Prognostic Impact of Interleukin-27 in Peripheral Artery Disease
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Enrollment and Follow-Up
3.2. Baseline Patient Characteristics
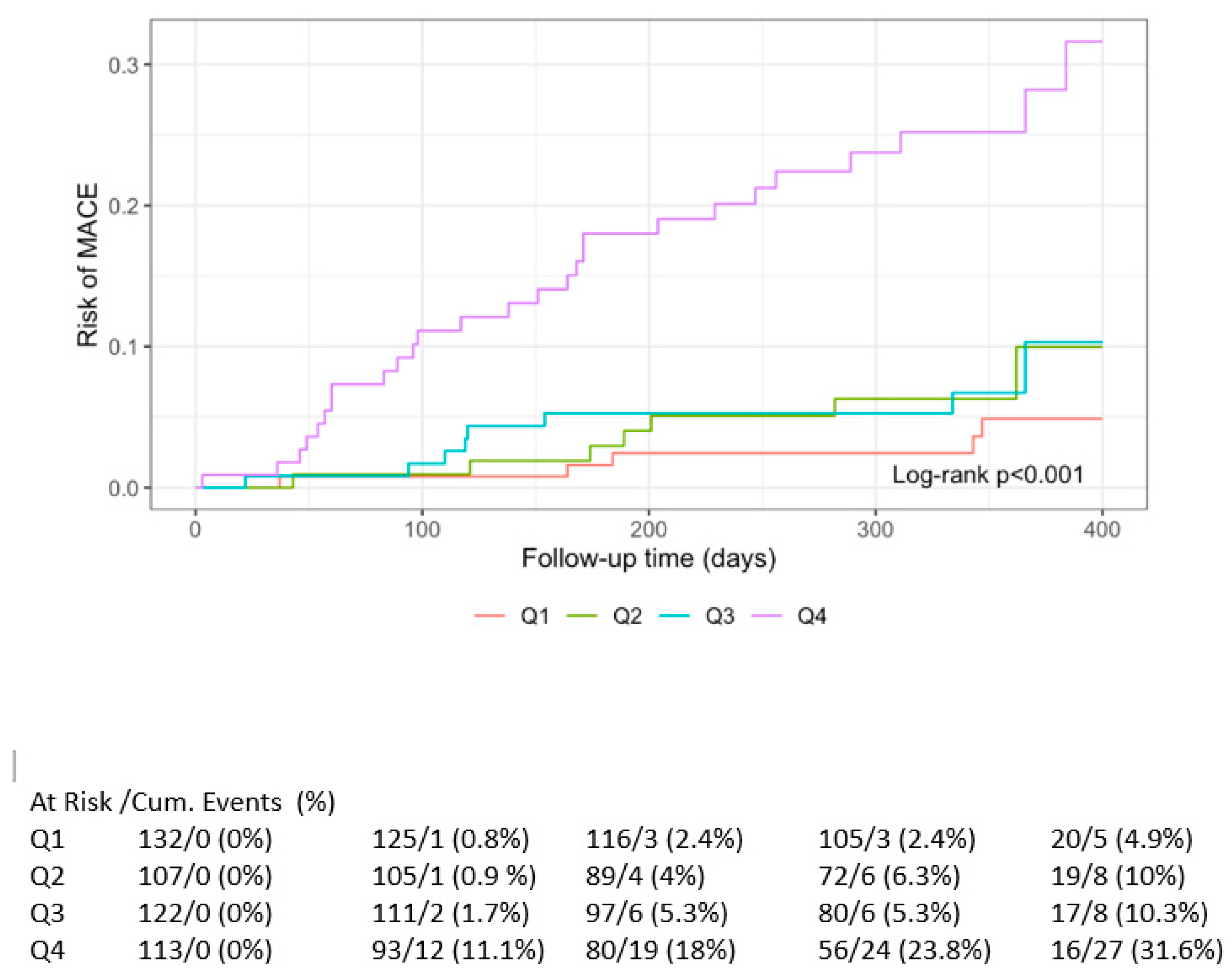
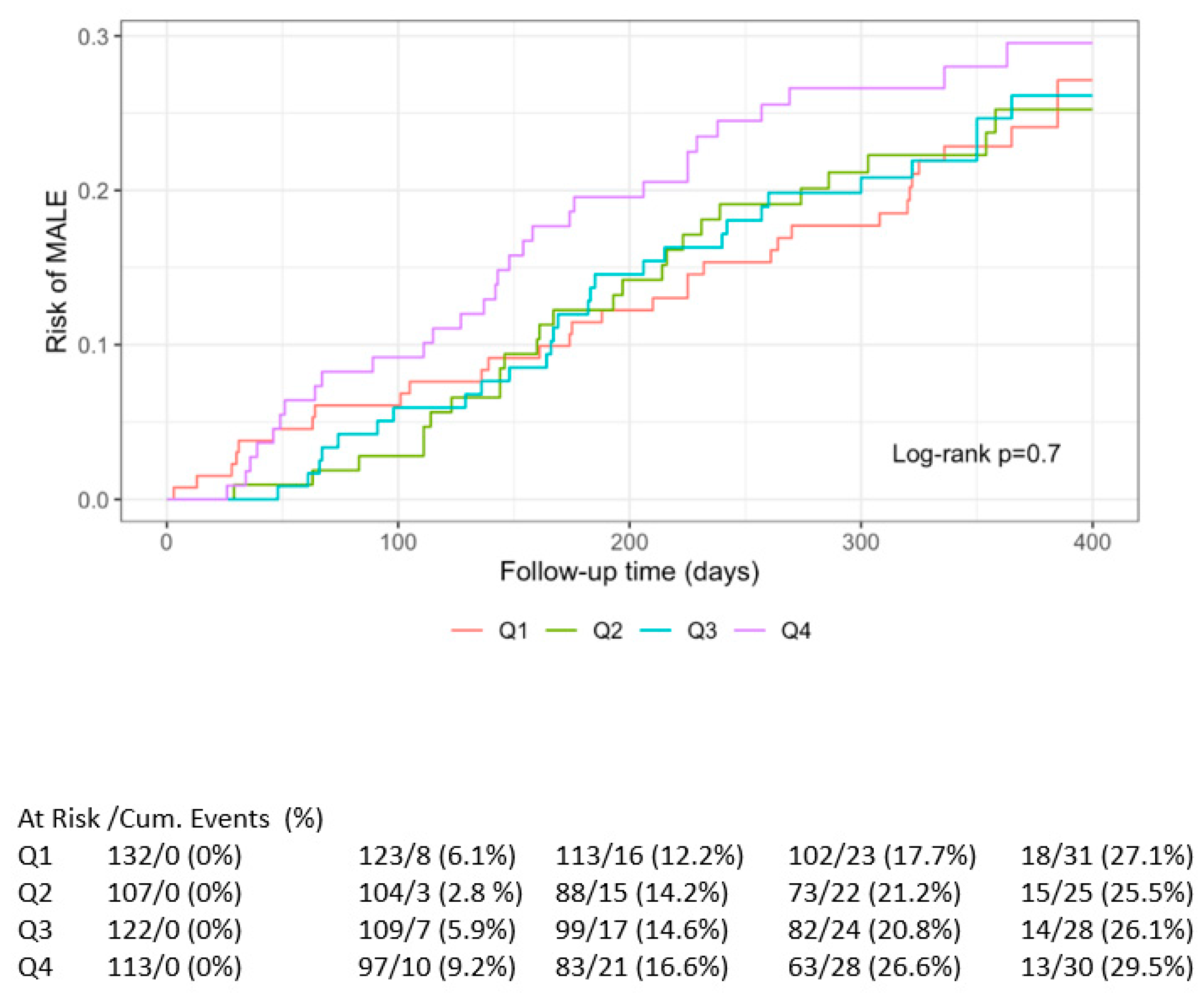
3.3. Primary Outcome
3.4. Survival Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | Coronary artery disease |
| CVD | Cardiovascular disease |
| IL | Interleukin |
| MACE | Major adverse cardiovascular events |
| MALE | Major adverse limb events |
| MI | Myocardial infarction |
| PAD | Peripheral artery disease |
| PTA | Percutaneus transluminal angioplasty |
| TIA | Transient ischaemic attack |
References
- Frostegård, J.; Ulfgren, A.-K.; Nyberg, P.; Hedin, U.; Swedenborg, J.; Andersson, U.; Hansson, G.K. Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999, 145, 33–43. [Google Scholar] [CrossRef]
- Yoshida, H.; Nakaya, M.; Miyazaki, Y. Interleukin 27: A double-edged sword for offense and defense. J. Leukoc. Biol. 2009, 86, 1295–1303. [Google Scholar] [CrossRef]
- Onea, H.-L.; Homorodean, C.; Lazar, F.-L.; Negrea, M.O.; Calin, T.; Bitea, I.C.; Teodoru, M.; Nechita, V.I.; Olteanu, A.L.; Olinic, D.-M. Galectin-3 Reflects Systemic Atherosclerosis in Patients with Coronary Artery Disease. Medicina 2025, 61, 1388. [Google Scholar] [CrossRef]
- Damen, M.S.; Popa, C.D.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Interleukin-32 in chronic inflammatory conditions is associated with a higher risk of cardiovascular diseases. Atherosclerosis 2017, 264, 83–91. [Google Scholar] [CrossRef]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002, 16, 779–790. [Google Scholar]
- Awasthi, A.; Carrier, Y.; Peron, J.P.S.; Bettelli, E.; Kamanaka, M.; Flavell, R.A.; Kuchroo, V.K.; Oukka, M.; Weiner, H.L. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007, 8, 1380–1389. [Google Scholar]
- Carl, J.W.; Bai, X.-F. IL27: Its roles in the induction and inhibition of inflammation. Int. J. Clin. Exp. Pathol. 2008, 1, 117–123. [Google Scholar] [PubMed]
