Human Responses to Magnetic and Hypomagnetic Fields: Available Evidence and Potential Risks for Deep Space Travel
Abstract
1. Introduction
2. Methods
3. Hypomagnetic Field
4. The Human Body’s Response to MF and hypoMF
5. Molecular Mechanisms of hypoMF Effects
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVS | cardiovascular system |
| DNA | deoxyribonucleic acid |
| EEG | electroencephalography |
| ECG | electrocardiography |
| ELF | extremely low frequency |
| fMRI | functional Magnetic Resonance Imaging |
| FMBA | Federal Medical-Biological Agency |
| geoMF | geomagnetic field |
| HR | heart rate |
| HRV | heart rate variability |
| hypoMF | hypomagnetic field |
| MF | magnetic field |
| MRI | magnetic resonance imaging |
| RF | radio frequency |
| RPM | radical pair mechanism |
| RZD | Russian Railways (Rossiyskie Zheleznye Dorogi) |
References
- Barnothy, M.F. Biological Effects of Magnetic Fields; Plenum: New York, NY, USA, 1964. [Google Scholar]
- Buchachenko, A.L. Magnetic field-dependent molecular and chemical processes in biochemistry, genetics and medicine. Russ. Chem. Rev. 2014, 83, 1–12. [Google Scholar] [CrossRef]
- Greenebaum, B.; Barnes, F. Biological and Medical Aspects of Electromagnetic Fields, 4th ed.; Handbook of Biological Effects of Electromagnetic Fields; CRC Press: Boca Raton, FL, USA, 2019; Volume 1. [Google Scholar]
- Tekutskaya, E.E.; Baryshev, M.G.; Gusaruk, L.R.; Ilchenko, G.P. Oxidative damage to DNA under the action of an alternating magnetic field. Biophysics 2020, 65, 564–568. [Google Scholar] [CrossRef]
- Tian, L.; Luo, Y.; Ren, J.; Zhao, C. The Role of Oxidative Stress in Hypomagnetic Field Effects. Antioxidants 2024, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Morgan, L.L.; Udasin, I.; Davis, D.L. Cancer epidemiology update, following the 2011 IARC evaluation of radiofrequency electromagnetic fields (Monograph 102). Environ. Res. 2018, 167, 673–683. [Google Scholar] [CrossRef]
- Friedman, H.; Becker, R.O.; Bachman, C.H. Geomagnetic parameters and psychiatric hospital admissions. Nature 1963, 200, 626–628. [Google Scholar] [CrossRef]
- Burch, J.B.; Reif, J.S.; Yost, M.G. Geomagnetic activity and human melatonin metabolite excretion. Neurosci. Lett. 2008, 438, 76–79. [Google Scholar] [CrossRef]
- Close, J. Are stress responses to geomagnetic storms mediated by the cryptochrome compass system? Proc. R. Soc. B 2012, 279, 2081–2090. [Google Scholar] [CrossRef]
- Vencloviene, J.; Babarskiene, R.; Slapikas, R. The association between solar particle events, geomagnetic storms, and hospital admissions for myocardial infarction. Nat. Hazards 2012, 65, 1–12. [Google Scholar] [CrossRef]
- Breus, T.K.; Boiko, E.R.; Zenchenko, T.A. Magnetic storms and variations in hormone levels among residents of North Polar area—Svalbard. Life Sci. Space Res. 2015, 4, 17–21. [Google Scholar] [CrossRef]
- Krylov, V.V. Biological effects related to geomagnetic activity and possible mechanisms. Bioelectromagnetics 2017, 38, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Pishchalnikov, R.Y.; Gurfinkel, Y.I.; Sarimov, R.M.; Vasin, A.L.; Sasonko, M.L.; Matveeva, T.A.; Binhi, V.N.; Baranov, M.V. Cardiovascular response as a marker of environmental stress caused by variations in geomagnetic field and local weather. Biomed. Signal Process. Control 2019, 51, 401–410. [Google Scholar] [CrossRef]
- Kiznys, D.; Vencloviene, J.; Milvidaita, I. The associations of geomagnetic storms, fast solar wind, and stream interaction regions with cardiovascular characteristic in patients with acute coronary syndrome. Life Sci. Space Res. 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Binhi, V.N. Low-frequency magnetic fields in cars and office premises and the geomagnetic field variations. Bioelectromagnetics 2020, 41, 360–368. [Google Scholar] [CrossRef]
- Pavlovich, S.A. Magnetic Susceptibility of Organisms; Nauka i Tekhnika: Minsk, Republic of Belarus, 1985. (In Russian) [Google Scholar]
- Schott, H. Zur Geschichte der Elektrotherapie und ihrer Beziehung zum Heilmagnetismus. In Naturheilverfahren und Unkonventionelle Medizinische Richtunge; Fialka und andere, V., Ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Webb, S.J.; Dodds, D.E. Microwave inhibition of bacterial growth. Nature 1968, 218, 374–375. [Google Scholar] [CrossRef]
- Kholodov, Y.A. Magnetism in Biology; Nauka: Moscow, Russia, 1970. (In Russian) [Google Scholar]
- Bawin, S.M.; Gavalas-Medici, R.J.; Adey, W.R. Effects of modulated very high frequency fields on specific brain rhythms in cats. Brain Res. 1973, 58, 365–384. [Google Scholar] [CrossRef]
- Devyatkov, N.D. Influence of millimeter-band electromagnetic radiation on biological objects. Physics–Uspekhi 1974, 16, 568–569. [Google Scholar] [CrossRef]
- Binhi, V.N.; Rubin, A.B. On the quantum nature of magnetic phenomena in biology. Phys. Biol. Med. 2023, 1, 44–73. (In Russian) [Google Scholar] [CrossRef]
- Wiltschko, R.; Wiltschko, W. Magnetic Orientation in Animals; Springer: Berlin, Germany, 1995. [Google Scholar] [CrossRef]
- Lohmann, K.J.; Lohmann, C.M.; Ehrhart, L.M.; Bagley, D.A.; Swing, T. Animal behaviour: Geomagnetic map used in sea-turtle navigation. Nature 2004, 428, 909–910. [Google Scholar] [CrossRef]
- Phillips, J.; Painter, M. Small differences in weak electromagnetic fields disrupt magnetic compass orientation of C57 BL/6 mice (Rodentia: Muridae). Lynx Ser. Nova 2022, 53, 219–234. [Google Scholar] [CrossRef]
- Breus, T.K.; Binhi, V.N.; Petrukovich, A.A. Magnetic factor of the solar terrestrial relations and its impact on the human body: Physical problems and prospects for research. Physics–Uspekhi 2016, 59, 502–510. [Google Scholar] [CrossRef]
- Novikov, V.V.; Sheiman, I.M.; Fesenko, E.E. Effect of weak static and low-frequency alternating magnetic fields on the fission and regeneration of the planarian Dugesia (Girardia) tigrina. Bioelectromagnetics 2008, 29, 387–393. [Google Scholar] [CrossRef]
