Warkentin, T.E. Autoimmune heparin-induced thrombocytopenia. J. Thromb. Haemost. 2017, 15, 2099–2114. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Makris, M.; Jay, R.M.; Kelton, J.G. A Spontaneous Prothrombotic Disorder Resembling Heparin-induced Thrombocytopenia. Am. J. Med. 2008, 121, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Pipalia, A.; Weil, A. Refractory Heparin-Induced Thrombocytopenia with Cerebral Venous Sinus Thrombosis Treated with IVIg, Steroids, and a Combination of Anticoagulants: A Case Report. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619832324. [Google Scholar] [CrossRef] [PubMed]
- Seiler, J.A.; Durrani, A.K.; Ahmeti, M. A Case of Argatroban Refractory Heparin Induced Thrombocytopenia and Thrombosis. Am. Surg. 2023, 89, 3574–3575. [Google Scholar] [CrossRef]
- Mian, H.; Warkentin, T.E.; Sheppard, J.A.I.; MacDonald, A.; Linkins, L.A.; Benger, A.; Foley, R. Autoimmune HIT due to apheresis catheter heparin flushes for stem cell harvesting before autotransplantation for myeloma. Blood 2017, 130, 1679–1682. [Google Scholar] [CrossRef]
- Refaai, M.; Warkentin, T.; Axelson, M.; Matevosyan, K.; Sarode, R. Delayed-onset heparin-induced thrombocytopenia, venous thromboembolism, and cerebral venous thrombosis: A consequence of heparin “flushes”. Thromb. Haemost. 2007, 98, 1139–1140. [Google Scholar] [CrossRef]
- Te, W. Heparin-Induced Thrombocytopenia Associated with Fondaparinux. N. Engl. J. Med. 2007, 356, 2653–2655. [Google Scholar]
- Warkentin, T.; Sheppard, J.A.; Manheim, J. HIT complicating fondaparinux prophylaxis: Fondaparinux-dependent platelet activation as a marker for fondaparinux- induced HIT. Thromb. Haemost. 2014, 112, 1319–1322. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Greinacher, A. Spontaneous HIT syndrome: Knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia. Thromb. Res. 2021, 204, 40–51. [Google Scholar] [CrossRef]
- Perrin, J.; Barraud, D.; Toussaint-Hacquard, M.; Bollaert, P.E.; Lecompte, T. Rapid onset heparin-induced thrombocytopenia (HIT) without history of heparin exposure: A new case of so-called ‘spontaneous’ HIT. Thromb. Haemost. 2012, 107, 795–797. [Google Scholar]
- Jay, R.M.; Warkentin, T.E. Fatal heparin-induced thrombocytopenia (HIT) during warfarin thromboprophylaxis following orthopedic surgery: Another example of ‘spontaneous’ HIT? J. Thromb. Haemost. 2008, 6, 1598–1600. [Google Scholar] [CrossRef]
- Baker, K.; Lim, M.Y. Spontaneous Heparin-Induced Thrombocytopenia and Venous Thromboembolism following Total Knee Arthroplasty. Case Rep. Hematol. 2017, 2017, 4918623. [Google Scholar] [CrossRef]
- Tardy-Poncet, B.; Tardy, B.; Grelac, F.; Reynaud, J.; Mismetti, P.; Bertrand, J.C.; Guyotat, D. Pentosan polysulfate-induced thrombocytopenia and thrombosis. Am. J. Hematol. 1994, 45, 252–257. [Google Scholar] [CrossRef]
- Greinacher, A.; Michels, I.; Schäfer, M.; Kiefel, V.; Mueller-Eckhardt, C. Heparin-associated thrombocytopenia in a patient treated with polysulphated chondroitin sulphate: Evidence for immunological crossreactivity between heparin and polysulphated glycosaminoglycan. Br. J. Haematol. 1992, 81, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Warkentin, T.E. Thrombotic anti-PF4 immune disorders: HIT, VITT, and beyond. Hematology 2023, 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Krauel, K.; Weber, C.; Brandt, S.; Zähringer, U.; Mamat, U.; Greinacher, A.; Hammerschmidt, S. Platelet factor 4 binding to lipid A of Gram-negative bacteria exposes PF4/heparin-like epitopes. Blood 2012, 120, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Gruel, Y.; Watier, H. Bacteria and HIT: A close connection? Blood J. Am. Soc. Hematol. 2011, 117, 1105–1106. [Google Scholar] [CrossRef]
