Study of the Physicochemical and Phytochemical Parameters Together with Antibacterial Properties of Conventionally and Organically Cultivated Spirulina (Arthrospira platensis) in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Spirulina Samples
2.2. Chemicals and Reagents
2.3. Extraction of Phytochemical Compounds
2.4. Determination of Antioxidant Activity
2.4.1. Preparation of DPPH Standard Solution
2.4.2. Determination of In Vitro Antioxidant Activity
2.5. Total Phenolic Content of Spirulina Extracts
2.6. Determination of Quercetin Content in Spirulina Extracts
2.7. Determination of Caffeic Acid Content
2.8. Determination of pH
2.9. Determination of Color Parameters
2.10. Determination of C-Phycocyanin Content
2.11. Estimation of Antibacterial Activity of Spirulina Extracts
2.12. Statistical Analysis
3. Results
3.1. Antioxidant Activity
3.2. Total Phenolic Content
3.3. Secondary Metabolites
3.3.1. Caffeic Acid
3.3.2. Quercetin
3.4. C–Phycocyanin
3.5. Effective Acidity (pH)
3.6. Yellow Color Tone
3.7. Blue Color Tone
3.8. Red Color Tone
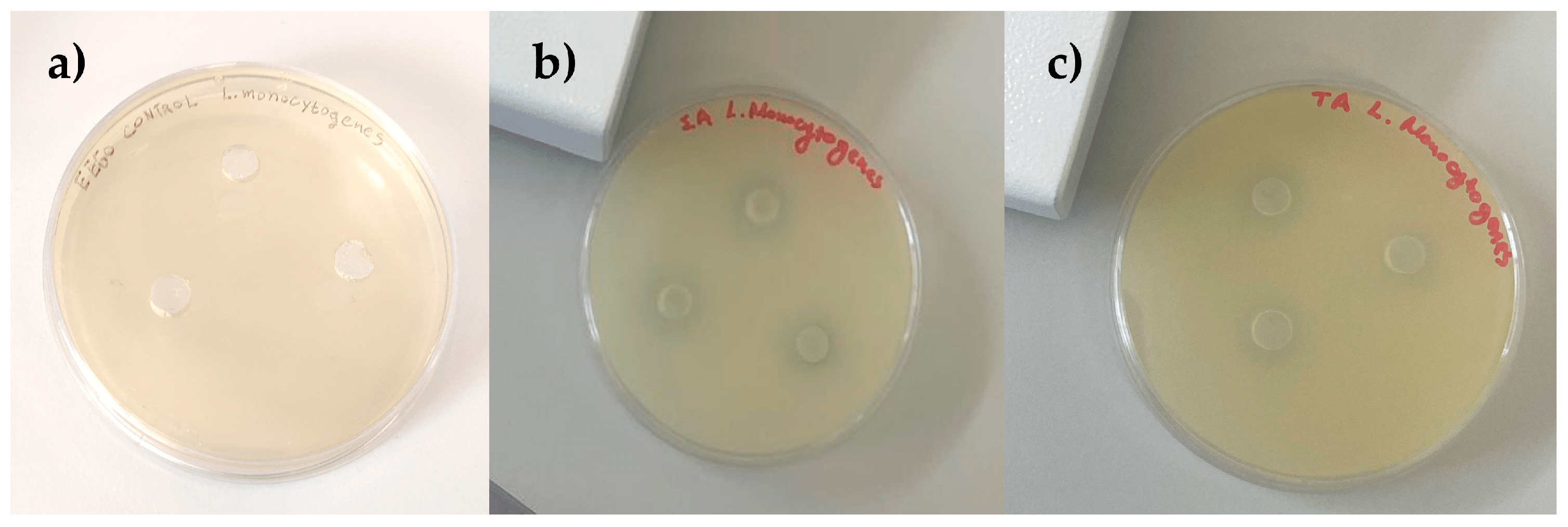
3.9. Estimation of Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Withana, T.N.; Ranasinghe, Y.S.; Rathnayake, A.I.; Bamunuarachchige, T.C.; de Zoysa, H.K.S. Applications of Spirulina in the Food Industry: Scientometric and Systematic Review. Food Humanit. 2025, 5, 100644. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.B.; Sant’Anna, E.S. Cultivation of Arthrospira (Spirulina) platensis in Desalinator Wastewater and Salinated Synthetic Medium: Protein Content and Amino-Acid Profile. Braz. J. Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Comparative Study on the Growth Performance of Spirulina platensis on Modifying Culture Media. Energy Rep. 2019, 5, 327–336. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.-M.E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and Antimicrobial Activities of Spirulina platensis Extracts and Biogenic Selenium Nanoparticles against Selected Pathogenic Bacteria and Fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in Bread Formulated with Wheat Flours of Different Alveograph Strength. Foods 2023, 12, 3724. [Google Scholar] [CrossRef] [PubMed]
- Taiti, C.; Stefano, G.; Percaccio, E.; Di Giacomo, S.; Iannone, M.; Marianelli, A.; Di Sotto, A.; Garzoli, S. Addition of Spirulina to Craft Beer: Evaluation of the Effects on Volatile Flavor Profile and Cytoprotective Properties. Antioxidants 2023, 12, 1021. [Google Scholar] [CrossRef]
- Bosnea, L.; Terpou, A.; Pappa, E.; Kondyli, E.; Mataragas, M.; Markou, G.; Katsaros, G. Incorporation of Spirulina platensis on Traditional Greek Soft Cheese with Respect to Its Nutritional and Sensory Perspectives. Proceedings 2021, 70, 99. [Google Scholar] [CrossRef]
- Elkot, W.F.; El-Mahdy, A.; El-Sawah, T.H.; Alghamdia, O.A.; Alhag, S.K.; Al-Shahari, E.A.; AL-Farga, A.; Ismail, H.A. Development and Characterization of a Novel Flavored Functional Fermented Whey-Based Sports Beverage Fortified with Spirulina platensis. Int. J. Biol. Macromol. 2023, 258, 128999. [Google Scholar] [CrossRef]