- Yoshida, H.; Hamano, S.; Senaldi, G.; Covey, T.; Faggioni, R.; Mu, S.; Xia, M.; Wakeham, A.C.; Nishina, H.; Potter, J.; et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 2001, 15, 569–578. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Yoshimoto, T.; Yasuda, K.; Mizuguchi, J.; Nakanishi, K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: A novel therapeutic way for Th2-mediated allergic inflammation. J. Immunol. 2007, 179, 4415–4423. [Google Scholar] [CrossRef] [PubMed]
- Posadas-Sánchez, R.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Coral-Vázquez, R.M.; Roque-Ramírez, B.; Llorente, L.; Lima, G.; Flores-Dominguez, C.; Villarreal-Molina, T.; Posadas-Romero, C.; et al. Interleukin-27 polymorphisms are associated with premature coronary artery disease and metabolic parameters in the Mexican population: The genetics of atherosclerotic disease (GEA) Mexican study. Oncotarget 2017, 8, 64459–64470. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Rezayati, M. Serum levels of interleukin-27 in patients with ischemic heart disease. Cytokine 2011, 56, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Jafarizade, M.; Kahe, F.; Sharfaei, S.; Momenzadeh, K.; Pitliya, A.; Tajrishi, F.Z.; Singh, P.; Chi, G. The role of interleukin-27 in atherosclerosis: A contemporary review. Cardiology 2021, 146, 517–530. [Google Scholar] [CrossRef]
- Gregersen, I.; Sandanger, Ø.; Askevold, E.T.; Sagen, E.L.; Yang, K.; Holm, S.; Pedersen, T.M.; Skjelland, M.; Krohg-Sørensen, K.; Hansen, T.V.; et al. Interleukin 27 is increased in carotid atherosclerosis and promotes NLRP3 inflammasome activation. PLoS ONE 2017, 12, e0188387. [Google Scholar] [CrossRef]
- Grufman, H.; Yndigegn, T.; Gonçalves, I.; Nilsson, J.; Schiopu, A. Elevated IL-27 in patients with acute coronary syndrome is associated with adverse ventricular remodeling and increased risk of recurrent myocardial infarction and cardiovascular death. Cytokine 2019, 122, 154208. [Google Scholar] [CrossRef]
- Jin, W.; Zhao, Y.; Yan, W.; Cao, L.; Zhang, W.; Wang, M.; Zhang, T.; Fu, Q.; Li, Z. Elevated circulating interleukin-27 in patients with coronary artery disease is associated with dendritic cells, oxidized low-density lipoprotein, and severity of coronary artery stenosis. Mediat. Inflamm. 2012, 2012, 506283. [Google Scholar] [CrossRef]
