Swinging Mass Through the Pulmonary Valve: A Rare Case of Right Ventricular Myxoma
Abstract
1. Introduction
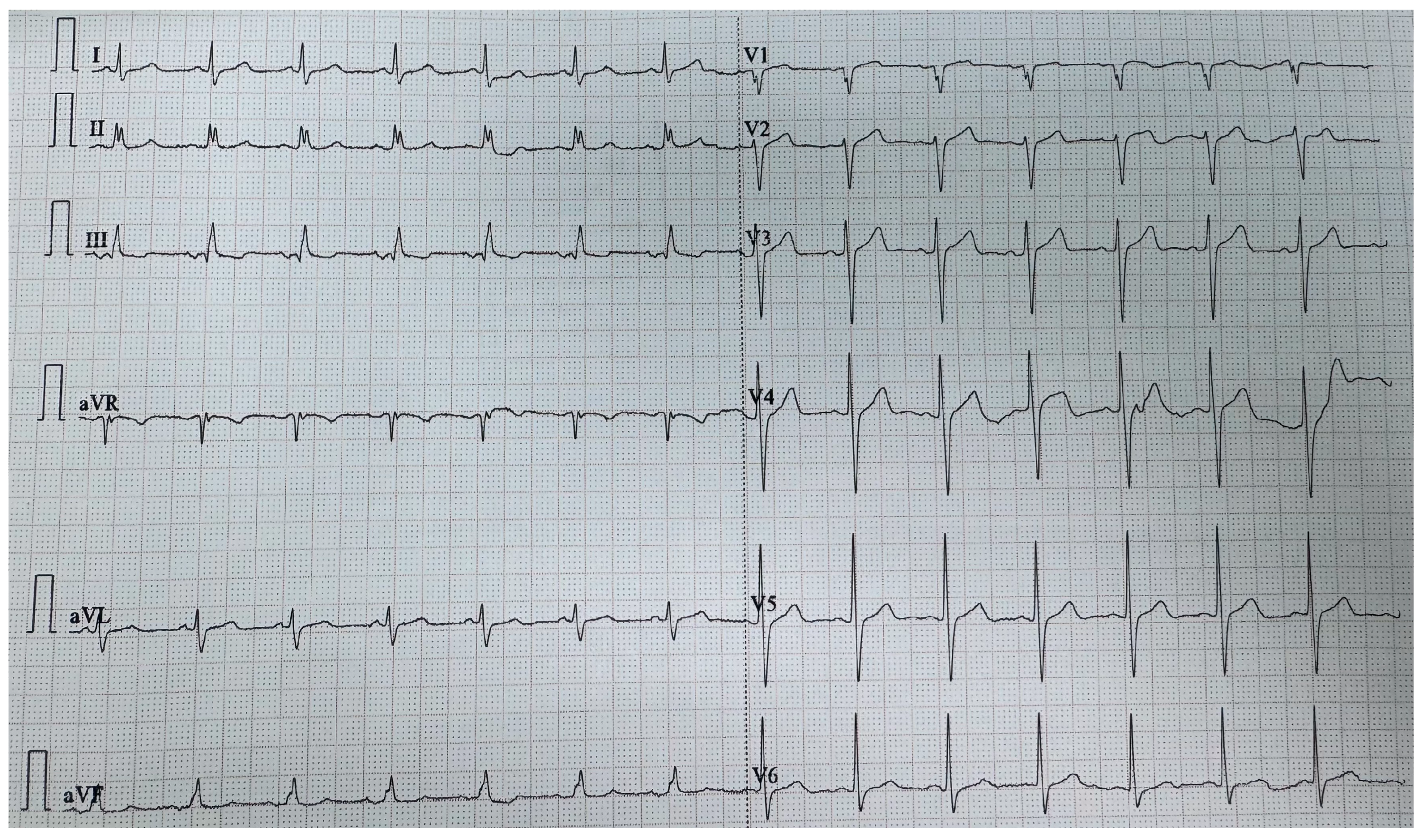
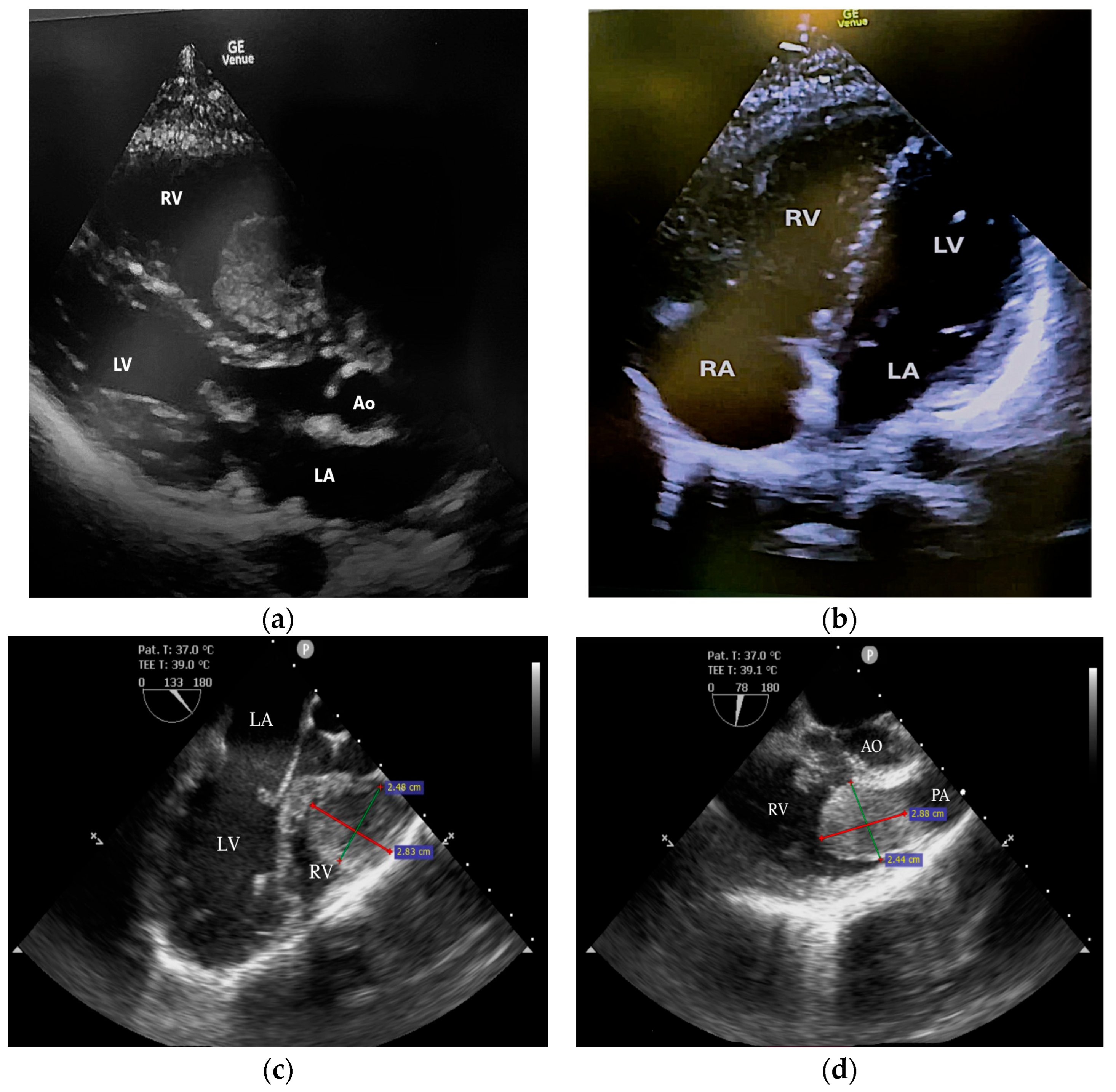
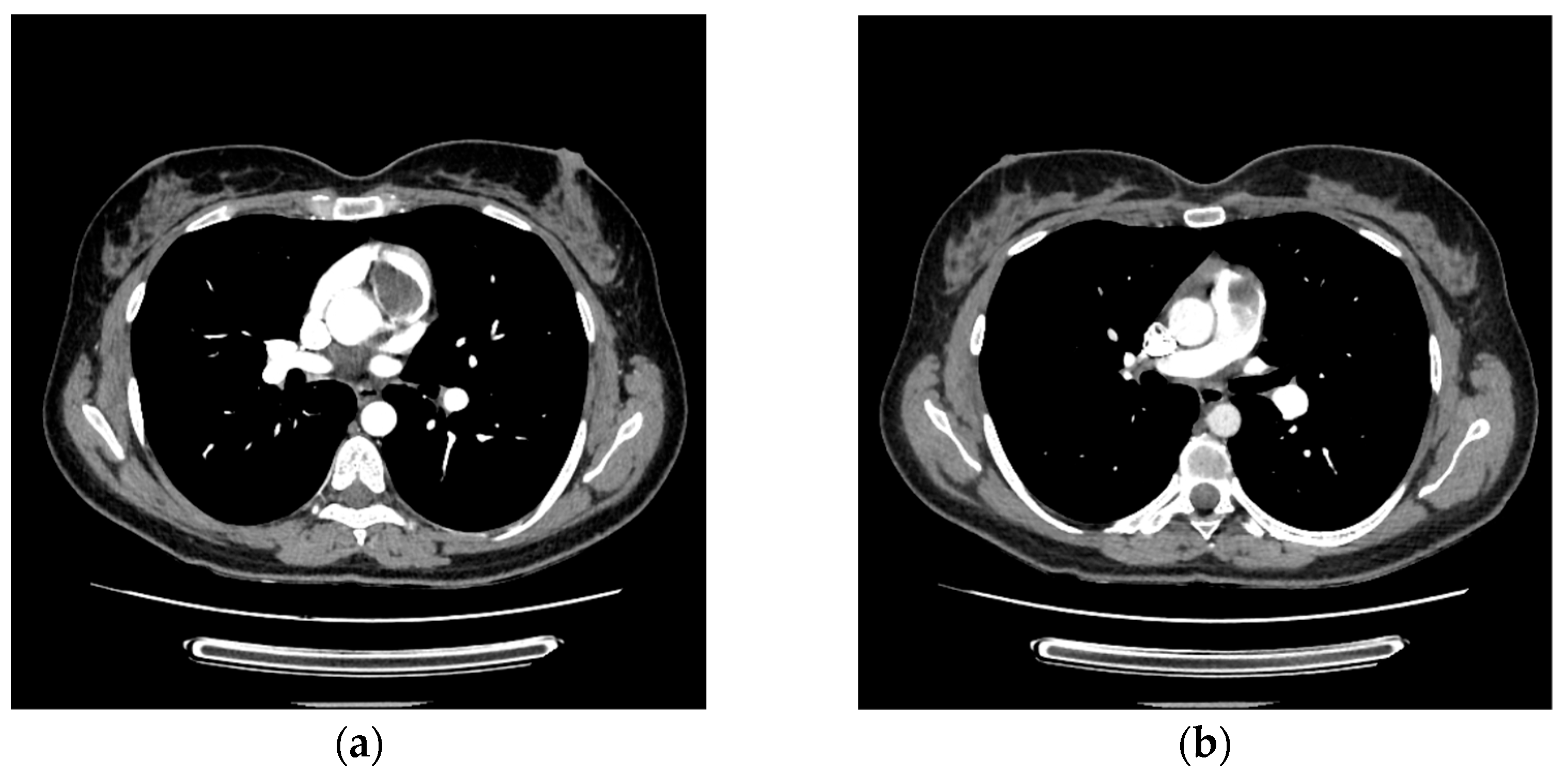
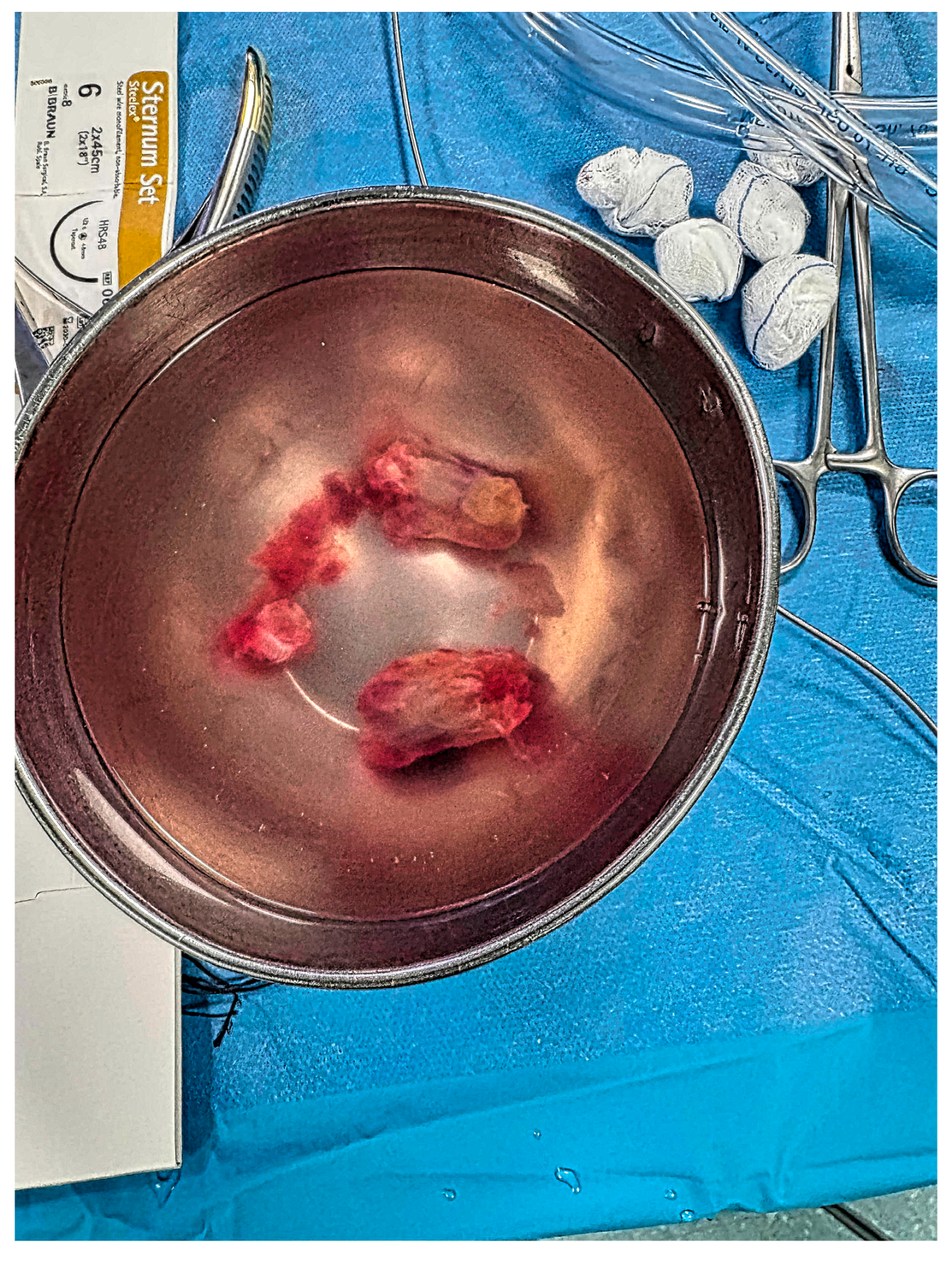