- Prato, F.S. Non-thermal extremely low frequency magnetic field effects on opioid related behaviors: Snails to humans, mechanisms to therapy. Bioelectromagnetics 2015, 36, 333–348. [Google Scholar] [CrossRef]
- Binhi, V.N. Random effects in magnetobiology and a way to summarize them. Bioelectromagnetics 2021, 42, 501–515. [Google Scholar] [CrossRef]
- Huss, A.; Peters, S.; Vermeulen, R. Occupational exposure to extremely low-frequency magnetic fields and the risk of ALS: A systematic review and meta-analysis. Bioelectromagnetics 2018, 39, 156–163. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. Biological effects of the hypomagnetic field: An analytical review of experiments and theories. PLoS ONE 2017, 12, e0179340. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, E.A.; Vassilieva, S.A.; Schegolev, B.F.; Savvateyeva-Popova, E.V. Weak static magnetic field: Impact on nervous system. I.P. Pavlov J. High. Nerv. Act. 2022, 72, 783–799. (In Russian) [Google Scholar] [CrossRef]
- Sarimov, R.M.; Serov, D.A.; Gudkov, S.V. Hypomagnetic conditions and their biological action (review). Biology 2023, 12, 1513. [Google Scholar] [CrossRef] [PubMed]
- Sinčák, M.; Sedlakova-Kadukova, J. Hypomagnetic fields and their multilevel effects on living organisms. Processes 2023, 11, 282. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Ahmad, N.; Shi, Z.; Raza, A.; Wang, X.; Li, J. Growth, physiological, biochemical and molecular changes in plants by magnetic field: A review. Plant Biol. 2023, 25, 8–23. [Google Scholar] [CrossRef]
- Saletnik, B.; Saletnik, A.; Slysz, E.; Zagula, G.; Bajcar, M.; Puchalska-Sarna, A.; Puchalski, C. The static magnetic field regulates the structure, biochemical activity, and gene expression of plants. Molecules 2022, 27, 5823. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.L.; Haggren, W.; Thomas, W.J.; Ishida-Jones, T.; Adey, W.R. Magnetic field-induced changes in specific gene transcription. Biochim. Biophys. Acta 1992, 1132, 140–144. [Google Scholar] [CrossRef]
- Volpe, P.; Eremenko, T. Gene expression in a space-simulating magnetically shielded environment. Environmentalist 2005, 25, 83–92. [Google Scholar] [CrossRef]
- Wang, X.K.; Ma, Q.F.; Jiang, W.; Lv, J.; Pan, W.D.; Song, T.; Wu, L.F. Effects of hypomagnetic field on magnetosome formation of Magnetospirillum magneticum AMB-1. Geomicrobiol. J. 2008, 25, 296–303. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Gul, Y.V.; Zelenskaya, A.Y.; Pan, E.S.; Krivova, N.A. Effect of long-term geomagnetic field weakening on aggressiveness of rats and opioidergic neurons activation. Tomsk. State Univ. J. Biol. 2013, 1, 146–160. (In Russian) [Google Scholar]
- Wan, G.J.; Wang, W.J.; Xu, J.J.; Yang, Q.F.; Dai, M.J.; Zhang, F.J.; Sword, G.A.; Pan, W.D.; Chen, F.J. Cryptochromes and hormone signal transduction under near-zero magnetic fields: New clues to magnetic field effects in a rice planthopper. PLoS ONE 2015, 10, e0132966. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, Y.; Zhang, Y.; Li, Y.; Wei, S. Gibberellins are involved in effect of near-null magnetic field on Arabidopsis flowering. Bioelectromagnetics 2017, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cremer-Bartels, G.; Krause, K.; Küchle, H.J. Influence of low magnetic-field-strength variations on the retina and pineal gland of quail and humans. ALbrecht Von Graefe’S Arch. Clin. Exp. 1983, 220, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Kopanev, V.I.; Shakula, A.V. Effect of a Hypomagnetic field on Biological Systems; Nauka: Moscow, Russia, 1986. (In Russian) [Google Scholar]
- Serdyukov, Y.A.; Novitskii, Y.I. Impact of weak permanent magnetic field on antioxidant enzyme activities in radish seedlings. Russ. J. Plant Physiol. 2013, 60, 69–76. [Google Scholar] [CrossRef]
- Kuz’mina, V.V.; Ushakova, N.V.; Krylov, V.V. The effect of magnetic fields on the activity of proteinases and glycosidases in the intestine of the crucian carp Carassius carassius. Biol. Bulletin 2015, 42, 61–66. [Google Scholar] [CrossRef]
- Kim, Y.; Bertagna, F.; D’Souza, E.M.; Heyes, D.J.; Johannissen, L.O.; Nery, E.T.; Pantelias, A.; Sanchez-Pedreño Jimenez, A.; Slocombe, L.; Spencer, M.G.; et al. Quantum Biology: An Update and Perspective. Quantum Rep. 2021, 3, 80–126. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. A physical mechanism of magnetoreception: Extension and analysis. Bioelectromagnetics 2017, 38, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S.; Matveeva, M.I.; Mukhametov, A.; Shtemberg, A.S. Risk of defeats in the central nervous system during deep space missions. Neurosci. Biobehav. Rev. 2016, 71, 621–632. [Google Scholar] [CrossRef]
- Erdmann, W.; Kmita, H.; Kosicki, J.Z.; Kaczmarek, L. How the geomagnetic field influences life on Earth – An integrated approach to geomagnetobiology. Orig. Life Evol. Biosph. 2021, 51, 231–257. [Google Scholar] [CrossRef]
- Xu, Y.; Pei, W.; Hu, W. A current overview of the biological effects of combined space environmental factors in mammals. Front. Cell Dev. Biol. 2022, 10, 861006. [Google Scholar] [CrossRef]
- Hart, D.A. Homo sapiens—A Species not designed for space flight: Health risks in low Earth orbit and beyond, including potential risks when traveling beyond the geomagnetic field of Earth. Life 2023, 13, 757. [Google Scholar] [CrossRef]
- Kaspranski, R.R.; Binhi, V.N.; Koshel, I.V. Is the weakening of the magnetic field in space linked to the risk of mistakes in the work of astronauts? Phys. Biol. Med. 2024, 2, 77–90. (In Russian) [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Syunyaev, R.A. Space Physics–Little Encyclopedia, 2nd ed.; Soviet Encyclopedia: Moscow, Russia, 1986. (In Russian) [Google Scholar]