- Miura, K.; Saita, E.; Suzuki-Sugihara, N.; Miyata, K.; Ikemura, N.; Ohmori, R.; Ikegami, Y.; Kishimoto, Y.; Kondo, K.; Momiyama, Y. Plasma interleukin-27 levels in patients with coronary artery disease. Medicine 2017, 96, e8260. [Google Scholar] [CrossRef]
- Bosmann, M.; Ward, P.A. Modulation of inflammation by interleukin-27. J. Leukoc. Biol. 2013, 94, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005, 5, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.N.; Liu, B.; Liu, W.; Liu, S. Interleukin-27 enhances TNF-α-mediated activation of human coronary artery endothelial cells. Mol. Cell. Biochem. 2016, 411, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Owaki, T.; Asakawa, M.; Morishima, N.; Hata, K.; Fukai, F.; Matsui, M.; Mizuguchi, J.; Yoshimoto, T. A role for IL-27 in early regulation of Th1 differentiation. J. Immunol. 2005, 175, 2191–2200. [Google Scholar] [CrossRef]
- Koltsova, E.K.; Kim, G.; Lloyd, K.M.; Saris, C.J.; Von Vietinghoff, S.; Kronenberg, M.; Ley, K. Interleukin-27 receptor limits atherosclerosis in Ldlr-/- mice. Circ. Res. 2012, 111, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Hirase, T.; Hara, H.; Miyazaki, Y.; Ide, N.; Nishimoto-Hazuku, A.; Fujimoto, H.; Saris, C.J.M.; Yoshida, H.; Node, K. Interleukin 27 inhibits atherosclerosis via immunoregulation of macrophages in mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H420–H429. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, R.; Zhu, Z.; Yu, K.; Wang, Y.; Ding, Y.; Yu, J.; Tang, H.; Zeng, Q.; Zhong, Y. Interleukin-27 ameliorates atherosclerosis in ApoE-/- mice through regulatory T Cell augmentation and dendritic cell tolerance. Mediat. Inflamm. 2022, 2022, 2054879. [Google Scholar] [CrossRef]