- Sharoba, A.M. NUTRITIONAL VALUE of SPIRULINA and ITS USE in the PREPARATION of SOME COMPLEMENTARY BABY FOOD FORMULAS. J. Food Dairy Sci. 2014, 5, 517–538. [Google Scholar] [CrossRef]
- Ahmad, A.M.R.; Intikhab, A.; Zafar, S.; Farooq, U.; Shah, H.B.U.; Akram, S.; Abid, J.; Parveen, Z.; Iqbal, S. Spirulina, an FDA-Approved Functional Food: Worth the Hype? Cell Mol. Biol. 2023, 69, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Gogna, S.; Kaur, J.; Sharma, K.; Prasad, R.; Singh, J.; Bhadariya, V.; Kumar, P.; Jarial, S. Spirulina- an Edible Cyanobacterium with Potential Therapeutic Health Benefits and Toxicological Consequences. JANA 2022, 43, 559–572. [Google Scholar] [CrossRef]
- Spínola, M.P.; Mendes, A.R.; Prates, J.A.M. Chemical Composition, Bioactivities, and Applications of Spirulina (Limnospira platensis) in Food, Feed, and Medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Hatami, E.; Ghalishourani, S.-S.; Najafgholizadeh, A.; Pourmasoumi, M.; Hadi, A.; Clark, C.C.T.; Assaroudi, M.; Salehi-sahlabadi, A.; Joukar, F.; Mansour-Ghanaei, F. The Effect of Spirulina on Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Diabetes Metab. Disord. 2021, 20, 883–892. [Google Scholar] [CrossRef]
- Chauhan, B.; Kumar, G.; Kalam, N.; Shahid, A. Current Concepts and Prospects of Herbal Nutraceutical: A Review. J. Adv. Pharm. Technol. Res. 2013, 4, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical Properties of Phycocyanin. J. Funct. Foods. 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Zeece, M. Chapter Eight—Food Colorants. In Introduction to the Chemistry of Food; Zeece, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 313–344. [Google Scholar] [CrossRef]
- Lazaridis, D.G.; Karabagias, V.K.; Karabagias, I.K.; Andritsos, N.D.; Giannakas, A.E. Physicochemical and Phytochemical Characterization of Green Coffee, Cinnamon Clove, and Nutmeg EEGO, and Aroma Evaluation of the Raw Powders. Eur. Food Res. Technol. 2024, 250, 83–96. [Google Scholar] [CrossRef]
- Lazaridis, D.G.; Karabagias, V.K.; Andritsos, N.D.; Giannakas, A.E.; Karabagias, I.K. A Grape-Derived Solvent for the Recovery of Phenolic Compounds from Food Waste By-Products Using Ultrasonic-Assisted and Overnight Extraction. Molecules 2025, 30, 3878. [Google Scholar] [CrossRef] [PubMed]
- Kitsios, A.-P.M.; Lazaridis, D.G.; Koutoulis, A.S.; Karabagias, V.K.; Andritsos, N.D.; Karabagias, I.K. Geographical Origin Estimation of Miniaturized Samples of “Nova” Mandarin Juice Based on Multiple Physicochemical and Biochemical Parameters Conjointly with Bootstrapping. Eur. Food Res. Technol. 2025, 251, 1005–1020. [Google Scholar] [CrossRef]