- Oliveira, J.S.; Wieczorek, M.A. Testing the axial dipole hypothesis for the Moon by modeling the direction of crustal magnetization. J. Geophys. Res. Planets 2017, 122, 383–399. [Google Scholar] [CrossRef]
- Klokočník, J.; Kostelecký, J.; Cílek, V.; Bezděk, A.; Kletetschka, G. Atlas of the Gravity and Magnetic Fields of the Moon; Springer Geophysics: Cham, Switzerland, 2022. [Google Scholar]
- Breus, T.K.; Verigin, M.I.; Kotova, G.A.; Slavin, J.A. Characteristics of the martian magnetosphere according to the data of the Mars 3 and Phobos 2 satellites: Comparison with MGS and MAVEN result. Cosm. Res. 2021, 59, 478–492. [Google Scholar] [CrossRef]
- Binhi, V.N.; Rubin, A.B. Theoretical concepts in magnetobiology after 40 years of research. Cells 2022, 11, 274. [Google Scholar] [CrossRef]
- Martino, C.F.; Perea, H.; Hopfner, U.; Ferguson, V.L.; Wintermantel, E. Effects of weak static magnetic fields on endothelial cells. Bioelectromagnetics 2010, 31, 296–301. [Google Scholar] [CrossRef]
- Xu, C.; Yin, X.; Lv, Y.; Wu, C.; Zhang, Y.; Song, T. A near-null magnetic field affects cryptochrome-related hypocotyl growth and flowering in Arabidopsis. Adv. Space Res. 2012, 49, 834–840. [Google Scholar] [CrossRef]
- Mo, W.C.; Liu, Y.; Bartlett, P.F.; He, R.Q. Transcriptome profile of human neuroblastoma cells in the hypomagnetic field. Sci. China–Life Sci. 2014, 57, 448–461. [Google Scholar] [CrossRef]
- Dhiman, S.K.; Galland, P. Effects of weak static magnetic fields on the gene expression of seedlings of Arabidopsis thaliana. J. Plant Physiol. 2018, 231, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Thun-Battersby, S.; Mevissen, M.; Loscher, W. Exposure of Sprague-Dawley rats to a 50-Hertz, 100-μTesla magnetic field for 27 weeks facilitates mammary tumorigenesis in the 7,12- dimethylbenz[a]-anthracene model of breast cancer. Cancer Res. 1999, 59, 3627–3633. [Google Scholar]
- Cameron, I.L.; Sun, L.Z.; Short, N.; Hardman, W.E.; Williams, C.D. Therapeutic electromagnetic field (TEMF) and gamma irradiation on human breast cancer xenograft growth, angiogenesis and metastasis. Cancer Cell Int. 2005, 5, 23. [Google Scholar] [CrossRef]
- Politanski, P.; Rajkowska, E.; Brodecki, M.; Bednarek, A.; Zmyslony, M. Combined effect of X-ray radiation and static magnetic fields on reactive oxygen species in rat lymphocytes in vitro. Bioelectromagnetics 2013, 34, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Buchachenko, A.L.; Kuznetsov, D.A. Genes and cancer under magnetic control. Russ. J. Phys. Chem. B 2021, 15, 1–11. [Google Scholar] [CrossRef]
- Nieminen, V.; Seppälä, J.; Virén, T.; Juutilainen, J.; Naarala, J.; Luukkonen, J. Static magnetic field exposure causes small cell cycle disruptions and changes in reactive oxygen species levels in ionizing radiation exposed human neuroblastoma cells. Bioelectromagnetics 2025, 46, e22538. [Google Scholar] [CrossRef]
- Xue, X.; Ali, Y.F.; Liu, C.; Hong, Z.; Luo, W.; Nie, J.; Li, B.; Jiao, Y.; Liu, N.A. Geomagnetic shielding enhances radiation resistance by promoting DNA repair process in human bronchial epithelial cells. Int. J. Mol. Sci. 2020, 21, 9304. [Google Scholar] [CrossRef]
- Erdmann, W.; Idzikowski, B.; Kowalski, W.; Kosicki, J.Z.; Kaczmarek, L. Tolerance of two anhydrobiotic tardigrades Echiniscus testudo and Milnesium inceptum to hypomagnetic conditions. PeerJ 2021, 9, e10630. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, Y.; Yang, J.; Shang, P.; Yuan, X. Biological effects of hypomagnetic field: Ground-based data for space exploration. Bioelectromagnetics 2021, 42, 516–531. [Google Scholar] [CrossRef]
- Binhi, V.N. Statistical amplification of the effects of weak magnetic fields in cellular translation. Cells 2023, 12, 724. [Google Scholar] [CrossRef]
- Zel’dovich, Y.B.; Buchachenko, A.L.; Frankevich, E.L. Magnetic-spin effects in chemistry and molecular physics. Phys. Usp. 1988, 31, 385–408. [Google Scholar] [CrossRef]
- Jauchem, J.R. Exposure to extremely-low-frequency electromagnetic fields and radiofrequency radiation: Cardiovascular effects in humans. Int. Arch. Occup. Environ. Health 1997, 70, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ahlbom, A.; Bridges, J.; Mattsson, M.; de Seze, R.; Hillert, L.; Juutilainen, J.; Neubauer, G.; Schüz, J.; Simko, M. Possible effects of Electromagnetic Fields (EMF) on Human Health; SCENIHR: Brussels, Belgium, 2007. [Google Scholar] [CrossRef]
- Karimi, A.; Moghaddam, F.G.; Valipour, M. Insights in the biology of extremely low-frequency magnetic fields exposure on human health. Mol. Biol. Rep. 2020, 47, 5621–5633. [Google Scholar] [CrossRef]
- Chernetsov, N.; Nikishena, I.; Zavarzina, N.; Kulbach, O. Perception of static magnetic field by humans: A review. Biol. Commun. 2021, 66, 171–178. [Google Scholar] [CrossRef]
- Raines, J.K. Electromagnetic Field Interactions with the Human Body: Observed Effects and Theories; National Aeronautics and Space Administration, Goddard Space Flight Center: Greenbelt, UK, 1981; NASA CR 166661. [Google Scholar]
- Baker, R. Goal orientation by blindfolded humans after long-distance displacement: Possible involvement of a magnetic sense. Science 1980, 210, 555–557. [Google Scholar] [CrossRef]
- Gould, J.L.; Able, K.P. Human homing: An elusive phenomenon. Science 1981, 212, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, J.L.; Jones, D.S.; MacFadden, B.J. (Eds.) Magnetite Biomineralization and Magnetoreception in Organisms. A New Biomagnetism; Plenum: New York, NY, USA, 1985. [Google Scholar]
- Baaken, D.; Dechent, D.; Blettner, M.; Drießen, S.; Merzenich, H. Occupational exposure to extremely low-frequency magnetic fields and risk of amyotrophic lateral sclerosis: Results of a feasibility study for a pooled analysis of original data. Bioelectromagnetics 2021, 42, 271–283. [Google Scholar] [CrossRef]