- Eric, S.; Zaric, R.Z.; Jevdjic, J.; Drakulic, S.M.; Stanojevic, I.; Vojvodic, D.; Arsenijevic, P.; Stojanovic, B.; Jakovljevic, S.; Markovic, N.; et al. Interleukin 33, soluble suppression of tumorigenicity 2, interleukin 27, and galectin 3 as predictors for outcome in patients admitted to intensive care units. Open Med. 2023, 18, 20230859. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.-M.; Cao, P.; Zhang, C.; Feng, C.-M.; Zheng, L.; Xu, D.-X.; Fu, L.; Zhao, H. Serum IL-27 predicts the severity and prognosis in patients with community-acquired pneumonia: A prospective cohort study. Int. J. Med. Sci. 2022, 19, 74–81. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Q.; Lin, S.; Shen, N.; Yin, Y.; Cao, J. IL-27 is elevated in acute lung injury and mediates inflammation. J. Clin. Immunol. 2013, 33, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Saita, E.; Kishimoto, Y.; Ohmori, R.; Kondo, K.; Momiyama, Y. Association between Plasma Interleukin-27 Levels and Cardiovascular Events in Patients Undergoing Coronary Angiography. J. Cardiovasc. Dev. Dis. 2024, 11, 139. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N = 474 |
|---|---|
| Age (years) 1 | 73 (65, 81) |
| Age group | |
| ≤60 | 53 (11%) |
| 61–70 | 152 (32%) |
| 71–80 | 150 (32%) |
| >80 | 119 (25%) |
| Sex (female) | 166 (35%) |
| Rutherford stage ≥ 4 | 222 (47%) |
| Primary segment involvement | |
| Iliac | 90 (19%) |
| Femoropopliteal | 135 (28%) |
| Distal | 79 (17%) |
| Complex (femoropopliteal and distal) | 170 (36%) |
| Diabetes mellitus | 224 (47%) |
| Cerebrovascular disease | 112 (24%) |
| CVI/Transient ischaemic attack | 77 (17%) |
| Revascularization of carotid arteries | 16 (3%) |
| >50% stenosis of carotid arteries | 19 (4%) |
| Coronary artery disease | 153 (32%) |
| Chronic kidney disease | 140 (30%) |
| Acetylsalicylic acid | 388 (82%) |
| Additional antithrombotic drug | |
| Clopidogrel | 331 (71%) |
| Ticagrelor | 3 (0.6%) |
| Prasugrel | 1 (0.2%) |
| Statin therapy | |
| Less potent statins | 125 (28%) |
| Atorvastatin/Rosuvastatin | 233 (50%) |
| Ezetimib/PCSK9 inhibitors | 4 (1%) |
| Combination of antilipemic therapy | 35 (7%) |
| ACEi/ARB | 328 (69%) |
| Amputation | 30 (6%) |
| Unplanned next revascularisation | 14 (3%) |
| Coronary event | 14 (3%) |
| Cerebrovascular event | 14 (3%) |
| Cardiovascular death | 33 (7%) |
| Death from any cause | 70 (15%) |
| Major cardiovascular event | 48 (10%) |
| Major limb event | 114 (24%) |