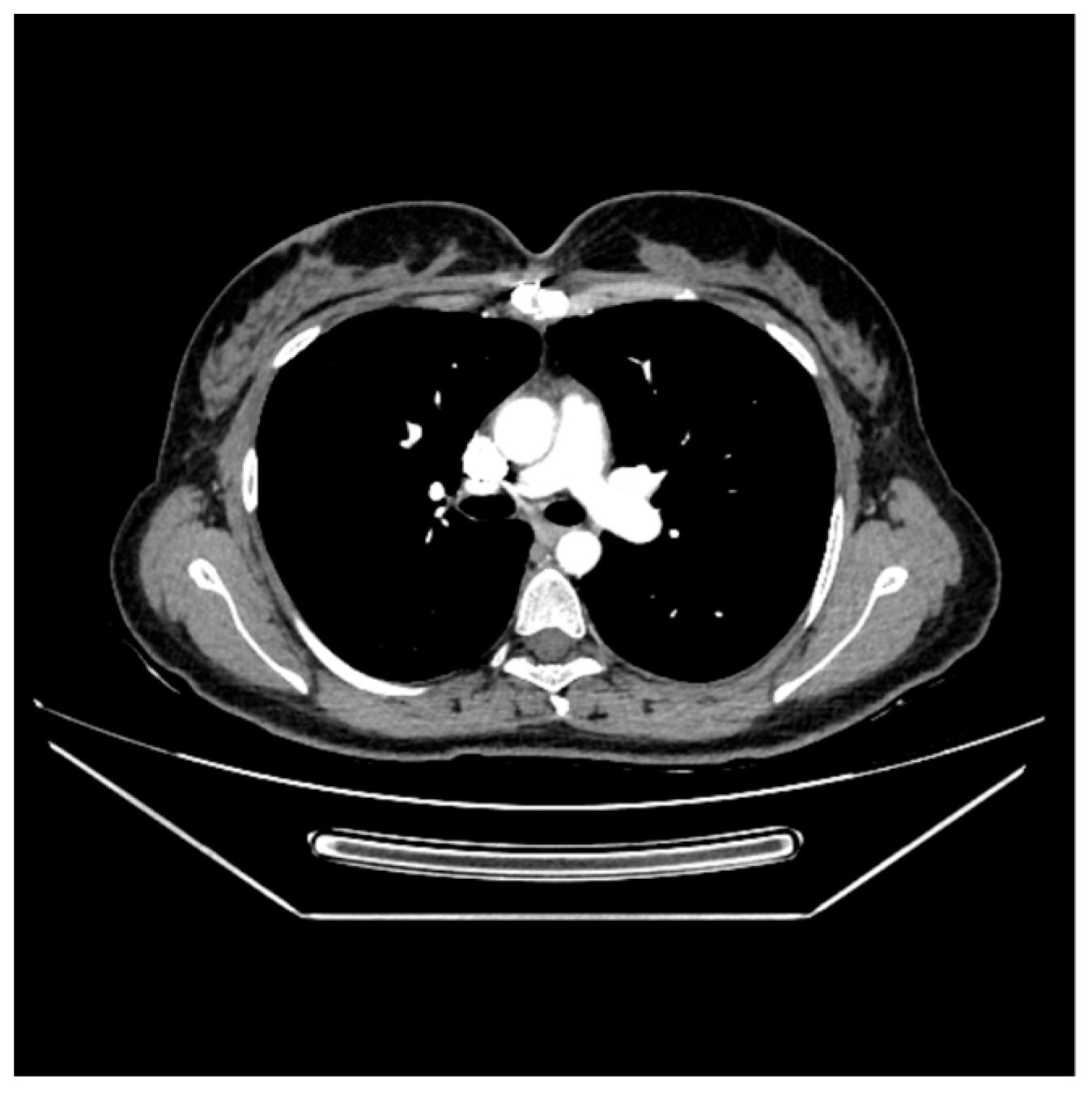
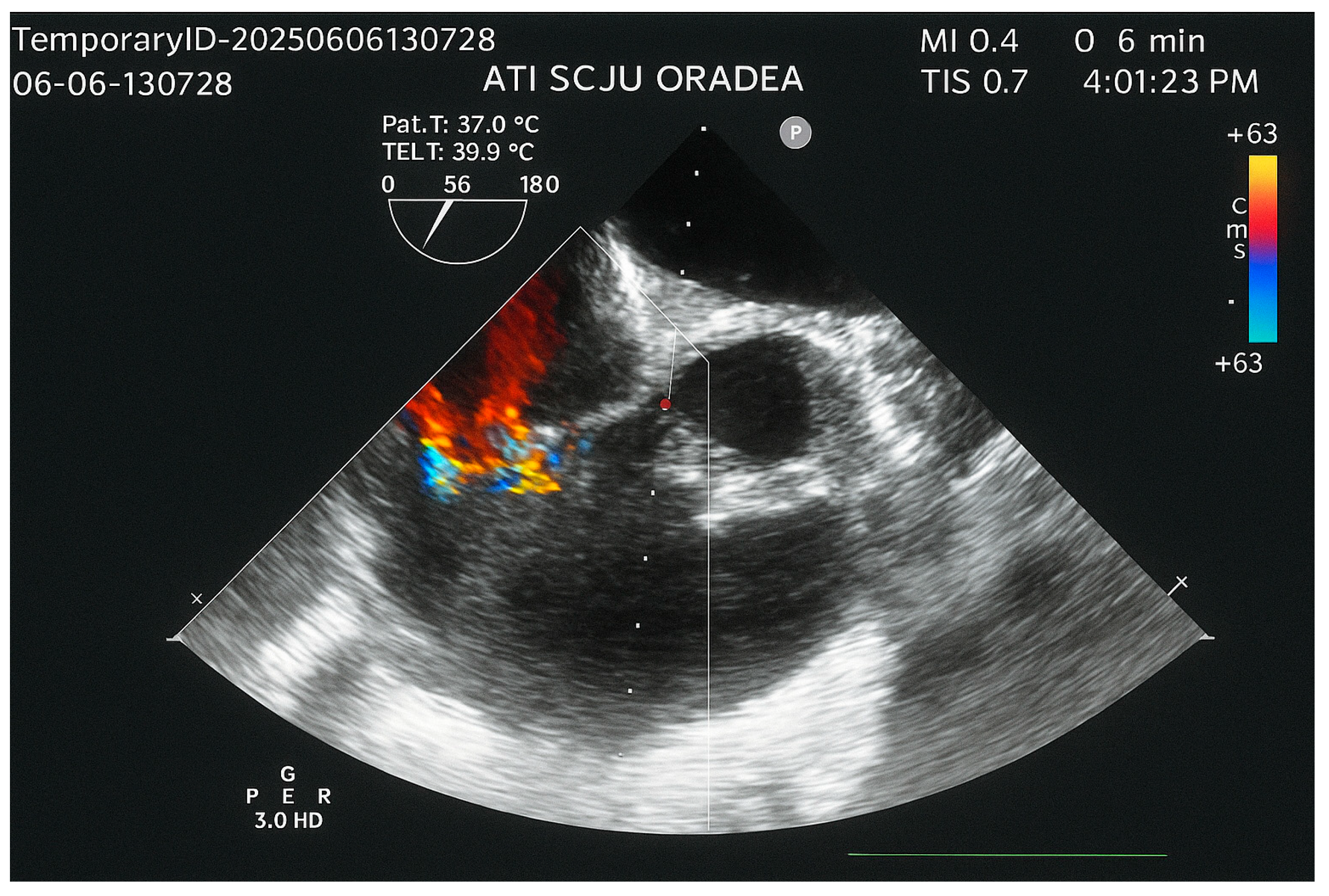
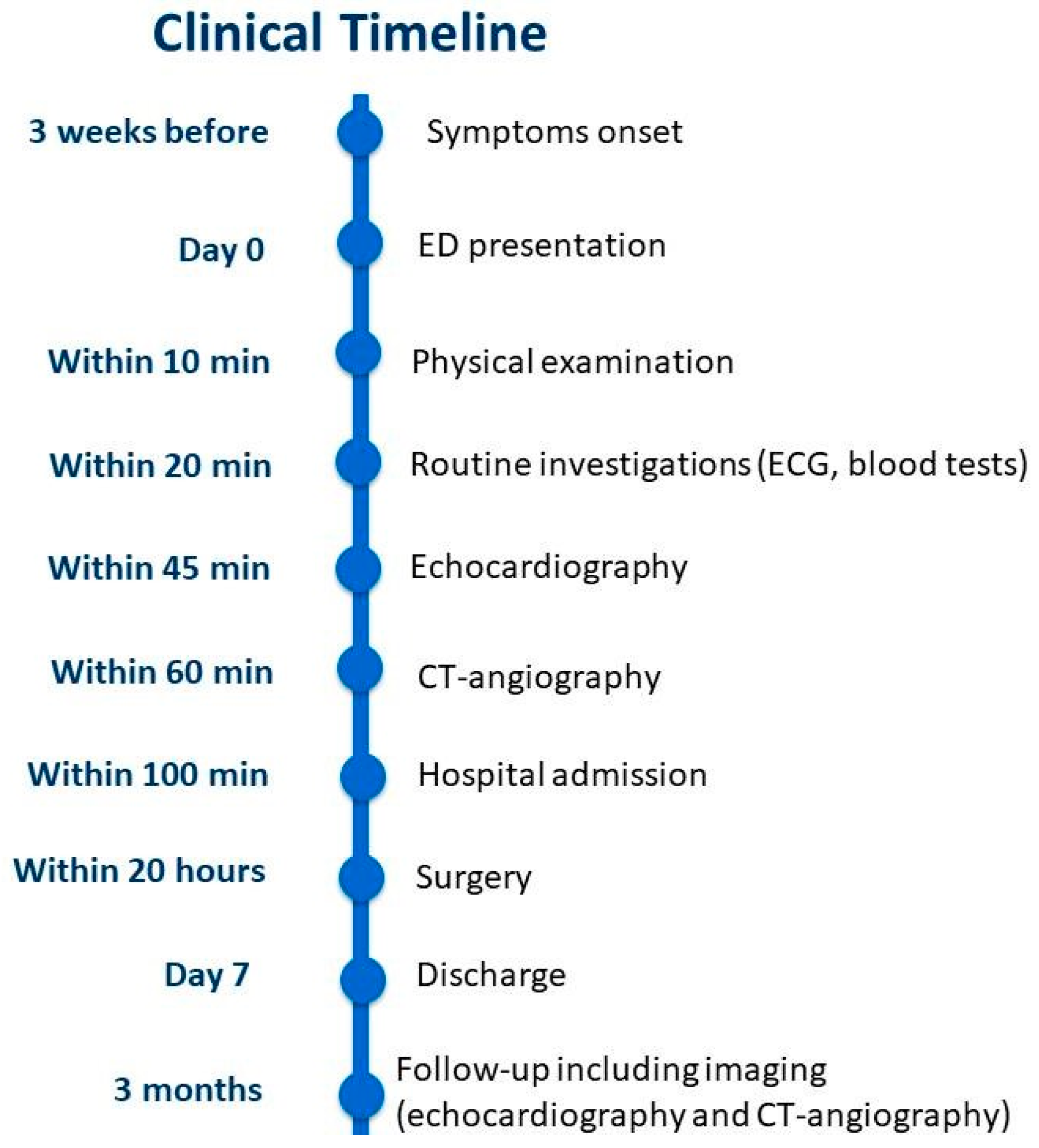
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| RV | Right ventricle |
| RA | Right atrium |
| LV | Left ventricle |
| LA | Left atrium |
| PA | Main pulmonary artery |