- Makinistian, L.; Vives, L. Devices, facilities, and shielding for biological experiments with static and extremely low frequency magnetic fields. IEEE J. Electromagn. RF Microwaves Med. Biol. 2025, 9, 141–156. [Google Scholar] [CrossRef]
- Friedman, H.; Becker, R.O.; Bachman, C.H. Effect of magnetic fields on reaction time performance. Nature 1967, 213, 949–950. [Google Scholar] [CrossRef]
- Podd, J.V.; Whittington, C.J.; Barnes, G.R.G.; Page, W.H.; Rapley, B.I. Do ELF magnetic fields affect human reaction time? Bioelectromagnetics 1995, 16, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kazantzis, N.; Podd, J.; Whittington, C. Acute effects of 50 Hz, 100 μT magnetic field exposure on visual duration discrimination at two different times of the day. Bioelectromagnetics 1998, 19, 310–317. [Google Scholar] [CrossRef]
- Ghione, S.; Seppia, C.D.; Mezzasalma, L.; Bonfiglio, L. Effects of 50 Hz electromagnetic fields on electroencephalographic alpha activity, dental pain threshold and cardiovascular parameters in humans. Neurosci. Lett. 2005, 382, 112–117. [Google Scholar] [CrossRef]
- Carrubba, S.; Frilot, C.F., II; Chesson, A.L., Jr.; Marino, A.A. Evidence of a nonlinear human magnetic sense. Neuroscience 2007, 144, 356–367. [Google Scholar] [CrossRef]
- Nevelsteen, S.; Legros, J.J.; Crasson, M. Effects of information and 50 Hz magnetic fields on cognitive performance and reported symptoms. Bioelectromagnetics 2007, 28, 53–63. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Capone, F.; Apollonio, F.; Borea, P.A.; Cadossi, R.; Fassina, L.; Grassi, C.; Liberti, M.; Paffi, A.; Parazzini, M.; et al. A consensus panel review of central nervous system effects of the exposure to low-intensity extremely low-frequency magnetic fields. Brain Stimul. 2013, 6, 469–476. [Google Scholar] [CrossRef]
- Villard, S.; Allen, A.; Bouisset, N.; Corbacio, M.; Thomas, A.; Guerraz, M.; Legros, A. Impact of extremely low-frequency magnetic fields on human postural control. Exp. Brain Res. 2018, 237, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.B.; Marino, A.A. Exposure System for Production of Uniform Magnetic Fields. Electromagn. Biol. Med. 1989, 8, 147–158. [Google Scholar] [CrossRef]
- Beischer, D.E.; Miller, E.F., II; Knepton, J.C., Jr. Exposure of Man to Low Intensity Magnetic Fields in a Coil, System; Naval Aerospace Medical Institute, NAMI-1018, NASA Order R-39: Pensacola, FL, USA, 1967. [Google Scholar]
- Selmaoui, B.; Lambrozo, J.; Touitou, Y. Magnetic fields and pineal function in humans: Evaluation of nocturnal acute exposure to extremely low frequency magnetic fields on serum melatonin and urinary 6-sulfatoxymelatonin circadian rhythms. Life Sci. 1996, 58, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.W.; Armstrong, S.M.; Sait, M.L.; Devine, L.; Martin, M.J. Changes in human plasma melatonin profiles in response to 50 Hz magnetic field exposure. J. Pineal Res. 1998, 25, 116–127. [Google Scholar] [CrossRef]
- Doynov, P.; Cohen, H.D.; Cook, M.R.; Graham, C. Test facility for human exposure to AC and DC magnetic fields. Bioelectromagnetics 1999, 20, 101–111. [Google Scholar] [CrossRef]
- Thomas, A.W.; Drost, D.J.; Prato, F.S. Magnetic field exposure and behavioral monitoring system. Bioelectromagnetics 2001, 22, 401–407. [Google Scholar] [CrossRef]
- Griefahn, B.; Künemund, C.; Blaszkewicz, M.; Golka, K.; Mehnert, P.; Degen, G. Experiments on the effects of a continuous 16.7 Hz magnetic field on melatonin secretion, core body temperature, and heart rates in humans. Bioelectromagnetics 2001, 22, 581–588. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Nitta, H.; Imai, H.; Kabuto, M. Acute exposure to 50 Hz magnetic fields with harmonics and transient components: Lack of effects on nighttime hormonal secretion in men. Bioelectromagnetics 2003, 24, 12–20. [Google Scholar] [CrossRef]
- Legros, A.; Beuter, A. Effect of a low intensity magnetic field on human motor behavior. Bioelectromagnetics 2005, 26, 657–669. [Google Scholar] [CrossRef]
- Bae, J.E.; Bang, S.; Min, S.; Lee, S.H.; Kwon, S.H.; Lee, Y.; Lee, Y.H.; Chung, J.; Chae, K.S. Positive geotactic behaviors induced by geomagnetic field in Drosophila. Mol. Brain 2016, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Hilburn, I.A.; Wu, D.; Mizuhara, Y.; Cousté, C.P.; Abrahams, J.N.H.; Bernstein, S.E.; Matani, A.; Shimojo, S.; Kirschvink, J.L. Transduction of the geomagnetic field as evidenced from alpha-band activity in the human brain. eNeuro 2019, 6, e0483. [Google Scholar] [CrossRef]
- Kukanov, V.Y.; Vasin, A.L.; Demin, A.V.; Schastlivtseva, D.V.; Bubeev, Y.A.; Suvorov, A.V.; Popova, J.A.; Luchitskaya, E.S.; Niiazov, A.R.; Polyakov, A.V.; et al. Effect of simulated hypomagnetic conditions on some physiological parameters under 8-hour exposure. Experiment Arfa-19. Hum. Physiol. 2023, 49, 138–146. [Google Scholar] [CrossRef]
- Shupak, N.M.; Prato, F.S.; Thomas, A.W. Human exposure to a specific pulsed magnetic field: Effects on thermal sensory and pain thresholds. Neurosci. Lett. 2004, 363, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; Theberge, J.; Weller, J.; Drost, D.J.; Prato, F.S.; Thomas, A.W. Low-frequency pulsed electromagnetic field exposure can alter neuroprocessing in humans. J. R. Soc. Interface 2010, 7, 467–473. [Google Scholar] [CrossRef]
- Legros, A.; Beuter, A. Individual subject sensitivity to extremely low frequency magnetic field. NeuroToxicology 2006, 27, 534–546. [Google Scholar] [CrossRef]
- Sartucci, F.; Bonfiglio, L.; Del Seppia, C.; Luschi, P.; Ghione, S.; Murri, L.; Papi, F. Changes in pain perception and pain-related somatosensory evoked potentials in humans produced by exposure to oscillating magnetic fields. Brain Res. 1997, 769, 362–366. [Google Scholar] [CrossRef]
- Chae, K.S.; Kim, S.C.; Kwon, H.J.; Kim, Y. Human magnetic sense is mediated by a light and magnetic field resonance-dependent mechanism. Sci. Rep. 2022, 12, 8997. [Google Scholar] [CrossRef]