| Characteristic | No MALE, N = 349 1 | MALE, N = 140 1 | p-Value 2 |
|---|---|---|---|
| Age (years) | 73 (65, 80) | 72 (65, 81) | 0.7 |
| Age group | 0.5 | ||
| ≤60 | 43 (12%) | 10 (8.8%) | |
| 61–70 | 113 (31%) | 39 (34%) | |
| 71–80 | 118 (33%) | 32 (28%) | |
| >80 | 86 (24%) | 33 (29%) | |
| Sex (female) | 128 (36%) | 38 (33%) | 0.7 |
| Rutherford stage ≥ 4 | 152 (42%) | 70 (61%) | <0.001 |
| Primary segment involvement | 0.072 | ||
| Iliac | 76 (21%) | 14 (12%) | |
| Femoropoliteal | 104 (29%) | 31 (27%) | |
| Distal | 61 (17%) | 18 (16%) | |
| Complex (femoropopliteal and distal) | 119 (33%) | 51 (45%) | |
| Diabetes mellitus | 162 (45%) | 62 (54%) | 0.080 |
| Chronic kidney disease | 94 (26%) | 46 (40%) | 0.004 |
| Cerebrovascular disease | 85 (24%) | 27 (24%) | >0.9 |
| Coronary artery disease | 122 (34%) | 31 (27%) | 0.2 |
| Acetylsalicilic acid | 295 (82%) | 93 (82%) | >0.9 |
| P2Y12/rivaroxaban | 251 (70%) | 84 (74%) | 0.4 |
| Statin therapy | 305 (85%) | 92 (81%) | 0.3 |
| ACEi/ARB | 260 (72%) | 68 (60%) | 0.011 |
| Characteristic | No MACE, N = 426 1 | MACE, N = 48 1 | p-Value 2 |
|---|---|---|---|
| Age (years) | 72 (65, 79) | 81 (73, 86) | <0.001 |
| Age group | <0.001 | ||
| ≤60 | 50 (12%) | 3 (6.3%) | |
| 61–70 | 145 (34%) | 7 (15%) | |
| 71–80 | 137 (32%) | 13 (27%) | |
| >80 | 94 (22%) | 25 (52%) | |
| Sex (female) | 147 (35%) | 19 (40%) | 0.5 |
| Rutherford stage ≥ 4 | 185 (43%) | 37 (77%) | <0.001 |
| Primary segment involvement | 0.6 | ||
| Iliac | 84 (20%) | 6 (13%) | |
| Femoropoliteal | 121 (28%) | 14 (29%) | |
| Distal | 69 (16%) | 10 (21%) | |
| Complex (femoropopliteal and distal) | 152 (36%) | 18 (38%) | |
| Diabetes mellitus | 198 (46%) | 26 (54%) | 0.3 |
| Chronic kidney disease | 110 (26%) | 30 (63%) | <0.001 |
| Cerebrovascular disease | 99 (23%) | 13 (27%) | 0.6 |
| Coronary artery disease | 130 (31%) | 23 (48%) | 0.015 |
| Acetylsalicilic acid | 354 (83%) | 34 (71%) | 0.037 |
| P2Y12/rivaroxaban | 306 (72%) | 29 (60%) | 0.10 |
| Statin therapy | 367 (86%) | 30 (63%) | <0.001 |
| ACEi/ARB | 301 (71%) | 27 (56%) | 0.040 |
| MACE | MALE | |||||