| RVOT | Right ventricular outflow tract |
| CT | Computed tomography |
| SGLT2 | Sodium-Glucose Cotransporter-2 |
| ARNI | Angiotensin receptor-neprilysin inhibitor |
| ALL | Acute lymphoblastic leukemia |
References
- Çobankent Aytekin, E.; Behzatoğlu, K.; Akçay, A.; Özgün Şahin, A.; Kökbudak, N.; Kılınç, F.; Okçu Heper, A.; Kurtulan, O.; Özbilim, G.; Eğilmez, R.; et al. Primary Cardiac Tumors: Clinical Presentations and Pathological Features in a Multicenter Cohort. Diagnostics 2025, 15, 1951. [Google Scholar] [CrossRef]
- Islam, A.K.M.M. Cardiac Myxomas: A Narrative Review. World J. Cardiol. 2022, 14, 206–219. [Google Scholar] [CrossRef]
- Abdul-Hafez, H.A.; Sarama, A.; Safadi, T.; Khadra, M.N.; Salman, H.H.; Darwazah, A.K.; Alkhatib, H. Surgically Treated Left Ventricular Myxomas: A 75-Year Systematic Review of Patient Demographics, Tumor Characteristics, and Outcomes. Interdiscip. Cardiovasc. Thorac. Surg. 2025, ivaf248. [Google Scholar] [CrossRef]
- Alahmadi, M.H.; Rodriguez Ziccardi, M.; Tariq, M.A.; Limaiem, F.; Ahmed, S.W. Cardiac Cancer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537144/ (accessed on 10 October 2025).