- Gurfinkel, Y.I.; Vasin, A.L.; Pishchalnikov, R.Y.; Sarimov, R.M.; Sasonko, M.L.; Matveeva, T.A. Geomagnetic storm under laboratory conditions: Randomized experiment. Int. J. Biometeorol. 2017, 62, 501–512. [Google Scholar] [CrossRef]
- Graham, C.; Cook, M.R. Human sleep in 60 Hz magnetic fields. Bioelectromagnetics 1999, 20, 277–283. [Google Scholar] [CrossRef]
- Cook, C.M.; Saucier, D.M.; Thomas, A.W.; Prato, F.S. Exposure to ELF magnetic and ELF-modulated radiofrequency fields: The time course of physiological and cognitive effects observed in recent studies (2001–2005). Bioelectromagnetics 2006, 27, 613–627. [Google Scholar] [CrossRef]
- Sastre, A.; Graham, C.; Cook, M.R.; Gerkovich, M.M.; Gailey, P. Human EEG responses to controlled alterations of the Earth’s magnetic field. Clin. Neurophysiol. 2002, 113, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P. Affective response to 5 μT ELF magnetic field-induced physiological changes. Bioelectromagnetics 2007, 28, 109–114. [Google Scholar] [CrossRef]
- Tian, L.; Ren, J.; Luo, Y.; Li, Y.; Guo, W.; Zhang, B.; Pan, Y. Potential health risks of hypomagnetic field for manned deep-space explorations. Natl. Sci. Rev. 2024, 11, nwae395. [Google Scholar] [CrossRef]
- Hart, D.A. The influence of magnetic fields, including the planetary magnetic field, on complex life forms: How do biological systems function in this field and in electromagnetic fields? Biophysica 2024, 4, 1–21. [Google Scholar] [CrossRef]
- Beischer, D.E. The null magnetic field as reference for the study of geomagnetic directional effects in animals and man. Ann. N. Y. Acad. Sci. 1971, 188, 324–330. [Google Scholar] [CrossRef]
- Thoss, F.; Bartsch, B. The human visual threshold depends on direction and strength of a weak magnetic field. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003, 189, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Thoss, F.; Bartsch, B. The geomagnetic field influences the sensitivity of our eyes. Vis. Res. 2007, 47, 1036–1041. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Binhi, V.N.; Milyaev, V.A. The influence of geomagnetic field compensation on human cognitive processes. Biophysics 2008, 53, 433–441. [Google Scholar] [CrossRef]
- Binhi, V.N.; Sarimov, R.M. Zero magnetic field effect observed in human cognitive processes. Electromagn. Biol. Med. 2009, 28, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.M. Influence of hypomagnetic conditions on the size of a human eye pupil. Phys. Biol. Med. 2024, 2, 24–40. (In Russian) [Google Scholar] [CrossRef]
- Sarimov, R.M.; Binhi, V.N. The effect of a hypomagnetic field on the pupil size of the eye in humans. Opera Medica Physiol. 2025, 12, 127–135. [Google Scholar] [CrossRef]
- Gurfinkel, Y.I.; At’kov, O.Y.; Vasin, A.L.; Breus, T.K.; Sasonko, M.L.; Pishchalnikov, R.Y. Effect of zero magnetic field on cardiovascular system and microcirculation. Life Sci. Space Res. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Demin, A.V.; Suvorov, A.V.; Orlov, O.I. Characteristics of healthy men hemodynamics in a hypomagnetic environment. Aerosp. Environ. Med. 2021, 55, 63–68. (In Russian) [Google Scholar] [CrossRef]
- Markin, A.A.; Zhuravleva, O.A.; Zhuravleva, T.V.; Kuzichkin, D.C.; Markina, E.A.; Polyakov, A.V.; Vostrikova, L.V.; Zabolotskaya, I.V.; Loginov, V.I. Influence of a hypomagnetic environment on the metabolism and psychophysiological reactions of a healthy person. Hum. Physiol. 2023, 49, 84–91. (In Russian) [Google Scholar] [CrossRef]
- Kashirina, D.N.; Pastushkova, L.K.; Brzhozovskiy, A.G.; Kononikhin, A.S.; Rusanov, V.B.; Kukanov, V.Y.; Popova, O.V.; Tyuzhin, M.G.; Nikolaev, E.N.; Larina, I.M.; et al. Study of the protein composition of dry blood spots of healthy volunteers in the experiment with hypomagnetic conditions. Hum. Physiol. 2023, 49, 104–115. (In Russian) [Google Scholar] [CrossRef]
- Kovrov, G.V.; Popova, O.V.; Chernikova, A.G.; Orlov, O.I. The psychophysiological state of a person in altered magnetic conditions. Extrem. Med. 2024, 26, 57–64. (In Russian) [Google Scholar] [CrossRef]
- Popova, O.; Rusanov, V.B.; Orlov, O.I. Vegetative regulation of blood circulation and bioelectric processes in the human myocardium under simulated hypomagnetic conditions. Extrem. Med. 2024, 26, 89–96. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. Rotations of macromolecules affect nonspecific biological responses to magnetic fields. Sci. Rep. 2018, 8, 13495. [Google Scholar] [CrossRef]
- Breus, T.K.; Baevskii, R.M.; Chernikova, A.G. Effects of geomagnetic disturbances on humans functional state in space flight. J. Biomed. Sci. Eng. 2012, 5, 341–355. [Google Scholar] [CrossRef]
- Kim, J.S.; Zilli Vieira, C.L.; Koutrakis, P.; Lee, K. Impact of geomagnetic disturbances and air pollution on total mortality in South Korea. Sci. Total Environ. 2025, 997, 180201. [Google Scholar] [CrossRef]
- Binhi, V.N.; Savin, A.V. Molecular gyroscopes and biological effects of weak extremely low-frequency magnetic fields. Phys. Rev. E 2002, 65, 051912. [Google Scholar] [CrossRef]
- Zhang, B.; Yuan, X.; Lv, H.; Che, J.; Wang, S.; Shang, P. Biophysical mechanisms underlying the effects of static magnetic fields on biological systems. Prog. Biophys. Mol. Biol. 2022, 177, 14–23. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Binhi, V.N. Nonspecific magnetic biological effects: A model assuming the spin-orbit coupling. J. Chem. Phys. 2019, 151, 204101. [Google Scholar] [CrossRef]
- Binhi, V.N. The radical-pair mechanism in magnetobiology: State of the art. Physics–Uspekhi 2025. accepted. [Google Scholar] [CrossRef]
- Binhi, V.N. Nuclear spins in the primary mechanisms of biological action of magnetic fields. Biophysics 1995, 40, 671–685. [Google Scholar]