- Lazaridis, D.G.; Giannoulis, S.D.; Simoni, M.; Karabagias, V.K.; Andritsos, N.D.; Triantafyllidis, V.; Karabagias, I.K. Physicochemical and Phytochemical Determinations of Greek “Kollitsida’’ (Arctium lappa L.) from Different Regions and Evaluation of Its Antimicrobial Activity. Separations 2025, 12, 151. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. COMPLEMENTARY CHROMATIC ADAPTATION in a FILAMENTOUS BLUE-GREEN ALGA. JCB 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Koutelidakis, A.E.; Andritsos, N.D.; Kabolis, D.; Kapsokefalou, M.; Drosinos, E.H.; Komaitis, M. Antioxidant and antimicrobial properties of tea and aromatic plant extracts against bacterial foodborne pathogens: A comparative evaluation. CTNR 2016, 14, 133–142. [Google Scholar]
- Vollmannová, A.; Bojňanská, T.; Musilová, J.; Lidiková, J.; Cifrová, M. Quercetin as One of the Most Abundant Represented Biological Valuable Plant Components with Remarkable Chemoprotective Effects—A Review. Heliyon 2024, 10, e33342. [Google Scholar] [CrossRef] [PubMed]
- Stunda-Zujeva, A.; Berele, M.; Lece, A.; Skesters, A. Comparison of Antioxidant Activity in Various Spirulina Containing Products and Factors Affecting It. Sci. Rep. 2023, 13, 4529. [Google Scholar] [CrossRef]
- Rebey, I.B.; Bourgou, S.; Debez, I.B.S.; Karoui, I.J.; Sellami, I.H.; Msaada, K.; Limam, F.; Marzouk, B. Effects of Extraction Solvents and Provenances on Phenolic Contents and Antioxidant Activities of Cumin (Cuminum cyminum L.) Seeds. Food Bioprocess Technol. 2012, 5, 2827–2836. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Melkis, K.; Dalewski, B.; Janda-Milczarek, K. Assessment of Antioxidant Properties and Mycotoxin Profile of Commercial Spirulina Supplements. Food Biosci. 2024, 62, 105461. [Google Scholar] [CrossRef]
- Rahim, A.; Çakir, C.; Ozturk, M.; Şahin, B.; Soulaimani, A.; Sibaoueih, M.; Nasser, B.; Eddoha, R.; Essamadi, A.; El Amiri, B. Chemical Characterization and Nutritional Value of Spirulina platensis Cultivated in Natural Conditions of Chichaoua Region (Morocco). S. Afr. J. Bot. 2021, 141, 235–242. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Yu, B.; Xu, Q.; Bai, Z.; Ji, K.; Gao, X.; Wang, B.; Aadil, R.M.; Ma, H.; Xiao, R. The Physicochemical Properties and Antioxidant Activity of Spirulina (Artrhospira platensis) Chlorophylls Microencapsulated in Different Ratios of Gum Arabic and Whey Protein Isolate. Foods 2022, 11, 1809. [Google Scholar] [CrossRef]
- Chu, W.-L.; Lim, Y.-W.; Radhakrishnan, A.K.; Lim, P.-E. Protective Effect of Aqueous Extract from Spirulina platensis against Cell Death Induced by Free Radicals. BMC Complement Altern Med. 2010, 10, 53. [Google Scholar] [CrossRef]
- Park, K.A.; Choi, Y.; Kang, S.; Kim, M.-R.; Hong, J. Effects of Proteins on the Reactivity of Various Phenolic Compounds with the Folin-Ciocalteu Reagent. Korean J. Food Sci. Technol. 2015, 47, 299–305. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. eCAM 2016, 1, 7803846. [Google Scholar] [CrossRef] [PubMed]