- Pongas, G.; Dasgupta, S.K.; Thiagarajan, P. Antiplatelet factor 4/heparin antibodies in patients with gram negative bacteremia. Thromb. Res. 2013, 132, 217–220. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Wang, J.J.; Van Der Neut Kolfschoten, M.; Rutten, L.; Armour, B.; Tan, C.W.; Chataway, T.; Bos, R.; Koornneef, A.; Abeywickrema, P.; Kapur, R.; et al. Characterization of reverse-engineered anti-PF4 stereotypic antibodies derived from serum of patients with VITT. Blood 2024, 143, 370–374. [Google Scholar] [CrossRef]
- Huynh, A.; Kelton, J.G.; Arnold, D.M.; Daka, M.; Nazy, I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021, 596, 565. [Google Scholar] [CrossRef]
- Wagner, D.D.; Heger, L.A. Thromboinflammation: From Atherosclerosis to COVID-19. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.; Liu, X.; Kong, Y.; Qi, M.; Perdomo, J.; Fenwick, J.; Tieng, J.; Johnston, B.; Shi, Q.S.; Larance, M.; et al. Endothelial cell activation enhances thromboinflammation in vaccine-induced immune thrombotic thrombocytopenia. Blood Adv. 2025, 9, 2891–2906. [Google Scholar] [CrossRef] [PubMed]
- Kanack, A.J.; Bayas, A.; George, G.; Abou-Ismail, M.Y.; Singh, B.; Kohlhagen, M.C.; Splinter, N.P.; Christ, M.; Naumann, M.; Moser, K.A.; et al. Monoclonal and oligoclonal anti-platelet factor 4 antibodies mediate VITT. Blood 2022, 140, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Paré, G.; Nazy, I. Genetic predisposition to vaccine-induced immune thrombotic thrombocytopenia. Br. J. Haematol. 2025, 206, 387–388. [Google Scholar] [CrossRef]
- Schönborn, L.; Esteban, O.; Wesche, J.; Dobosz, P.; Broto, M.; Puig, S.R.; Fuhrmann, J.; Torres, R.; Serra, J.; Llevadot, R.; et al. Anti-PF4 immunothrombosis without proximate heparin or adenovirus vector vaccine exposure. Blood 2023, 142, 2305–2314. [Google Scholar] [CrossRef]
- Wang, J.J.; Schönborn, L.; Warkentin, T.E.; Chataway, T.; Grosse, L.; Simioni, P.; Moll, S.; Greinacher, A.; Gordon, T.P. Antibody Fingerprints Linking Adenoviral Anti-PF4 Disorders. N. Engl. J. Med. 2024, 390, 1827–1829. [Google Scholar] [CrossRef]
- Johansen, S.; Lægreid, I.J.; Ernstsen, S.L.; Azrakhsh, N.A.; Kittang, A.O.; Lindås, R.; Gjertsen, B.T.; Vetti, N.; Mørtberg, T.V.; Sørvoll, I.H.; et al. Thrombosis and thrombocytopenia after HPV vaccination. J. Thromb. Haemost. 2022, 20, 700–704. [Google Scholar] [CrossRef]
- Lin, T.C.; Fu, P.A.; Hsu, Y.T.; Chen, T.Y. Vaccine-Induced Immune Thrombotic Thrombocytopenia following BNT162b2 mRNA COVID-19 Booster: A Case Report. Vaccines 2023, 11, 1115. [Google Scholar] [CrossRef]
- Lambert, M.P.; Warkentin, T.E. Mini-clusters of postadenovirus VITT. Res. Pract. Thromb. Haemost. 2025, 9, 102641. [Google Scholar] [CrossRef]
- Dimopoulou, D.; Mentesidou, L.; Dettoraki, A.; Karastathi, C.; Berikopoulou, M.; Katsouli, P.; Anastasopoulou, I.; Stamatakis, I.G.; Bachou, T.; Tzifi, F.; et al. A cluster of pediatric VITT-like cases with thrombosis and thrombocytopenia following respiratory infections-Case series. Res. Pract. Thromb. Haemost. 2024, 8, 102589. [Google Scholar] [CrossRef]