- Binhi, V.N. Magnetic biological effect: Quantum constraints. Biophysics 2025, 70, 353–360. [Google Scholar] [CrossRef]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Zhang, L.; Malkemper, E.P. Cryptochromes in mammals: A magnetoreception misconception? Front. Physiol. 2023, 14, 1250798. [Google Scholar] [CrossRef] [PubMed]
- Thoradit, T.; Thongyoo, K.; Kamoltheptawin, K.; Tunprasert, L.; El-Esawi, M.A.; Aguida, B.; Jourdan, N.; Buddhachat, K.; Pooam, M. Cryptochrome and quantum biology: Unraveling the mysteries of plant magnetoreception. Front. Plant Sci. 2023, 14, 1266357. [Google Scholar] [CrossRef]
- Henbest, K.B.; Maeda, K.; Hore, P.J.; Joshi, M.; Bacher, A.; Bittl, R.; Weber, S.; Timmel, C.R.; Schleicher, E. Magnetic-field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc. Natl. Acad. Sci. USA 2008, 105, 14395–14399. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Todo, T. The cryptochromes. Genome Biol. 2005, 6, 220. [Google Scholar] [CrossRef]
- Messiha, H.L.; Wongnate, T.; Chaiyen, P.; Jones, A.R.; Scrutton, N.S. Magnetic field effects as a result of the radical pair mechanism are unlikely in redox enzymes. J. R. Soc. Interface 2015, 12, 20141155. [Google Scholar] [CrossRef]
- Binhi, V. Magnetic effects in biology: Crucial role of quantum coherence in the radical pair mechanism. Phys. Rev. E 2025, 112, 014409. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Dertinger, H. Stochastic resonance as a possible mechanism of amplification of weak electric signals in living cells. Bioelectromagnetics 1994, 15, 539–547. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Evans, E.W.; Dejean, V.; Dodson, C.A.; Wallace, M.I.; Mackenzie, S.R.; Timmel, C.R.; Hore, P.J. Chemical amplification of magnetic field effects relevant to avian magnetoreception. Nat. Chem. 2016, 8, 384–391. [Google Scholar] [CrossRef]
- Player, T.C.; Baxter, E.D.A.; Allatt, S.; Hore, P.J. Amplification of weak magnetic field effects on oscillating reactions. Sci. Rep. 2021, 11, 9615. [Google Scholar] [CrossRef]
- Binhi, V.N. Two types of magnetic biological effects: Individual and batch effects. Biophysics 2012, 57, 237–243. [Google Scholar] [CrossRef]
- Verbeek, J.; Zeeb, H.; van Deventer, E.; Ijaz, S.; Doré, J.; Driessen, S.; Roth, N.; Whaley, P. Systematic reviews and meta-analyses for the WHO assessment of health effects of exposure to radiofrequency electromagnetic fields, an introduction. Environ. Int. 2025, 203, 109751. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Xie, L.; Zheng, Q.; Yang, P.F.; Zhang, W.J.; Ding, C.; Qian, A.R.; Shang, P. A hypomagnetic field aggravates bone loss induced by hindlimb unloading in rat femurs. PLoS ONE 2014, 9, e105604. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Meng, X.; Dong, D.; Xue, Y.; Chen, X.; Wang, S.; Shen, Y.; Zhang, G.; Shang, P. Iron overload involved in the enhancement of unloading-induced bone loss by hypomagnetic field. Bone 2018, 114, 235–245. [Google Scholar] [CrossRef]
- Ramsay, J.; Kattnig, D.R. Radical triads, not pairs, may explain effects of hypomagnetic fields on neurogenesis. PLoS Comput. Biol. 2022, 18, e1010519. [Google Scholar] [CrossRef] [PubMed]
- Ziegenbalg, L.; Güntürkün, O.; Winklhofer, M. Extremely low frequency magnetic field distracts zebrafish from a visual cognitive task. Sci. Rep. 2025, 15, 8589. [Google Scholar] [CrossRef] [PubMed]
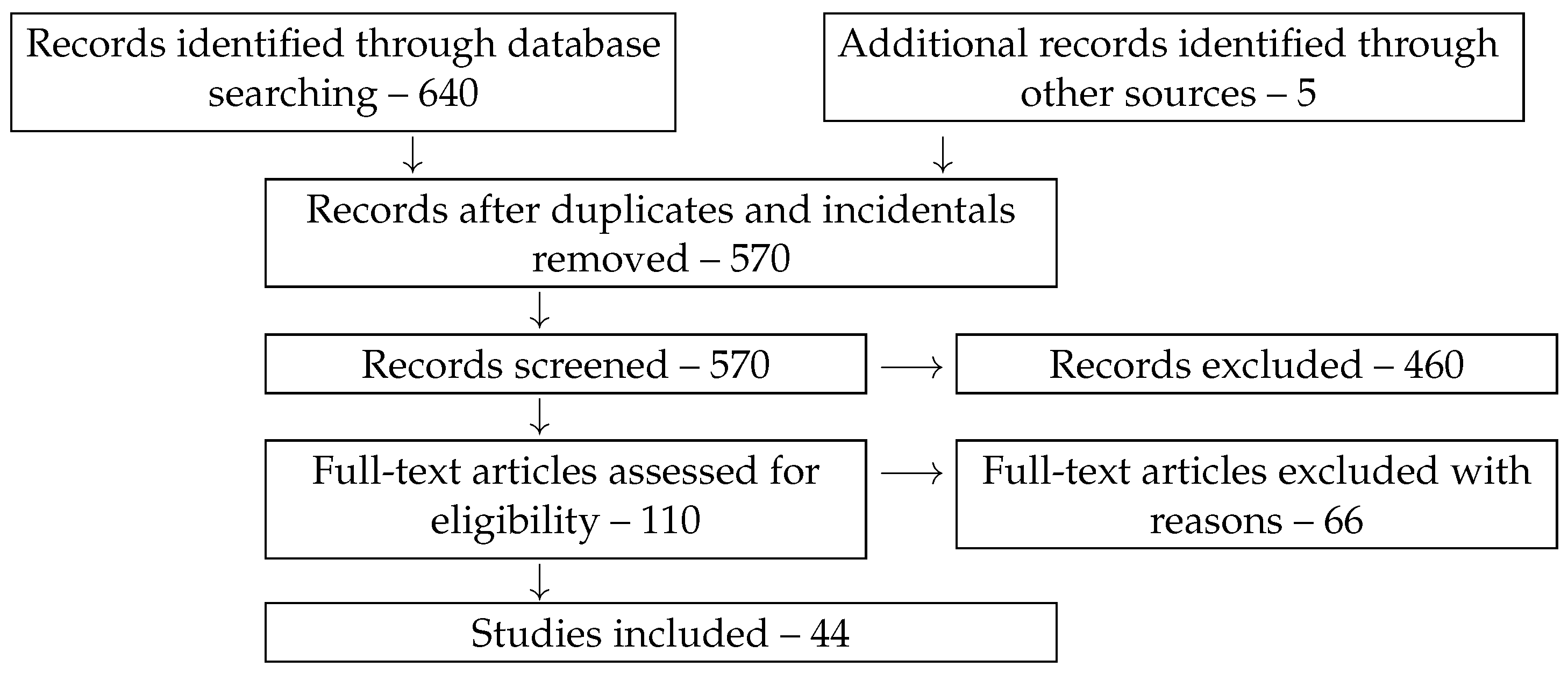
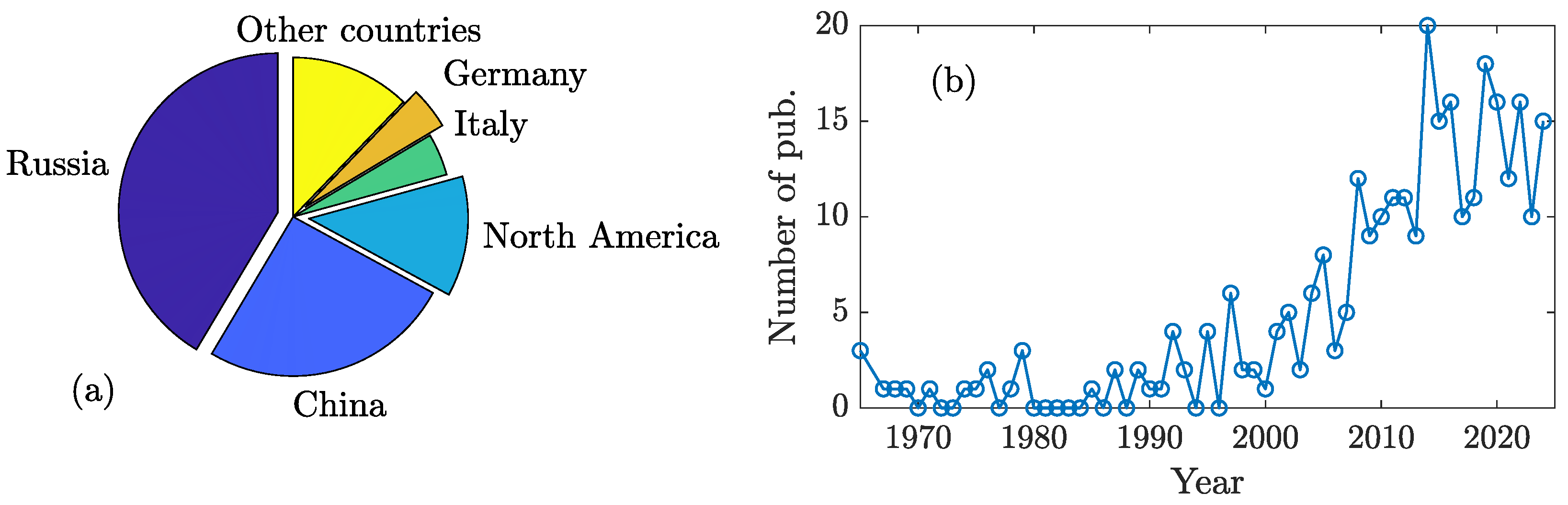


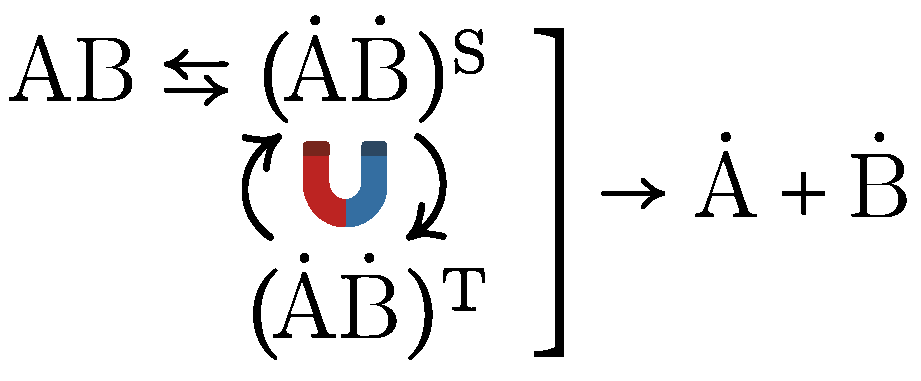
| System | S 1 | N | P | M | C | Reference |
|---|---|---|---|---|---|---|
| Naval Aerospace Medical Institute, USA | 8 | 3 | SL | H | − | [93] |
| Faculté de Médecine Pitié-Salpêtrière, France | 1.2 | 3 | L | AC | − | [94] |
| Swinburne University of Technology, Australia | 2 | 2 | S | G | − | [95] |
| Midwest Research Institute, USA | 4 | 3 | SL | G | − | [96] |
| Lawson Health Research Institute, Canada | 1.5 | 3 | S | AC | − | [97] |
| Institute for Occupational Physiology, Germany | 1.8 | 1 | L | AC | − | [98] |
| National Institute for Environ. Studies, Japan | 2.7 | 3 | SL | G | − | [99] |
| Institut de Recherche d’Hydro-Québec, Canada | 1.6 | 1 | S | AC | − | [100] |
| Kyungpook National University, Korea | 1.9 | 3 | S | G | − | [101] |
| California Institute of Technology, USA | 2 | 3 | S | G | − | [102] |
| Scientific Clinical Center RZD, RF, Faradey | 2.7 | 3 | SL | G | + | [13] |
| Institute of Biomedical Problems, RF, Arfa | 1.2 | 1 | S | H | + | [103] |
| Exp | Property | MF | Result | Comment | Ref |
|---|---|---|---|---|---|
| hypo B y | cognition/ vision | 0.05-week-4 | CVS measurands in four subjects in hypoMF have shown no effect. Critical flicker frequency of the visual system gave a 30% effect in three subjects at p < 0.001. | No compensation of the MF variations. Doubtful hypoMF value because of uncontrolled MF variations. | [93] |
| hypo B n | cognition/ vision | 0.05-10 days-24 | Neither the functions nor the behavior of man changed significantly during a two-week exposure to MFs below 50 nT. | There were no compensation of the geo and technogenic MF variations. | [116] |
| hypo H y | vision/ threshold | 1-15 min-55 | The photopic sensitivity of the human visual system decreased by 6–7% in hypoMF, . | The hypoMF magnitude was not quantified; however, based on the reported measurement accuracy, it should not exceed 1 μT. | [118] |
| hypo B y | cognition/ performance | 0.4-44 min-40 | HypoMF increased the number of errors and the time required to perform tasks in cognitive tests; an average effect 2.1% for all tests, p < 0.002. | Feedback compensation of the MF technogenic variations over z axis of the exposure system only. The standard MF deviations over x–y axes might be about 0.4 μT. | [119] |
| hypo B y | cognition/ performance | 0.4-44 min-40 | The effect magnitude in average was 1.49% at p < 0.004. | Elimination of the data falling out of the “three-sigma” range had no influence on the existence of the effect. | [120] |