- Alshuniaber, M.A.; Krishnamoorthy, R.; AlQhtani, W.H. Antimicrobial Activity of Polyphenolic Compounds from Spirulina against Food-Borne Bacterial Pathogens. Saudi J. Biol. Sci. 2021, 28, 459–464. [Google Scholar] [CrossRef]
- Sakai, E.; Farhana, F.; Yamaguchi, Y.; Tsukuba, T. Potentials of Natural Antioxidants from Plants as Antiosteoporotic Agents. Stud. Nat. Prod. Chem. 2022, 72, 1–28. [Google Scholar] [CrossRef]
- Ilieva, Y.; Zaharieva, M.M.; Najdenski, H.; Kroumov, A.D. Antimicrobial Activity of Arthrospira (Former Spirulina) and Dunaliella Related to Recognized Antimicrobial Bioactive Compounds. Int. J. Mol. Sci. 2024, 25, 5548. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, J.; Sun, M.; Xiong, S.; Xu, C.; Zhang, Z.; Li, M.; Li, C.; Lin, L. Plant-Derived Caffeic Acid and Its Derivatives: An Overview of Their NMR Data and Biosynthetic Pathways. Molecules 2024, 29, 1625. [Google Scholar] [CrossRef]
- Kuntzler, S.G.; Maria, A.; Alberto, J.; Greque, M. Polyhydroxybutyrate and Phenolic Compounds Microalgae Electrospun Nanofibers: A Novel Nanomaterial with Antibacterial Activity. Int. J. Biol. Macromol. 2018, 113, 1008–1014. [Google Scholar] [CrossRef]
- Pyne, S.; Paria, K. Optimization of Extraction Process Parameters of Caffeic Acid from Microalgae by Supercritical Carbon Dioxide Green Technology. BMC Chem. 2022, 16, 31. [Google Scholar] [CrossRef]
- El-Baky, H.; Baz, F.E.; GS El-Baroty, G.S. Production of Phenolic Compounds from Spirulina maxima Microalgae and Its Protective. Effects. Afr. J. Biotechnol. 2009, 8, 7059–7067. [Google Scholar]
- Bortolini, D.G.; Maciel, G.M.; de Andrade Arruda Fernandes, I.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional Properties of Bioactive Compounds from Spirulina spp.: Current Status and Future Trends. Food Chem.-Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional Composition, Nutritional Properties, and Biological Activities of Moroccan Spirulina Microalga. J. Food Qual. 2019, 2019, 3707219. [Google Scholar] [CrossRef]
- Guldas, M.; Ziyanok-Demirtas, S.; Sahan, Y.; Yildiz, E.; Gurbuz, O. Antioxidant and Anti-Diabetic Properties of Spirulina platensis Produced in Turkey. Food Sci. Technol. 2021, 41, 615–625. [Google Scholar] [CrossRef]
- Su, C.-H.; Liu, C.-S.; Yang, P.-C.; Syu, K.-S.; Chiuh, C.-C. Solid–Liquid Extraction of Phycocyanin from Spirulina platensis: Kinetic Modeling of Influential Factors. Sep. Purif. Technol. 2014, 123, 64–68. [Google Scholar] [CrossRef]
- Vali Aftari, R.; Rezaei, K.; Mortazavi, A.; Bandani, A.R. The Optimized Concentration and Purity of Spirulina platensis C-Phycocyanin: A Comparative Study on Microwave-Assisted and Ultrasound-Assisted Extraction Methods. J. Food Process. Preserv. 2015, 39, 3080–3091. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Teixeira, I.R.; Marczak, L.D.F.; Mercali, G.D. Phycocyanin from Spirulina: A Review of Extraction Methods and Stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef]