- Liu, Y.; Li, X.; Liu, Z.; Jiang, Y.; Lu, C.; Ge, S.; Zhang, C. Clinical Characteristics and Surgical Outcomes of Right Heart Myxoma and Its Comparison with Left Heart Myxoma: A Single-Center Retrospective Study. Anatol. J. Cardiol. 2023, 27, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Yasin, Y.; Darwazah, A.K.; Rajabi, I.; Al-Ali, F.H.; Subhi, R.; Hasani, A.; Yasin, D.; Helou, M. Large Ventricular Myxoma Causing Inflow and Outflow Obstruction of the Right Ventricle; A Case Report. J. Cardiothorac. Surg. 2024, 19, 540. [Google Scholar] [CrossRef]
- Floria, M.; Burlacu, A.; Morariu, P.C.; Oancea, A.-F.; Iov, D.-E.; Baroi, G.L.; Stafie, C.S.; Scripcariu, V.; Tănase, D.M. Multimodality Imaging in Right Heart Tumors: Proposed Algorithm towards an Appropriate Diagnosis. J. Clin. Med. 2024, 13, 1000. [Google Scholar] [CrossRef]
- Luo, C.; Zhu, J.; Bao, C.; Ding, F.; Mei, J. Minimally Invasive and Conventional Surgical Treatment of Primary Benign Cardiac Tumors. J. Cardiothorac. Surg. 2019, 14, 76. [Google Scholar] [CrossRef]
- Rahouma, M.; Mohsen, H.; Morsi, M.; Khairallah, S.; Azab, L.; Abdelhemid, M.; Kumar, A.; El-Sayed Ahmed, M.M. Prevalence, Diagnosis, and Treatment of Cardiac Tumors: A Narrative Review. J. Clin. Med. 2025, 14, 3392. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Malla, S.R.; Mahapatra, R.P.; Barik, R.; Sethy, M.; Acharya, D.; Malla, H.S.; Panda, D.; Singh, P.K.; Kumar, M. Right Ventricular Myxoma Mimicking Heart Failure. JACC Case Rep. 2025, 30, 105148. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, G.; Vernekar, J.A.; Lawande, A.; Caculo, V. Cardiac MRI in Right Ventricular Outflow Tract Myxoma: Case Report with Review of Literature. J. Cardiol. Cases 2020, 22, 128–131. [Google Scholar] [CrossRef]
- Bruce, C.J. Cardiac Tumours: Diagnosis and Management. Heart 2011, 97, 151–160. [Google Scholar] [CrossRef]
- Hoffmeier, A.; Sindermann, J.R.; Scheld, H.H.; Martens, S. Cardiac Tumors—Diagnosis and Surgical Treatment. Dtsch. Arztebl. Int. 2014, 111, 205–211. [Google Scholar]
- Okongwu, C.C.; Olaofe, O.O. Cardiac Myxoma: A Comprehensive Review. J. Cardiothorac. Surg. 2025, 20, 151. [Google Scholar] [CrossRef]
- Pinede, L.; Duhaut, P.; Loire, R. Clinical Presentation of Left Atrial Cardiac Myxoma. A Series of 112 Consecutive Cases. Medicine 2001, 80, 159–172. [Google Scholar] [CrossRef]
- Patel, J.; Sheppard, M.N. Pathological Study of Primary Cardiac and Pericardial Tumours in a Specialist UK Centre: Surgical and Autopsy Series. Cardiovasc. Pathol. 2010, 19, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Kim, J.-H.; Na, C.-Y.; Oh, S.-S. Eleven Years’ Experience with Korean Cardiac Myxoma Patients: Focus on Embolic Complications. Cerebrovasc. Dis. 2012, 33, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Iovanovici, D.-C.; Negru, A.G.; Nistor-Cseppento, C.; Tit, D.M.; Niculescu, V.; Bungau, S.; Popescu, M. Association of the Echocardiographic Parameters with the Physical Dimension of Quality of Life. Balneo PRM Res. J. 2024, 15, 722. [Google Scholar] [CrossRef]