| hypo B y | cardio/ HRV | 0.4-1 h-32 | HypoMF exposure demonstrates a clear effect on CVS and microcirculation, . | The MF effect manifests specifically 40–60 min after the onset of hypoMF exposure. | [123] |
| hypo B y | cardio/ hemodyna- mics | 0.4-8 h-8 | Resting in a hypoMF led to a significant reduction in heart rate and blood pressure in most cases. | No compensation of the x–y MF variations, no statistical treatment. | [124] |
| hypo B y | behavior/ orientation | 1-few min-17 | Groups of subjects were classified with different magnetic orientation tendencies. Magnetic orientation of the subjects was sensitive to the wavelength of incident light. | No information on hypoMF; it is assumed to be about 1 μT conditionally. Overcomplicated design of experiment difficult for interpretation. | [108] |
| hypo B n | blood/ proteome | 0.1-24 h-8 | Chromatography and mass spectrometry analysis of dry blood spots: results either mean the absence of the hypoMF effects, or they are unreliable due to small number of subjects. | No description of MF values and the way of MF measurements. | [126] |
| hypo B n | physiology/ EEG, HRV, etc. | 0.4-8 h-8 | Experiment, conducted at time periods with natural MF variations within 40 nT, did not reveal significant changes in EEG, cognitive tests, and auditory evoked potentials. | No compensation of the technogenic x–y MF variations, small sample. | [103] |
| hypo B y | blood/ biochemistry | 0.1-24 h-8 | A hypoMF significantly altered multiple venous blood biochemical parameters, . | HypoMF z-component was reported to be about 0.1 μT. However, there were no compensation of the x–y MF variations. | [125] |
| hypo B y | cognition/ drowsiness | 0.4-24 h-6 | Based on self-assessment surveys, an increase in daytime sleepiness level was observed under the hypoMF in 72% of observations, by sign test. | The z-component of the hypoMF was 50–140 nT at no compensation of the x–y MF variations. | [127] |
| hypo B y | cardio/ ECG | 0.4-32 h-6 | In volunteers with predominance of sympathetic modulatory effects, the CVS response was significant in hypoMF lower than 0.15 μT. | No statistics, no hypoMF values, no compensation of the x-y MF components. | [128] |
| hypo B n/y | vision/ pupil size | 0.4-44 min-40 | The pupil size increased in the hypoMF. Effect of 1.6%, . | Almost insignificant hypoMF effect. Inter-individual variability among participants was accounted for in the analysis. | [122] |
| ELF H y | behavior/ reaction time | 1100-5 min-30 | MFs can significantly affect reaction time performance. | Nonuniform MF 0.2 Hz. | [84] |
| ELF H n | behavior/ reaction time | 1100-5 min-24 | MF had no effect on reaction time at any time during the exposure. | Replication study. ELF 0.1–0.2 Hz superimposed on the geoMF. Nonuniform MF. | [85] |
| ELF B n | blood/ melatonin | 10-9 h-32 | The levels of serum melatonin and its metabolite in urine in exposed men did not differ significantly from those in sham-exposed subjects. | 15 s pulses of the ELF MF by zero-crossing switch, linear polarization. | [94] |
| ELF B y | behavior/ pain | 50-2 h-11 | The reduction in the amplitude of pain-related stress-induced analgesia was observed after MF exposure. | Pain perception threshold, , ELF 0.05 Hz. Incomplete description of the position of human in the exposure system; nonuniformity; incomplete data on the MF. | [107] |
| ELF H n | vision/ discriminati- on | 100-8 min-99 | MF exposure had an insignificant effect on the accuracy of estimating the duration of light flashes. | 50 Hz intermittent 1 s on 1 s off, zero switch, nonuniform MF. | [86] |
| ELF B y | blood/ melatonin | 20-2 h-30 | MF exposure resulted in a half-hour delay in the onset of increased night-time melatonin concentration. | 15 s pulses of the ELF MF by zero-crossing switch, circular polarization, . | [95] |
| ELF B n | physiology/ melatonin, HR | 200-8 h-7 | Salivary melatonin levels determined hourly did not reveal alterations that can be related to the MF effect. | Sinusoidal MF 16.7 Hz. Large individual variability of the onset and amplitude of the hormone level and small sample could mask the MF effect. | [98] |
| ELF B n | blood/ hormone | 20-12 h-10 | No significant difference in the levels of four plasma hormones between blood samples collected during nights with MF exposure and those under control conditions. | Superimposed harmonics and 1-kHz 100 μT-amplitude MF transients per sec make interpretation difficult. | [99] |
| ELF H y | brain, cardio/ EEG | 80-1.5 h-40 | Alpha activity after 80 μT 50 Hz MF exposure doubled compared to sham. | Significance , blood pressure and HR were not changed. Pain threshold was almost insignificant. | [87] |
| ELF H n | cognition/ performance | 400-30 min-74 | No significant difference was found in cognitive performance, psychological and physiological parameters. | 50 Hz, continuous nonuniform MF. Large MF intensity might cause the absence of the effect. | [89] |
| ELF B y | physiology/ EEG | 5-1 h-20 | MF of 0–5 μT 8–12 Hz (DC-offset sinusoid) changes self-reported emotional state. | No details about exposure system, no information on the geoMF. | [113] |
| ELF H n | behavior/ vestibular balance | 50,000-25 min-22 | The parameters of human standing balance was analyzed to investigate postural modulation. There were no significant effects of the time-varying MF. | Nonuniform 20–160 Hz MF, 5 s pulses | [91] |
| var B y | cardio/ hemodynamics | 0.1-24 h-27 | MF storms affect regulation of blood circulation in cosmonauts during the flight dependently on the state of vegetative regulation. | Daily means of the most of HRV indices in control and exposed to MF storms groups differed at . This seems to exclude indirect effects via radiation, electric fields, etc. | [130] |