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and Light Stability of Three Natural Blue Colorants for Use in Confectionery and Beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- Rhoades, J.; Fotiadou, S.; Paschalidou, G.; Papadimitriou, T.; Álvarez Ordóñez, A.; Kormas, K.; Vardaka, E.; Likotrafiti, E. Microbiota and Cyanotoxin Content of Retail Spirulina Supplements and Spirulina Supplemented Foods. Microorganisms 2023, 11, 1175. [Google Scholar] [CrossRef]
- AlFadhly, K.Z.N.; Alhelfi, N.; Altemimi, B.A.; Verma, D.K.; Cacciola, F. Tendencies Affecting the growth and Cultivation of Genus Spirulina: An Investigative Review on Current Trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, N.; Pabbi, S.; Walia, S.; Dhar, D.W. Protocol Optimization for Enhanced Production of Pigments in Spirulina. Indian J. Plant Physiol. 2013, 18, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, V.; Filippini, R.; Zusso, M.; Caniato, R.; Piovan, A. Monitoring of Spirulina Flakes and Powders from Italian Companies. Molecules 2022, 27, 3155. [Google Scholar] [CrossRef] [PubMed]
- Işık, O.; Uslu, L.; Ak Çimen, B.; Gökpınar, Ş.; Reddad, C.; Sayın, S. Blue colored pigment phycocyanin extraction from Spirulina platensis. Acta Aqua. Tr. 2020, 16, 506–510. [Google Scholar] [CrossRef]
- Costa, B.R.; Rodrigues, M.C.K.; Rocha, S.F.; Pohndorf, R.S.; Larrosa, A.P.Q.; Pinto, L.A.A. Optimization of Spirulina sp. Drying in Heat Pump: Effects on the Physicochemical Properties and Color Parameters. J. Food Process. Preserv. 2015, 40, 934–942. [Google Scholar] [CrossRef]
- Thoisen, C.; Hansen, B.W.; Nielsen, S.L. A Simple and Fast Method for Extraction and Quantification of Cryptophyte Phycoerythrin. MethodsX 2017, 4, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Firoozabad, M.S.M.; Nasr, M.M. Antimicrobial activities of microbial essential fatty acid against foodborne pathogenic bacteria. Iran. J. Microbiol. 2022, 14, 214–218. [Google Scholar] [CrossRef]
- Cakmak, Y.S.; Kaya, M.; Asan-Ozusaglam, M. Biochemical composition and bioactivity screening of various extracts from Dunaliella salina, a green microalga. EXCLI J. 2014, 13, 679–690. [Google Scholar]


| Samples | Solvent | Antioxidant Activity (%) | Total Phenolic Content (mg/g DM) | Caffeic Acid Content (mg/g DM) | Quercetin Content (mg/g DM) | C-Phycocyanin Content (mg/mL) |
|---|---|---|---|---|---|---|
| Τablets | EEGO | 44.37 ±2.28 a | 10.10 ± 1.90 a | 2.34 ± 0.01 a | 2.60 ± 0.09 a | 1.42 ± 0.01 a |
| Powder | EEGO | 47.71 ± 1.28 a | 12.87 ± 0.95 b | 2.69 ± 0.01 b | 2.30 ± 0.03 b | 0.74 ± 0.01 b |
| Flakes | EEGO | 48.28 ± 0.39 ab | 4.83 ± 0.55 c | 1.05 ± 0.01 c | 1.40 ± 0.02 c | 0.07 ± 0.01 c |
| Τablets | Water | 19.60 ± 1.23 c | 15.40 ± 0.68 d | 2.88 ± 0.01 d | 1.99 ± 0.06 d | 1.65 ± 0.01 d |
| Powder | Water | 29.19 ± 1.37 d | 17.07 ± 0.66 d | 2.65 ± 0.01 e | 2.40 ± 0.01 b | 0.74 ± 0.01 b |
| Flakes | Water | 6.90 ± 0.80 e | 10.35 ± 0.20 a | 1.71 ± 0.01 f | 1.41 ± 0.02 c | 0.66 ± 0.01 e |