|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | p | Estimates | CI | p |
| qIL 27 [Q2] | 2.03 | 0.66–6.21 | 0.214 | 1.02 | 0.60–1.72 | 0.948 |
| qIL 27 [Q3] | 1.88 | 0.62–5.75 | 0.268 | 1.03 | 0.62–1.72 | 0.902 |
| qIL 27 [Q4] | 7.63 | 2.93–19.82 | <0.001 | 1.31 | 0.79–2.16 | 0.004 |
| Observations | 474 | 474 | ||||
| R2 Nagelkerke | 0.088 | 0.003 | ||||
| MACE | MALE | |||||
|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | p | Estimates | CI | p |
| qIL 27 [Q2] | 1.52 | 0.49–4.74 | 0.472 | 1.01 | 0.59–1.73 | 0.966 |
| qIL 27 [Q3] | 1.01 | 0.32–3.23 | 0.981 | 0.92 | 0.54–1.59 | 0.769 |
| qIL 27 [Q4] | 2.95 | 1.06–8.22 | 0.039 | 0.97 | 0.56–1.70 | 0.926 |
| Age | 1.06 | 1.02–1.10 | 0.004 | 1.00 | 0.98–1.02 | 0.830 |
| Sex | 0.93 | 0.48–1.81 | 0.835 | 0.80 | 0.51–1.24 | 0.319 |
| CLI | 2.97 | 1.32–6.67 | 0.008 | 2.05 | 1.29–3.26 | 0.002 |
| Segment (Femoropopliteal) | 1.10 | 0.40–2.98 | 0.857 | 1.41 | 0.74–2.70 | 0.295 |
| Segment (Distal) | 0.57 | 0.18–1.81 | 0.342 | 0.98 | 0.45–2.13 | 0.963 |
| Segment (Complex femoropopliteal and distal) | 0.45 | 0.15–1.35 | 0.154 | 1.38 | 0.71–2.70 | 0.345 |
| CAD | 1.60 | 0.89–2.89 | 0.119 | 0.75 | 0.49–1.15 | 0.187 |
| CVD | 1.22 | 0.63–2.36 | 0.545 | 1.12 | 0.72–1.74 | 0.616 |
| Diabetes | 1.27 | 0.68–2.39 | 0.457 | 1.12 | 0.75–1.67 | 0.579 |
| Smoking | 0.87 | 0.60–1.24 | 0.438 | 0.95 | 0.74–1.21 | 0.663 |
| Observations | 474 | 474 | ||||
| R2 Nagelkerke | 0.161 | 0.047 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokalj, N.; Jug, B. Prognostic Impact of Interleukin-27 in Peripheral Artery Disease. Life 2025, 15, 1768. https://doi.org/10.3390/life15111768
Kokalj N, Jug B. Prognostic Impact of Interleukin-27 in Peripheral Artery Disease. Life. 2025; 15(11):1768. https://doi.org/10.3390/life15111768
Chicago/Turabian StyleKokalj, Nataša, and Borut Jug. 2025. "Prognostic Impact of Interleukin-27 in Peripheral Artery Disease" Life 15, no. 11: 1768. https://doi.org/10.3390/life15111768
APA StyleKokalj, N., & Jug, B. (2025). Prognostic Impact of Interleukin-27 in Peripheral Artery Disease. Life, 15(11), 1768. https://doi.org/10.3390/life15111768