- Nicolson, P.L.R.; Montague, S.J.; Buka, R.J.; Calvert, A.; Sheppard, J.I.; Zhang, Y.; Wang, J.; Sharman, J.; Hassan, W.; Harrison, J.; et al. Anti-PF4 mediated thrombocytopenia and thrombosis associated with acute cytomegalovirus infection displays both HIT-like and VITT-like characteristics. Br. J. Haematol. 2025, 206, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Greinacher, A. Laboratory Testing for Heparin-Induced Thrombocytopenia and Vaccine-Induced Immune Thrombotic Thrombocytopenia Antibodies: A Narrative Review. Semin. Thromb. Hemost. 2023, 49, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Arnold, D.M.; Sheppard, J.A.I.; Moore, J.C.; Kelton, J.G.; Nazy, I. Investigation of anti-PF4 versus anti-PF4/heparin reactivity using fluid-phase enzyme immunoassay for 4 anti-PF4 disorders: Classic heparin-induced thrombocytopenia (HIT), autoimmune HIT, vaccine-induced immune thrombotic thrombocytopenia, and spontaneous HIT. J. Thromb. Haemost. 2023, 21, 2268–2276. [Google Scholar]
- Vayne, C.; Guery, E.; Kizlik-Masson, C.; Rollin, J.; Bauters, A.; Gruel, Y.; Pouplard, C. Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparin-induced thrombocytopenia antibodies. Br. J. Haematol. 2017, 179, 811–819. [Google Scholar] [CrossRef]
- Vayne, C.; Rollin, J.; Gruel, Y.; Pouplard, C.; Galinat, H.; Huet, O.; Mémier, V.; Geeraerts, T.; Marlu, R.; Pernod, G.; et al. PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 385, 376–378. [Google Scholar] [CrossRef]
- Schönborn, L.; Thiele, T.; Esefeld, M.; El Debuch, K.; Wesche, J.; Seck, S.E.; Kaderali, L.; Wolff, M.; Warkentin, T.E.; Greinacher, A. Quantitative interpretation of PF4/heparin-EIA optical densities in predicting platelet-activating VITT antibodies. J. Thromb. Haemost. 2022, 20, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimmer, J.; Kirscher, S.; Dolt, M.; Herb, A.; Pierre, L.; Grunebaum, L.; Feugeas, O.; Sattler, L.; Desprez, D. Diagnostic Reassessment of a Historical Case of Atypical Heparin-Induced Thrombocytopenia: Between Spontaneous Heparin-Induced Thrombocytopenia and a Vaccine-Induced Immune Thrombotic Thrombocytopenia-Like Syndrome. Life 2025, 15, 1767. https://doi.org/10.3390/life15111767
Wimmer J, Kirscher S, Dolt M, Herb A, Pierre L, Grunebaum L, Feugeas O, Sattler L, Desprez D. Diagnostic Reassessment of a Historical Case of Atypical Heparin-Induced Thrombocytopenia: Between Spontaneous Heparin-Induced Thrombocytopenia and a Vaccine-Induced Immune Thrombotic Thrombocytopenia-Like Syndrome. Life. 2025; 15(11):1767. https://doi.org/10.3390/life15111767
Chicago/Turabian StyleWimmer, Jordan, Solène Kirscher, Manon Dolt, Agathe Herb, Léa Pierre, Lélia Grunebaum, Olivier Feugeas, Laurent Sattler, and Dominique Desprez. 2025. "Diagnostic Reassessment of a Historical Case of Atypical Heparin-Induced Thrombocytopenia: Between Spontaneous Heparin-Induced Thrombocytopenia and a Vaccine-Induced Immune Thrombotic Thrombocytopenia-Like Syndrome" Life 15, no. 11: 1767. https://doi.org/10.3390/life15111767
APA StyleWimmer, J., Kirscher, S., Dolt, M., Herb, A., Pierre, L., Grunebaum, L., Feugeas, O., Sattler, L., & Desprez, D. (2025). Diagnostic Reassessment of a Historical Case of Atypical Heparin-Induced Thrombocytopenia: Between Spontaneous Heparin-Induced Thrombocytopenia and a Vaccine-Induced Immune Thrombotic Thrombocytopenia-Like Syndrome. Life, 15(11), 1767. https://doi.org/10.3390/life15111767