| var B n/y | cardio/ rate of admissions | 0.1-days-2000 | Two days after a solar proton flux event that going till MF storm, the risk of admission for myocardial infarction increased by 54%, . | What is the factor of influence, MF or protons? About 2000 persons. MF nT converted from Ap index. | [10] |
| var B y | physiology/ hormone | 0.1-days-900 | Cortisol level grows with the geomagnetic activity at summer and autumn, . | Observational study, geomagnetic activity is assumed to mean MF 0.01–0.1 μT. | [11] |
| var B y | cardio/ HRV | 0.2-24 h-9 | Artificial MF storm tends to randomize normal-normal RR intervals and decrease capillary blood velocity. | Incomplete statistics: no p-value for final statistical statement. | [109] |
| var B y | cardio/ HRV | 0.2-22 h-8 | Correlation of RR intervals with the Bx and By components of the MF was significantly higher during the artificial MF storm then in control, . | Averaged RR interval and capillary blood velocity were not sensitive to MF storms. Small number of tested persons; RR to Bx correlation might be a selection bias. | [13] |
| var B y | cardio/ rate of admissions | 0.1-days-4000 | Risk of acute coronary syndrome in obese patients is associated with the MF storms, . | Daily MF variations nT (converted from Ap index), about 4000 persons. | [14] |
| var B y | physiology/ mortality | 0.1-days-4,000,000 | A one-standard-deviation increase in the daily Kp index was associated with a 0.3% rise in total mortality in South Korea. | Cardio-vascular deceases, stroke, and myocardial infarction mortality across 237 administrative districts between 2001 and 2019. | [131] |
| puls B y | brain/EEG | 28-8 h-24 | Intermittent, but not continuous or sham exposure, was associated with less total sleep time, reduced sleep efficiency, increased time in stage II sleep, and decreased rapid sleep at . | Zero-crossing switch used to form 15 s pulses of the 60 Hz circularly polarized MF at night sleep. | [110] |
| puls B y | behavior/ pain | 200-30 min-70 | MF exposure does not affect basic human perception, but can increase pain thresholds. | No data on the pulse waveform. | [104] |
| puls B y | behavior/ tremor | 1000-5 min-24 | In postural tremor, the proportion of oscillations at frequencies between 2 and 4 Hz was higher during the real than during the sham exposure sequence, . | Pulses of the 50 Hz ELF MF, no description of pulse’s fronts. | [100] |
| puls H y | brain/EEG | 200-1680 s-17 | Human subjects responded to onset and to offset of 2 G, 60 Hz, 2 s MF pulses by exhibiting evoked potentials, in 16 of 17 subjects. | Zero-crossing switch to exclude the fronts of MF pulses. | [88] |
| puls H y | neuro/fMRI | 200-15 min-30 | The ELF MF produced by the fMRI procedure could induce electric currents and cause an increase in pain sensitivity. | Highly nonuniform MF pulses superimposed on 1.5 T fMRI MF. Complicated waveform of 5 s pulses impedes interpretation. Incomplete description of timing, time 15 min is conditional. | [105] |
| stat B y | behavior/ orientation | 50-min-64 | Blindfolded humans were able to orient toward home when subjected to displacement-release experiments. | Observational data. 86 trials, incomplete statistics, different number of persons in series of the experiments. | [79] |
| stat B n | behavior/ orientation | 50-min-100 | Attempts have been unsuccessful to replicate an ability of blindfolded humans transported from home to indicate the direction of displacement. | Near 200 trials, up to 100 persons | [80] |
| stat B y | vision/acuity | 19-few min-8 | Experiments show a delayed (about 1 min) reaction in night-vision acuity after the reverse of the horizontal MF component, . | Large MF effect. No information on the speed of the MF reverse. | [43] |
| stat B n/y | brain/EEG | 45-2 h-50 | MF alterations had no effect on EEG parameters. However, EEG could possibly change hundreds of ms after the start of the 4 s recording. | Exposition up to 2 h, MF μT, or μT. Overcomplicated design of MF exposures. | [112] |
| stat H y | vision/ threshold | 48-15 min-30 | Correspondence between viewing and MF direction results in a significant decrease of the visual discrimination threshold. | MF 70° rotation. 4% effect at . No information on the speed of the MF turn. | [117] |
| stat B y | brain/EEG | 35-1 h-36 | Following geoMF stimulation, a drop in amplitude of EEG alpha-oscillations occurred. | Rotation of MF, drop in alpha activity, mainly . | [102] |
| Mechanism | Critical MF Relation | Primary MF Target | /T−1s−1 | /s | Critical MF Estimate | Link to Chemistry |
|---|---|---|---|---|---|---|
| RPM | electron | 0.6 mT | known | |||
| Abstract precession | not applicable | – | – | not applicable | ||
| Quantum rotor | aminoacid residue | 0.1 | 5 T 50 nT | suggested | ||
| Proton mechanism | proton | 0.1 | 40 nT | suggested |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspranski, R.R.; Binhi, V.N.; Koshel, I.V. Human Responses to Magnetic and Hypomagnetic Fields: Available Evidence and Potential Risks for Deep Space Travel. Life 2025, 15, 1766. https://doi.org/10.3390/life15111766
Kaspranski RR, Binhi VN, Koshel IV. Human Responses to Magnetic and Hypomagnetic Fields: Available Evidence and Potential Risks for Deep Space Travel. Life. 2025; 15(11):1766. https://doi.org/10.3390/life15111766
Chicago/Turabian StyleKaspranski, Rustem R., Vladimir N. Binhi, and Ivan V. Koshel. 2025. "Human Responses to Magnetic and Hypomagnetic Fields: Available Evidence and Potential Risks for Deep Space Travel" Life 15, no. 11: 1766. https://doi.org/10.3390/life15111766
APA StyleKaspranski, R. R., Binhi, V. N., & Koshel, I. V. (2025). Human Responses to Magnetic and Hypomagnetic Fields: Available Evidence and Potential Risks for Deep Space Travel. Life, 15(11), 1766. https://doi.org/10.3390/life15111766