| Samples | Solvent | pH | YCT (%) | BCT (%) | RCT (%) |
|---|---|---|---|---|---|
| Tablets | EEGO | 7.32 ± 0.02 a | 23.46 ± 0.18 a | 14.28 ± 0.17 a | 62.25 ± 0.35 a |
| Powder | EEGO | 7.77 ± 0.01 b | 42.05 ± 0.03 b | 22.99 ± 0.09 b | 34.94 ± 0.08 b |
| Flakes | EEGO | 6.79 ± 0.02 c | 38.41 ± 0.08 c | 16.04 ± 0.11 c | 45.54 ± 0.03 c |
| Tablets | Water | 8.03 ± 0.00 d | 35.80 ± 0.08 d | 20.93 ± 0.03 d | 43.28 ± 0.06 d |
| Powder | Water | 8.24 ± 0.01 e | 47.01 ± 0.08 e | 24.91 ± 0.02 e | 27.99 ± 0.18 e |
| Flakes | Water | 7.26 ± 0.01 f | 42.19 ± 0.02 f | 24.05 ± 0.04 f | 33.76 ± 0.05 f |
| Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|
| Samples | Solvent | Salmonella Typhimurium | Staphylococcus aureus | Listeria monocytogenes | E. coli O157:H7 |
| Tablets | EEGO | 0.00 ± 0.00 a | 2.46 ± 0.06 a | 1.65 ± 0.14 a | 1.54 ± 0.09 a |
| Powder | EEGO | 2.15 ± 0.05 b | 2.55 ± 0.04 a | 2.88 ± 0.56 b | 1.86 ± 0.14 b |
| Flakes | EEGO | 0.51 ± 0.11 c | 0.00 ± 0.00 b | 0.23 ± 0.05 c | 0.32 ± 0.10 c |
| Tablets | Water | 2.30 ± 0.07 b | 3.18 ± 0.17 c | 4.22 ± 0.10 d | 0.32 ± 0.02 c |
| Powder | Water | 0.24 ± 0.04 d | 0.41 ± 0.01 d | 0.17 ± 0.01 c | 0.14 ± 0.02 c |
| Flakes | Water | 0.00 ± 0.00 a | 1.81 ± 0.03 e | 1.30 ± 0.04 a | 0.22 ± 0.03 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulopoulou, M.-A.; Lazaridis, D.G.; Simoni, M.; Intzirtzi, E.; Karabagias, V.K.; Andritsos, N.D.; Triantafyllidis, V.; Karabagias, I.K. Study of the Physicochemical and Phytochemical Parameters Together with Antibacterial Properties of Conventionally and Organically Cultivated Spirulina (Arthrospira platensis) in Greece. Life 2025, 15, 1761. https://doi.org/10.3390/life15111761
Christodoulopoulou M-A, Lazaridis DG, Simoni M, Intzirtzi E, Karabagias VK, Andritsos ND, Triantafyllidis V, Karabagias IK. Study of the Physicochemical and Phytochemical Parameters Together with Antibacterial Properties of Conventionally and Organically Cultivated Spirulina (Arthrospira platensis) in Greece. Life. 2025; 15(11):1761. https://doi.org/10.3390/life15111761
Chicago/Turabian StyleChristodoulopoulou, Maria-Athanasia, Dimitrios G. Lazaridis, Maria Simoni, Eirini Intzirtzi, Vassilios K. Karabagias, Nikolaos D. Andritsos, Vassilios Triantafyllidis, and Ioannis K. Karabagias. 2025. "Study of the Physicochemical and Phytochemical Parameters Together with Antibacterial Properties of Conventionally and Organically Cultivated Spirulina (Arthrospira platensis) in Greece" Life 15, no. 11: 1761. https://doi.org/10.3390/life15111761
APA StyleChristodoulopoulou, M.-A., Lazaridis, D. G., Simoni, M., Intzirtzi, E., Karabagias, V. K., Andritsos, N. D., Triantafyllidis, V., & Karabagias, I. K. (2025). Study of the Physicochemical and Phytochemical Parameters Together with Antibacterial Properties of Conventionally and Organically Cultivated Spirulina (Arthrospira platensis) in Greece. Life, 15(11), 1761. https://doi.org/10.3390/life15111761