- Griborio-Guzman, A.G.; Aseyev, O.I.; Shah, H.; Sadreddini, M. Cardiac Myxomas: Clinical Presentation, Diagnosis and Management. Heart 2022, 108, 827–833. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Graboń, K.; Paczesny, Ł.; Kruczyński, J. Avascular Necrosis of Femoral Condyles in a Patient Treated Due to Lymphoma. A Case Study. Ortop. Traumatol. Rehabil. 2017, 19, 469–480. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Dunham, A.; Huang, H.; Bukhari, S.M.A.; Mehta, A.; Awuah, W.A.; Ede-Imafidon, D.; Cantu-Herrera, E.; Talukder, S.; Joshi, A.; et al. Chemotherapy Induced Cardiotoxicity: A State of the Art Review on General Mechanisms, Prevention, Treatment and Recent Advances in Novel Therapeutics. Curr. Probl. Cardiol. 2023, 48, 101591. [Google Scholar] [CrossRef]
- Chin, V.; Finnegan, R.N.; Keall, P.; Otton, J.; Delaney, G.P.; Vinod, S.K. Overview of Cardiac Toxicity from Radiation Therapy. J. Med. Imaging Radiat. Oncol. 2024, 68, 987–1000. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Bai, Y.; Xu, H. Do Cancer Survivors Have an Increased Risk of Developing Subsequent Cancer? A Population-Based Study. J. Transl. Med. 2025, 23, 355. [Google Scholar] [CrossRef]
- Ushida, E.; Toyoda, H.; Kohso, A.; Okumura, Y.; Niwa, K.; Ito, T.; Morimoto, M.; Hanaki, R.; Amano, K.; Iwamoto, S.; et al. Secondary Neoplasms in Survivors of Pediatric Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma: A Single-Center, Retrospective Study. Front. Pediatr. 2025, 13, 1530832. [Google Scholar] [CrossRef]
- Poyer, F.; Dieckmann, K.; Dworzak, M.; Tamesberger, M.; Haas, O.; Jones, N.; Nebral, K.; Köhrer, S.; Moser, R.; Kropshofer, G.; et al. Second Malignant Neoplasms after Treatment of 1487 Children and Adolescents with Acute Lymphoblastic Leukemia-A Population-Based Analysis of the Austrian ALL-BFM Study Group. eJHaem 2022, 3, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. JACC Cardio Oncol. 2020, 2, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Campo-Rivera, N.; Aguilar-Molina, O.; Barbosa-Balaguera, S. Myxoma Mimic in a Patient With Acute Myeloid Leukemia. Cureus 2023, 15, e43714. [Google Scholar] [CrossRef]
- Lazar, D.R.; Maniu, D.; Lazar, F.-L.; Blag, C.; Bota, M.; Zdrenghea, M.; Cainap, S. Exploring the Baseline Cardiac Function and Its Correlation with Risk Stratification in Children Diagnosed with Acute Lymphoblastic Leukemia. Ann. Hematol. 2025, 104, 4157–4164. [Google Scholar] [CrossRef]
- Laird-Fick, H.; Tiwari, A.; Narayanan, S.; Qin, Y.; Vodnala, D.; Bhutani, M. A Case of Comorbid Myxoma and Chronic Lymphocytic Leukemia: Not Just a Coincidence? Case Rep. Oncol. Med. 2014, 2014, 142746. [Google Scholar] [CrossRef]
- Pui, C.-H.; Nichols, K.E.; Yang, J.J. Somatic and Germline Genomics in Paediatric Acute Lymphoblastic Leukaemia. Nat. Rev. Clin. Oncol. 2019, 16, 227–240. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustea, C.; Radu, A.-F.; Maghiar, P.B.; Brata, R.; Babes, E.E. Swinging Mass Through the Pulmonary Valve: A Rare Case of Right Ventricular Myxoma. Life 2025, 15, 1750. https://doi.org/10.3390/life15111750
Bustea C, Radu A-F, Maghiar PB, Brata R, Babes EE. Swinging Mass Through the Pulmonary Valve: A Rare Case of Right Ventricular Myxoma. Life. 2025; 15(11):1750. https://doi.org/10.3390/life15111750
Chicago/Turabian StyleBustea, Cristiana, Andrei-Flavius Radu, Paula Bianca Maghiar, Roxana Brata, and Elena Emilia Babes. 2025. "Swinging Mass Through the Pulmonary Valve: A Rare Case of Right Ventricular Myxoma" Life 15, no. 11: 1750. https://doi.org/10.3390/life15111750
APA StyleBustea, C., Radu, A.-F., Maghiar, P. B., Brata, R., & Babes, E. E. (2025). Swinging Mass Through the Pulmonary Valve: A Rare Case of Right Ventricular Myxoma. Life, 15(11), 1750. https://doi.org/10.3390/life15111750





